Abstract
Marine microheterotrophs thraustochytrids are emerging as a potential source for commercial production of polyunsaturated fatty acids (PUFA) that have nutritional and pharmacological values. With prospective demand for PUFAs increasing, biotechnological companies are looking for potential increases in those valuable products. However, high levels of NaCl in the culture media required for optimal thraustochytrid growth and PUFA production poses a significant problem to the biotechnological industry due to corrosion of fermenters calling for a need to reduce the amount of NaCl in the culture media, without imposing penalties on growth and yield of cultured organisms. Earlier, as reported by Shabala et al. (Environ Microbiol 11:1835–1843, 2009), we have shown that thraustochytrids use sodium predominantly for osmotic adjustment purposes and, as such, can be grown in low-salt environment without growth penalties, providing the media osmolality is adjusted. In this study, we verify if that conclusion, made for one specific strain and osmolyte only, is applicable to the larger number of strains and organic osmotica, as well as address the issue of yield quality (e.g., PUFA production in low-saline media). Using mannitol and sucrose for osmotic adjustment of the growth media enabled us to reduce NaCl concentration down to 1 mM; this is 15–100-fold lower than any method proposed so far. At the same time, the yield of essential PUFAs was increased by 15 to 20 %. Taken together, these results suggest that the proposed method can be used in industrial fermenters for commercial PUFA production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
An interest in the nutritional importance of long chain polyunsaturated fatty acids (PUFAs) has increased markedly in recent years. As the importance of the presence and proportions of various PUFAs in human and animal diets becomes better understood (Horrocks and Yeo 1999; Simopoulos 1999; James et al. 2003), demand for, and hence the value of, these dietary components is expected to increase further. At present, fish oils and cultured phototrophic microalgae are the main commercial sources of PUFA. The possible decline of commercial fish stocks and the relatively complex technology required to commercially produce microalgae have prompted research into possible alternative sources of PUFA. The culture of thraustochytrids and other PUFA-producing microheterotrophs is seen as one such alternative (Lewis et al. 1999; Barclay et al. 2005; Ward and Singh 2005). Thraustochytrids, heterotrophic marine protists, are now established candidates for commercial production of the omega-3 polyunsaturated fatty acids, docosahexaenoic acid (DHA), that are important in human health and aquaculture (Ward and Singh 2005; Raghukumar 2008; Batbatan et al. 2011). Thraustochytrid DHA is now commercially available as nutritional supplements for adults and as feeds to enhance DHA levels in larvae of aquaculture animals (Ward and Singh 2005; Raghukumar 2008; Ratledge 2012).
Optimisation of growth conditions is a crucial step in the development of microbial technologies (Kamagata and Tamaki 2005; Raghukumar 2008; Shene et al. 2010). The effect of salinity is of a particular importance. It was assumed that, being marine organisms, thraustochytrids needed NaCl to maintain optimal metabolism and growth. However, high levels of NaCl in the culture media pose a significant problem to biotechnological industry due to corrosion of fermenters (Barclay et al. 1994) and, more prosaically, disposal of effluent. Therefore, it is highly desirable to reduce the amount of NaCl (and chloride specifically) in the culture media to levels at which corrosion is minimal and, at the same time, provide conditions enabling the optimal growth and yield of cultured organisms.
Our early physiological studies (Shabala et al. 2009) revealed that thraustochytrids use NaCl primarily for osmotic adjustment and suggested that they can be grown in low-salt environment (as low as 1 mM NaCl) without growth penalties, providing the media osmolality is adjusted. The above conclusion was made for one specific strain (ACEM-C) and one specific osmolyte (mannitiol) only. In this follow-up work, we extend these findings for a broader range of thraustochytrid strains and a range of organic osmotica. We show that, regardless the genotype and a type of organic osmolyte used for osmtotic adjustment of the media, organisms can be grown in low-salt media without any negative impact on growth and yield. Moreover, we show that substitution of NaCl with organic osmolytes such as sucrose or mannitol results in a 15 to 20 % yield increase, achieving the same ratio of DHA. In practical terms, our method allows thraustochyrid culture at NaCl concentrations as low as 1 mM, 15 to 100-fold lower than any method proposed. Thus, corrosion of fermentation tanks is prevented.
Materials and Methods
Microheterotroph Culture and Cell Preparation
The new Australian strains used in this study (ACEM 6063, ACEM A, and ACEM E) were isolated by Lewis (2001) as part of his Ph.D. study. This included development of a phylogenetic tree based on 18SrDNA sequences of 15 isolates and a culture derived from Algamac-2000, a commercially available preparation. The sequences were aligned with those of 18 thraustochytrid strains (Honda et al. 1999) and one thraustochytrid clone (Lopez-Garcia et al. 2001). Strain ACEM E clustered with Japonochytrium sp. ATCC 28207; strain ACEM 6063 clustered close to Schizochytrium limnacium and the Algamac-2000 strain. Strain ACEM A was most closely related to strain ACEM 0004, but these were widely separated from all other strains included in the analysis. The fact that the strains isolated by Lewis (2001) were spread widely across the spectrum of thraustochytrid-like taxa including strains in three genera strongly suggests that the ability to grow in low-salt media is also a taxonomically widely distributed trait. The strains used are notable for producing high levels of the ω-3 fatty acid DHA (22:6ω3). Thraustochytrid cultures were grown for 7 days essentially as described in our earlier publications (Carter et al. 2003; Shabala et al. 2001, 2009).
Culture Maintenance and Growth
Cultures were maintained in a medium adapted from Iida et al. (1996) and Singh and Ward (1996), essentially as described in our earlier work (Shabala et al. 2001, 2009). The same nutrient composition was used for inoculum preparation. Cells in conical flasks of 250 mL were inoculated by the addition of 1 mL of inoculum to 50 mL of medium. Cultures were grown in thermostatic conditions at 20 °C with shaking by the oscillatory motion of the incubator through an arc of 60° at a rate of 60 oscillations per minute (Toyo Kagaku Sangyo, Tokyo, Japan) for up to 7 days (unless peak of biomass was achieved). For biomass determination experiments, cultures were grown in a modified medium containing different amounts of NaCl (Shabala et al. 2009). The composition of the media was: 25 mM MgCl2; 10 mM MgSO4; 35 mM CaCl2; 30 mM glutamic acid; 15 mM KCl; 0.1 mM KH2PO4; 5 g/L bacteriological peptone; 5 g/L glucose; 3 g/L yeast extract; vitamins and trace metals were provided in amounts stated by Shabala et al. (2001, 2009). Growth studies were performed in the above growth medium containing various concentrations of NaCl (0, 1, 10, 50, 100, 200, 500 mM). Half of the cells grew at different levels of NaCl in the absence of compatible solutes (and therefore, in solutions with different osmolality), while the other half had been grown in isotonic solutions of ~0.98 Os kg−1 (equivalent of the seawater, e.g., 500 mM NaCl) prepared using appropriate amounts of compatible solutes (mannitol or sucrose). The osmolality of growth medium for each treatment was measured and adjusted using 5520 Vapro Vapor Pressure osmometer (Wescor, Inc., USA) by adding an appropriate amount of mannitol or sucrose. To adjust osmolality of the growth media with sucrose, 251 and 245 g/L of sucrose was added to the medium containing 1 and 10 mM NaCl, respectively. Relevant amounts of mannitol used were 150 and 146 g/L for medium containing 1 and 10 mM NaCl, respectively. Flasks were inoculated with prepared thraustochytrid cultures to give starting OD650 = 0.063. Cultures were grown at 20 °C with shaking as described above. The sample size was four to six for each treatment, and experiments were repeated at least twice.
Biomass Determination
For biomass determination cultures were sampled after inoculum achieved peak biomass essentially as described in our earlier work (Carter et al. 2003). Samples (10 mL) were aseptically drawn into sterile, 15-mL screw-top plastic centrifuge tubes (Greiner, Germany), immediately centrifuged (5,000 × g, 10 min; EasySpin, Sorval Instruments, U.S.A.), resuspended in 10 mL of 1.0 % (w/v) NaCl, and recentrifuged. Cell pellets were frozen, freeze-dried (chamber temperature −110 °C, Mini Ultra Cold, Dynavac Australia; pressure <7 × 10−1 mbar, RV3 vacuum pump, Edwards High Vacuum International) for 15 h, weighed, and then stored at −30 °C. Culture biomass (milligramme dry weight per litre) was determined daily using freeze-dried samples. Dry weight of thraustochytrids grown under different salinities, with or without adjustment of medium osmolality using sucrose or mannitol, was expressed as percent of the dry weight of thraustochytrids grown in medium containing 500 mM (used as control).
Lipid Analysis
Analyses were performed on three thraustochytrid strains collected when cultures reached peak biomass essentially as described in our earlier publication (Lewis et al. 2000). Briefly, lipids were extracted in duplicate from each sample, using a 1-phase chloroform–methanol–water (1:2:0.8 v/v/v) extraction (White et al. 1979). Solvents were added to the freeze-dried biomass in order of increasing polarity, as this technique led to an increased lipid recovery from the cultures (Lewis et al. 2000). The biomass–solvent mixtures were left in darkness overnight. Phase separation was achieved the next day by adding chloroform and water to obtain a final chloroform–methanol–water ratio of 1:1:0.9 (v/v/v). Lipids were recovered from the lower chloroform phase. Solvents were removed under vacuum prior to the extracted lipids being stored in chloroform under N2 at −20 °C. Lipid fraction were analyzed by gas chromatography using a Hewlett Packard 5890 A gas chromatograph equipped with a 50-m × 0.32-mm internal diameter cross-linked HP5 methyl silicone (0.17-μm film thickness) fused silica capillary column, an HP7673A autosampler, a split/splitless injector, and a flame ionization detector (Carter et al. 2003). Peak areas were recorded and quantified using Millenium 32 version 3.05.01 (Waters Corporation, USA). Mass spectrometric data were obtained using a GCQ (Thermoquest, USA) GC–mass spectrometer, operated as described in Nichols et al. (1996).
Results
Thraustochytrid Yield is Improved When Media Osmolality is Adjusted to Optimal Values with Compatible Solutes
There was a clear relationship between dry weight of thraustochytrids and the amount of sodium present in the growth medium (Table 1; Fig. 1). Dry weight was 15 to 35 % lower in all thraustochytrid strains tested when they were grown in media containing low NaCl concentrations (1 to10 mM) compared to controls (cells grown at seawater salinity). However, when low NaCl media were complemented with compatible solutes (either mannitol or sucrose) to achieve the osmotic potential of the seawater, cell dry weight was substantially increased (Fig. 1; Table 1). The absolute values, however, differed for the strains with thraustochytrid strains ACEM E and ACEM A responding better than strain ACEM 6063. The highest yield (2.4-fold higher than control) was reported in strain ACEM E grown in medium containing 1 mM NaCl with osmolality adjusted with mannitol. In general, both 1 and 10 mM naCl concentrations were equally efficient when complemented with organic osmolytes. At the same time thraustochytrid strain ACEM 6063 underperformed in similar conditions suggesting that in this strain NaCl may be used for purposes other then osmotic adjustment, and that higher levels of NaCl are required for its optimal growth.
Dry weight of thraustochytrid strains ACEM E (a), ACEM A (b), and ACEM 6063 (c). Thraustochytrids were grown in medium containing 1, 10, 500 mM NaCl and in low-osmolality medium (1 and 10 mM NaCl) supplemented with mannitol (150 and 146 g/L added to the medium containing 1 and 10 mM NaCl, respectively) or sucrose (251 and 245 g/L in 1 and 10 mM NaCl medium, respectively) to achieve the overall osmolality of 0.98 Os kg−1 (isoosmotic to control conditions; 500 mM NaCl). Data are average of two experiments with four to six replicates in each variant. Error bars are SEM (n = 8–12)
Optimal Osmolality of Growth Media Increases Proportion of PUFA in Total FAs
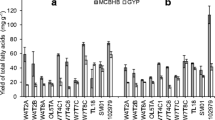
Decrease in medium osmolality resulted in a decrease in the overall amount of unsaturated fatty acids (both monounsaturated and PUFA) as shown for the strain ACEM A (Fig. 2a, b). Detailed analysis of selected FAs is shown in Table 2. Growth in a low osmolality medium (1 and 10 mM NaCl) reduced the proportion of PUFA among total FAs by 37 % and 28 %, respectively, compared to the control (Fig. 2a). At the same time, the proportion of saturated fatty acids substantially increased at the expense of decreased PUFAs (Table 2). Indeed, reducing the amount of NaCl in the growth media from 500 mM NaCl to 10 and 1 mM NaCl led to an increase of saturated FAs 14:0 by 38 % and 61 % over control, respectively. Saturated 16:0 FAs also increased with decreased medium osmolality by 6.5 % and 119 % for media containing 10 and 1 mM NaCl, respectively.
Fatty acid composition of thraustochytrid ACEM A grown at different NaCl concentrations (1 and 10 mM) and in the low-NaCl medium supplemented with amounts of mannitol and sucrose to achieve osmolality of 0.98 Os kg−1 (details on sucrose and mannitol used are given in a legend to Fig. 1). Medium containing 500 mM NaCl served as a control. Cultures were harvested at peak biomass and assessed for fatty acid composition. a Comparative levels of polyunsaturated, monounsaturated and saturated fatty acids. b Detailed compositions of fatty acid profiles. c Effects of compatible solutes on amount of ARA and DHA. Experiments were conducted twice with similar results
Addition of compatible solutes (such as sucrose and mannitol) to growth media to achieve osmolality isotonic to 500 mM NaCl increased amounts of PUFAs in all strains tested to a level similar to the control (500 mM NaCl) or higher. This is clearly demonstrated with thraustochytrid ACEM A (Fig. 2; Table 2). Indeed, while in control conditions PUFA accounted for 33 % of total FAs, this value increased to 45 % and 48 % when thraustochytrids were grown in a medium containing 10 mM NaCl supplemented with sucrose and mannitol, respectively (Fig. 2a). Moreover, amounts of PUFA in cultures grown in low (10 mM) NaCl that was balanced with sucrose and mannitol to optimal osmolality exceeded those of control by 28 % and 36 %, respectively. Increases in PUFAs were compensated by decreases in saturated FAs. Thus, amounts of saturated fatty acids 14:0 decreased by ~37 % when growth media containing 10 mM NaCl were supplemented with either mannitol or sucrose (Table 2). Similar decreases in 18:0 fatty acids were noted to be 19 % and 17 % in the presence of mannitol and sucrose, respectively.
Optimal Osmolality of Growth Medium Increases Proportion of ω-6 and ω-3 PUFAs
Of particular interest to biotechnology are nutritionally important ω-6 and ω-3 PUFAs. Thraustochytrids are a known source of those PUFAs, specifically of arachidonic acid (ARA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) (Ward and Singh 2005; Raghukumar 2008; Batbatan et al. 2011). We analyzed fatty acid composition of the studied thraustochytrids with special emphasis on important PUFAs (Fig. 2b, c; Table 2). We found that the amount of these important PUFAs decreased substantially when thraustochytrids were grown in low osmolality medium. Growth in media of 1 and 10 mM NaCl led to a decrease in EPA by 59 % and 40 %, respectively, compared to the control (500 mM NaCl). Similar growth conditions also resulted in decrease in an essential fatty acid, linoleic acid 18:2w6 by 26 % and 33 % in 1 and 10 mM NaCl, respectively. A relative decrease of the level of ARA in total FAs was 30 % and 2 % for 1 and 10 mM NaCl compared to control (Fig. 2c). At the same time, adjustment of osmolality with compatible solutes to the level of seawater salinity led to increase of the above PUFAs to the levels similar to control or even higher. For example, levels of ARA in thraustochytrids grown in low NaCl media (1 mM NaCl) supplemented with mannitol or sucrose were similar to the control or increased above control values by 40 to 60 % (10 mM NaCl) for the above compatible solutes, respectively. Likewise, amount of DHA exceeded the control culture by 57 % and 49 % when cells were grown in low-salinity media (10 mM NaCl) supplemented with mannitol or sucrose, respectively. Overall, we found that maintenance of optimal osmolality of growth media plays a crucial role in production of PUFAs and high levels can be maintained when thraustochytrids are grown in low-salinity media provided that the osmolality of the growth media is adjusted to the optimal level with compatible solutes.
Discussion
Thraustochytrids are emerging as a potential source of PUFAs that have nutritional and pharmacological values and highly sought after (Lewis et al. 1999; Ward and Singh 2005; Raghukumar 2008; Ratledge 2012). With prospective demand for PUFAs increasing, biotechnological companies are looking for potential increases in those valuable products. Being marine organisms, thraustochytrids require high levels of sea salts for optimal growth. Sea salt, however, is not suitable for large-scale commercial operation as its most abundant element is chloride, which is very corrosive to steel fermenters (Barclay et al. 1994).
It was reported that thraustochytrids can grow in salinities ranging from 10 to 100 % seawater but a number of publications showed that cell yield is substantially affected by amount of salt in the growth media (Goldstein 1963; Bahnweg 1979; Garrill et al. 1992; Zhou et al. 2007; Nagano et al. 2009). Likewise, we reported that thraustochytrid growth rate and yield decreased with a decrease in NaCl concentration in the growth media (Shabala et al. 2001, 2009). However, in our recent work we have suggested that thraustochytrid requirements for high Na+ and Cl− may be explained by their requirements for optimal osmotic adjustment (Shabala et al. 2009). This conclusion, made using only one strain and one osmolyte (Shabala et al. 2009) is now extended to include a range of new thraustochytrid strains (strains ACEM A, E, and 6063) and compatible solutes (sucrose, mannitol), and related to FA production. We confirm our earlier hypothesis and demonstrate that decreased yield of thraustochytrid cells at low NaCl concentration was due to low osmolality of the growth medium. We further demonstrate that increasing growth media osmolality to optimal levels with sucrose and mannitol substantially increased dry weight of thraustochytrids. The latter values exceeded controls in two out of three strains tested (ACEM E and ACEM A, Fig. 1). We show increasing osmolality of growth media with compatible solutes such as sucrose or mannitol enabled optimal growth of thraustochytrids in as low NaCl concentration as 1–10 mM. Such a low concentration of salt in the growth media would prevent corrosion of fermentors used for culturing thraustochytrids.
A number of other suggestions were made to minimize corrosion of fermentors used in biotechnology for growth of thraustochytrids and other marine organisms. As corrosion occurs mainly due to high concentration of chlorides, it was proposed to replace those salts with sulphates (Unagul et al. 2005, 2006). Similar claim was made in a US Patent N6410281 suggesting to reduce NaCl levels in the growth medium to about 3 g of chloride per litre (equivalent of about 100 mM NaCl or one out of five of the seawater) by substituting the remaining NaCl with Na2SO4 (Barclay 1999). There are at least two reasons, however, why this approach is not practical. First, 100 mM NaCl suggested is still very high to avoid corrosion problems to fermenters. Second, when chloride is replaced by sulphate (as proposed), a serious problem with solubility of sulphates (salt clamping) might occur at about 500 mM concentration required for optimal growth medium.
The major reason of thraustochytrid culturing is the high level of PUFAs found in the protist. It was reported that lipid production of the thraustochytrid isolates was highest at optimum salinity and correlated with biomass production (Leaño et al. 2003). Therefore, biomass levels might be used as a guide while screening thraustochytrid strains for their efficacy. Indeed, growth in medium containing 1 or 10 mM NaCl reduced amounts of PUFAs in the total fatty acids by 37 % and 27 %, respectively (Fig. 2a). Here we show, for the first time, that increasing growth media osmolality to optimal also correlated with increase in PUFA production. Indeed, PUFA content in total fatty acids exceeded relevant values of controls by 37 % and 29 % when growth media were supplemented with mannitol and sucrose, respectively (Fig. 2a). This is further supported by the high correlation (0.94) between cell dry weight and amount of PUFA produced at different concentrations of NaCl in the growth media.
Using several strains of thraustochytrids enabled us to show variability in responses to different salinity levels and osmolality adjustment. Observed differences in responses among strains tested suggest strain specificity and different sensitivity to variations in osmolality. Similar to dry weight data, changes in FA composition were more pronounced in strain ACEM E (Fig. 2a) suggesting that this strain is more sensitive to osmolality changes and therefore is more ‘responsive’ to compatible solute addition. These suggestions are in accord with the finding that the level of osmotolerance and the osmolyte systems varies among thraustochytrids (Jakobsen et al. 2007).
Essential PUFAs, arachidonic acid (20:4ω6, ARA) and docosahexaenoic acid (22:6ω3, DHA), are of particular interest to biotechnology as valuable sources of ω6 and ω3 fatty acids, respectively (Lewis et al. 1999; Ward and Singh 2005; Raghukumar 2008). However, the effect of salinity on these important PUFAs was not clearly defined (Unagul et al. 2005). Here we show that decreasing the salinity of growth media substantially decreased production of valuable ω3 and ω6 PUFAs (Fig. 2c). At the same time, maintenance of optimal osmolality by compatible solutes increased amounts of APA and DHA in total fatty acids. We found no difference in ARA content when thraustochytrids were grown in media containing 1 mM NaCl with osmolality adjusted to optimal using sucrose or mannitol. Moreover, ARA content increased by 40 and 60 % above control values in the presence of 10 mM NaCl and compatible solutes in the growth media. Similar results were observed for DHA, another important PUFA.
It is well-known that carbon source affects the lipid content in cultures of fungus and yeast (reviewed in Shene et al. 2010). Presence of monosaccharides (glucose and fructose), starch or glycerol in the growth media was shown to improve cell growth and positively affect PUFA production in thraustochytrids (Goldstein 1963; Bajpai et al. 1991; Li and Ward 1994; Yokochi et al. 1998; Zhou et al. 2007; Quilodrán et al. 2009). In contrast, di- and polysaccharides were shown to give poor cell growth and FA production (Yokochi et al. 1998; Burja et al. 2006) and no information is available for a relevant data on polyols. Indeed, more studies are required to establish specific role of compatible solutes when used for osmotic adjustment of low-osmolality growth medium. As for now, our data strongly suggest that sucrose and mannitol in our experiments were used for osmotic adjustment rather than as an additional carbon source. Recently, there has been increasing interest in using food processing waste or alternative carbon sources for thraustochytrid growth (Yamasaki et al. 2006; Shene et al. 2010; Arafiles et al. 2011). Those products provide additional carbon and nitrogen source for thraustochytrid growth. Importantly, often these are the preferable carbon sources such as glycerol and monosaccharides (Bahnweg 1979; Unagul et al. 2007; Shene et al. 2010). Published work strongly suggests that food processing waste or alternative products might be also used as a low-cost by-product for osmotic adjustment in thraustochytrids.
Overall, our results demonstrate that maintenance of osmolality is of paramount importance to efficient growth and PUFA production in thraustochytrids and strongly support a novel approach proposed to grow those marine protists in the media of low NaCl content providing that optimal osmolality is achieved by media supplementing with compatible solutes. As most of corrosion is apparently due to chloride, this approach will enable to avoid fermenter corrosion during thraustochytrid growth. The finding that cell growth characteristics and PUFA content appear to be unaffected or even benefit when cells are grown in low-salt media complemented with organic osmolytes may be of significant importance for biotechnology industry. In practice, the use of compatible solutes to adjust the media osmolality may provide an alternative to existing practices. Findings presented here may lead to large savings for companies involved in growth of thraustochytrids and possibly other organisms of marine origin.
References
Arafiles KHV, Alcantara JCO, Batoon JAL, Galura FS, Cordero PRF, Leaño EM, Dedeles GR (2011) Cultural optimization of thraustochytrids for biomass and fatty acid production. Mycosphere 2:521–531
Bahnweg G (1979) Studies on the physiology of Thraustochytriales. I. Growth requirements and nitrogen nutrition of Thraustochytrium spp., Schizochytrium sp., Japonochytrium sp., Ulkenia spp., and Labyrinthuloides spp. Veroff Inst Meeresforsch. Bremerhaven 17:245–268
Bajpai PK, Bajpai P, Ward OP (1991) Optimization of production of docosahexaenoic acid (DHA) by Thraustochytrium aureum ATCC 34304. J Amer Oil Chem Soc 68:509–514
Barclay WR (1999) Reducing corrosion in a fermentor by providing sodium with a non-chloride sodium salt. US Patent 6,410,281, 14 Dec 1999
Barclay WR, Meager KM, Abril JR (1994) Heterotrophic production of long chain omega-3 fatty acids utilizing algae and algaelike microorganisms. J Appl Phycol 6:123–129
Barclay WR, Weaver C, Metz J (2005) Development of a docosahexaenoic acid production technology using Schizochytrium: a historical perspective. In: Cohen Z, Ratledge C (eds) Single cell oil. AOCS, Champaign
Batbatan CG, Hepowit NL, Oclarit JM (2011) Developmental historicity and saccharide heterotrophy of Schizochytrium sp OT01: implication of docosahexaenoic acid production for biotechnological applications. Asia Life Sci 20:289–305
Burja AM, Radianingtyas H, Windust A, Barrow CJ (2006) Isolation and characterization of polyunsaturated fatty acid producing Thraustochytrium species: screening of strains and optimization of omega-3 production. Appl Microbiol Biotechnol 72:1161–1169
Carter CG, Bransden MP, Lewis TE, Nichols PD (2003) Potential of thraustochytrids to partially replace fish oil in Atlantic salmon feeds. Marine Biotechnol 5:480–492
Garrill A, Clipson NJW, Jennings DH (1992) Preliminary observations on the monovalent cation relations of Thraustochytrium aureum, a fungus requiring sodium for growth. Mycol Res 96:295–304
Goldstein S (1963) Development and nutrition of new species of Thraustochytrium. Am J Bot 50:271–279
Honda D, Yokochi T, Nakahara T, Ragukumar S, Nakagiri A, Schaumann K, Higashihara T (1999) Molecular phylogeny of labyrnthulids and thraustochytrids based on sequencing of 18S ribosomal RNA gene. J Eukar Microbiol 46:637–647
Horrocks LA, Yeo YK (1999) Health benefits of docosahexaenoic acid (DHA). Pharmacol Res 40:211–225
Iida I, Nakahara T, Yokochi T, Kamisaka Y, Yagi H, Yamaoka M, Suzuki O (1996) Improvement of docosahexaenoic acid production in a culture of Thraustochytrium aereum by medium optimisation. J Ferment Bioeng 81:76–78
Jakobsen AN, Aasen IM, Strom AR (2007) Endogenously synthesized (−)-proto-quercitol and glycine betaine are principal compatible solutes of Schizochytrium sp strain S8 (ATCC 20889) and three new isolates of phylogenetically related thraustochytrids. Appl Environ Microbiol 73:5848–5856
James MJ, Ursin VM, Cleland LG (2003) Metabolism of stearidonic acid in human subjects: comparison with the metabolism of other n-3 fatty acids. Am J Clin Nutrition 77:1140–1145
Kamagata Y, Tamaki H (2005) Cultivation of uncultured fastidious microbes. Microb Environ 20:85–91
Leaño EM, Gapasin RSJ, Polohan B, Vrijmoed LLP (2003) Growth and fatty acid production of thraustochytrids from Panay mangroves, Philippines. Fungal Diversity 12:111–122
Lewis TE, Nichols PD, McMeekin TA (1999) The biotechnological potential of thraustochytrids. Mar Biotechnol 1:580–587
Lewis T, Nichols PD, McMeekin TA (2000) Evaluation of extraction methods for recovery of fatty acids from lipidproducing microheterotrophs. J Microbiol Methods 43:107–116
Li ZY, Ward OP (1994) Production of docosahexaenoic acid (DHA) by Thraustocytrium roseum. J Ind Microbiol 13:238–341
Lopez-Garcia P, Rodriguez-Valera, Pedros-Allo C, Moreira D (2001) Unexpected diversity of small eukaryotes in deep-sea Antarctic plankton. Nature 409:603–607
Nagano N, Taoka Y, Honda D, Hayashi M (2009) Optimization of culture conditions for growth and docosahexaenoic acid production by a marine thraustochytrid, Aurantiochytrium limacinum mh0186. J Oleo Sci 58:623–628
Nichols DS, Hart P, Nichols PD, McMeekin TA (1996) Enrichment of the rotifer Brachionus plicatilis fed an Antarctic bacterium containing polyunsaturated fatty acids. Aquaculture 147:115–125
Quilodrán B, Hinzpeter I, Quiroz A, Shene C (2009) Evaluation of liquid residues from beer and potato processing for the production of docosahexaenoic acid (C22:6n-3, DHA) by native thraustochytrid strains. World J Microbiol Biotechnol 21:2121–2128
Raghukumar S (2008) Thraustochytrid marine protists: production of PUFAs and other emerging technologies. Mar Biotechnol 10:631–640
Ratledge C (2012) Omega-3 biotechnology: errors and omissions. Biotechnol Adv 30:1746–1747
Shabala L, Shabala S, Ross T, McMeekin T (2001) Membrane transport activity and ultradian ion flux oscillations associated with cell cycle of Thraustochytrium sp. Aust J Plant Physiol 28:87–99
Shabala L, McMeekin T, Shabala S (2009) Osmotic adjustment and requirement for sodium in marine protist thraustochytrid. Environ Microbiol 11:1835–1843
Shene C, Leyton A, Esparza Y, Flores L, Quilodrán B, Hinzpeter I, Rubilar M (2010) Microbial oils and fatty acids: effect of carbon source on docosahexaenoic acid (C22:6 N-3, DHA) production by thraustochytrid strains. J Soil Sci Plant Nutr 10(3):207–216
Simopoulos AP (1999) Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutrition 54:438–463
Singh A, Ward OP (1996) Production of high yields of docosahexaenoic acid by Thraustochytrium roseum ATCC 28210. J Indust Microbiol 16:370–373
Unagul P, Assantachai C, Phadungruengluij S, Suphantharika M, Verduyn C (2005) Properties of the docosahexaenoic acid-producer Schizochytrium mangrovei Sk-02: effects of glucose, temperature and salinity and their interaction. Bot Marina 48:387–394
Unagul P, Assantachai C, Phadungruengluij S, Pongsuteeragul T, Suphantharika M, Verduyn C (2006) Biomass and docosahexaenoic acid formation by Schizochytrium mangrovei Sk-02 at low salt concentrations. Bot Marina 49:182–190
Unagul P, Assantachai C, Phadungruengluij S, Suphantharika M, Tanticharoen M, Verduyn V (2007) Coconut water as a medium additive for the production of docosahexaenoic acid (C22:6n3) by Schizochytrium mangrovei Sk-02. Bioresource Technol 98:281–287
Ward OP, Singh A (2005) Omega-3/6 fatty acids: alternative sources of production. Process Biochem 40:3627–3652
White DC, Davis WM, Nickels JS, King JD, Bobbie RJ (1979) Determination of the sedimentary microbial biomass by extractable lipid phosphate. Oecologia 40:51–62
Yokochi T, Honda D, Higashihara T, Nakahara T (1998) Optimization of docosahexaenoic acid production by Schizochytrium limacinum SR21. Appl Microbiol Biotechnol 49:72–76
Yamasaki T, Aki T, Shinozaki M, Taguchi M, Kawamoto S, Ono K (2006) Utilization of shochu distillery wastewater for production of polyunsaturated fatty acids and xanthophylls using thraustochytrid. J Biosci Bioeng 102:323–327
Zhou L, Lu YH, Zhou MH, Zhao XW (2007) Enhanced production of docosahexaenoic acid using Schizochytrium sp by optimization of medium components. J Chem Eng Japan 40:1093–1100
Acknowledgments
This work was supported by ARC Discovery and University of Tasmania research grants to Prof. Sergey Shabala. We thank Dr. T. Lewis for his contribution to fatty acid analyses and information on strains origin.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shabala, L., McMeekin, T. & Shabala, S. Thraustochytrids Can Be Grown in Low-Salt Media Without Affecting PUFA Production. Mar Biotechnol 15, 437–444 (2013). https://doi.org/10.1007/s10126-013-9499-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-013-9499-y