Abstract
Because of the importance of shrimp in world aquaculture, there is much interest in understanding their immune system in order to improve their resistance to pathogenic microorganisms. An effective tool in studying genes involved in the immune response in shrimp is RNA interference (RNAi). RNAi, first recognized as an antiviral response against RNA viruses, is a cellular mechanism that is triggered by double-stranded RNAs and results in the degradation of homologous genes. In this review, we describe the current studies of genes in shrimp that employed RNAi technology to elucidate or confirm their functions. We also review the potential of RNAi to elicit antiviral response in shrimp.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Farmed shrimp are a popular food that fetch a high price in both local and international markets. Like other farmed organisms that are cultured at high density, shrimp are vulnerable to a wide array of bacterial and viral pathogens. One approach to controlling microbial pathogens is to better understand the shrimp immune system. While shrimp do not possess an adaptive immune system, they possess an innate immune system that is capable of protecting themselves against pathogens (Lee and Söderhäll 2002). It is a complex system that is poorly understood.
A recent technology for studying immune function is RNA interference or simply RNAi. RNAi is a process whereby small double-stranded RNAs (dsRNAs) trigger the post-transcriptional suppression or silencing of homologous genes in a sequence-specific manner (Fire et al. 1998; Hannon 2002; Tuschl et al. 1999). It has been recognized to be a natural defense mechanism in plants, nematode, insect, and mammals against foreign RNAs such as virus and transposons (Hannon 2002; Waterhouse et al. 2001). First observed in plants where it was referred to as post-transcriptional gene silencing (Vaucheret et al. 2001) or co-suppression (Jorgensen 1990), RNAi, gained considerable attention through the work of Fire et al.(1998) in Caenorhabditis elegans. Early studies showed that both sense and anti-sense RNA can effectively inhibit gene expression (Fire et al. 1991; Guo and Kemphues 1995). Fire and his colleagues found that injection of dsRNAs or a mixture of both RNA strands can silence genes with sequences complementary to the introduced dsRNAs and that injected dsRNAs are more potent as a silencing trigger than either sense or antisense RNA strands (Fire et al. 1998).
The RNAi silencing pathway involves the cleavage of long dsRNA by the RNAs-III-like enzyme Dicer into short RNA duplexes, around 21–28 bp in length, called short interfering RNAs (siRNAs) or microRNAs (miRNAs) in the case of miRNA precursors (Hammond et al. 2000; Elbashir et al. 2001). These short RNA duplexes will subsequently unwind and assemble into the RNA-induced silencing complex (RISC) (Hammond et al. 2000). The single-stranded siRNA in the RISC eventually guides the degradation of complimentary target mRNAs leading to translational repression of the target gene (Martinez et al. 2002).
Although relatively new, RNAi has become a staple in recent molecular studies as a functional genomics tool. RNAi has been used to elicit gene function in worms (Mori et al. 2008; Palakodeti et al. 2008), insects (Cruz et al. 2008; McGregor et al. 2008), to plants (Sappl et al. 2008; Bhuiyan et al. 2008) and livestock (Golding et al. 2006), as well as in cancerous tissues (Lam et al. 2008; Sun et al. 2008). Here we review the recent work in shrimp that has employed RNAi technology and discuss the potential of RNAi to further the understanding of the immune mechanisms in shrimp.
RNAi in Shrimp
The innate immune response in shrimp is mediated by cellular and humoral mechanisms (Tincu and Taylor 2004). Cellular responses include phagocytosis, nodule formation, and encapsulation while humoral responses are effected by three major mechanisms: melanization through the prophenoloxidase (proPO) cascade, clotting mechanism, and release of anti-microbial peptides present in the hemolymph. Activation of the humoral response in shrimp begins with the recognition of microbial cell wall components by pattern recognition proteins in the hemolymph which will trigger the release of antimicrobial peptides and activate the proPO cascade.
Clotting System
Hemolymph coagulation or clotting is an integral part of the invertebrate immune system and the hemolymph-clotting phenomenon was first identified as a prominent defense system in horseshoe crab (Limulus polyphemus) by Bang in 1956 (Iwanaga and Lee 2005). Clotting serves to prevent blood loss due to injury and prevent microbes from entering the hemocoel and may also be linked with the release of antimicrobial substances (Iwanaga 2002). The key components in hemocyte coagulation are the clotting protein (CP) and transglutaminase (TGase), which mediates the polymerization or crosslinking of CP to form stable clots (Kopacek et al. 1993; Hall et al. 1999). Clotting is essentially achieved through the activation of TGase, which is released in the hemocytes or tissues, by Ca2+ ions in the plasma leading to the crosslinking of plasma CP molecules into large aggregates (Hall et al. 1999).
CP in crustaceans, first cloned and characterized in crayfish, is homologous to vitellogenins, proteins present in females of egg-laying animals, but differs from vitellogenins in function and are expressed in both males and females (Hall et al. 1999). It was later cloned in a number of shrimp species (Perazzolo et al. 2005; Reyes-Izquierdo and Vargas-Albores 2001; Yeh et al. 1999). On the other hand, tissue localization and cloning of crustacean TGase was done in crayfish (Wang et al. 2001) and also identified in shrimp (Chen et al. 2005; Huang et al. 2004).
RNAi was used to show that the transcription and translation of TGase and CP were effectively silenced in shrimp. Injection of dsRNAs homologous to TGase and CP inhibit blood coagulation in vivo. The effects of the knockdown of both genes showed that TGase- and CP-depleted shrimp hemolymph failed to coagulate at room temperature in contrast to control samples where coagulation was observed moments after their hemolymph were withdrawn. TGase- and CP-depleted shrimp were also more susceptible to white spot virus and Vibrio penaeicida infection (Maningas et al. 2008). These results provided further evidence that both genes are involved in the shrimp immune response.
Antimicrobial Peptides
Antimicrobial peptides (AMPs) constitute another major component of the innate immune defense system in marine invertebrates. AMPs, which have masses less than 10 kDa, have a role in the first line of host defense in many animal species (Boman 1995). Among AMPs' advantages are their small size, which allows them to be easily synthesized and be rapidly diffused to the point of infection and their ability to function without either high specificity or memory (Relf et al. 1999). Many AMPs also show remarkable specificity for prokaryotes with low toxicity to eukaryotic cells, making them good candidates for development as potential new antibiotics (Zasloff 1992). Shrimp have a number of AMPs including penaedins (Destoumieux et al. 1997), histones (Patat et al. 2004), crustin (Bartlett et al. 2002; Supungul et al. 2004), anti-lipopolysaccharide factor (ALF) (Somboonwiwat et al. 2005; Liu et al. 2005), and hemocyanin fragments (Destoumieux-Garzon et al. 2001).
To date, RNAi was used to determine or confirm the functions of only two AMPs: ALF and crustin. ALF is a potent anticoagulant that acts by inhibiting the endotoxin- or lipopolysaccharide-mediated activation of the coagulation cascade (Tanaka et al. 1982). ALF gene expression was also found to increase in response to V. harveyi infection in black tiger shrimp, Penaeus monodon (Tharntada et al. 2008) and was proposed to be a viable prophylactic marker in shrimp (de Lorgeril et al. 2008). Silencing ALF in L. vannamei significantly increased the mortality of V. penaeicida- and Fusarium oxysporum-challenged shrimp, indicating the importance of ALF in bacterial clearance and defense against filamentous fungus infection (de la Vega et al. 2008). Furthermore, this work on ALF was also the first to demonstrate in vivo that a single antimicrobial peptide is necessary for shrimp resistance against diverse pathogens.
Crustin is a cysteine-rich antimicrobial peptide that acts against Gram-positive bacteria (Relf et al. 1999) including Vibrio harveyi, a major pathogenic bacterium in shrimp (Amparyup et al. 2008). Injection of dsRNA specific for crustin resulted in a significant increase in mortality of crustin-depleted shrimp after V. penaeicida challenge (Shockey et al. 2008). Interestingly, silencing crustin caused no significant mortality after F. oxysporum challenge (Shockey et al. 2008). Although both studies confirmed the importance of AMPs in the shrimp immune response, they also demonstrated that AMPs may vary in activity against various pathogenic organisms.
ProPO System
Melanization, through the proPO cascade, of pathogens and of damage tissues is regarded as a major innate response mechanism regulated by the enzyme phenoloxidase (PO). The proPO system is also considered a non-self recognition system since it is activated by small amounts of microbial components such as lipopolysaccharides, peptidoglycans, or β-1,3-glucans (Soderhall and Cerenius 1998). These microbial components or pathogen associated molecular patterns are recognized by pattern recognition receptors which will initiate a serine protease cascade leading to the eventual conversion of the inactive enzyme precursor, proPO, into phenoloxidase (PO). PO will in turn catalyze the oxidation of tyrosine to produce toxic quinones and finally leading to the formation of melanin (Cerenius et al. 2008; Soderhall and Cerenius 1998). Melanin could then bind to the surface of bacteria and increase the adhesion of hemocytes to bacteria, thus accelerating their removal by nodule formation (da Silva 2002). Invertebrate proPO was initially cloned and characterized in freshwater crayfish Pacifastacus leniusculus (Aspan et al. 1995), and to date, this gene has already been identified in shrimp and in several invertebrate species (Adachi et al. 1999; Ai et al. 2008, 2009; Aladaileh et al. 2007; Amparyup et al. 2009, reviewed in Cerenius and Soderhall 2004).
Silencing of proPO homologs in shrimp resulted in a significant decrease in endogenous proPO mRNA levels in the hemocytes and a substantial reduction in total PO activity (Amparyup et al. 2009; Fagutao et al. 2009). P. monodon proPO-silenced shrimp were also found to be more susceptible to V. harveyi infection (Amparyup et al. 2009). On the other hand, injection of dsRNA homologous to proPO in kuruma shrimp resulted in an abrupt increase in mortality of proPO-depleted samples even in the absence of a microbial challenge and a significant increase in endemic bacteria in the shrimp hemolymph (Fagutao et al. 2009). These reports confirm the importance of proPO in shrimp immune response.
Silencing of other Genes in Shrimp
RNAi technology also proved effective in analyzing the function of other genes in shrimp. Silencing of the lymphoid cell-expressed receptor for yellow head virus (YHV) in P. monodon (pmYRP65) conclusively proved its role as a receptor for YHV, and it resulted in the complete inhibition of virus entry (Assavalapsakul et al. 2006). Gene silencing of β-integrin, another prominent cell-surface receptor, effectively inhibited white spot syndrome virus (WSSV) infection, which suggests that β-integrin acts as a cellular receptor for WSSV (Li et al. 2007). RNAi has also been used to elucidate the function of genes involved in growth, molting, and reproduction in shrimp. Injection of P. monodon with dsRNA specific for the gonad-inhibiting hormone, an important peptide hormone that controls reproduction in crustaceans, increased the level of vitellogenin, the precursor of egg yolk protein levels in the ovary (Treerattrakool et al. 2008). In contrast, silencing of the molt-inhibiting hormone in Metapenaeus ensis resulted in decreased vitellogenin expression (Tiu and Chan 2007). RNAi studies also demonstrated the functions of the crustacean vitellogenin receptor (Tiu et al. 2008) and two enzymes that regulate molting and reproduction, hyperglycemic hormone (Lugo et al. 2006; Tiu et al. 2007), and farnesoic acid O-methyltransferase. Knockdown of caspase, a main effector for apoptosis in kuruma shrimp, resulted in the increase of WSSV copies, indicating that apoptosis plays a key role in the antiviral response in shrimp (Wang et al. 2008). A separate RNAi assay on a caspase homolog, caspase 3, in L. vannamei, however, showed that dsRNA for caspase 3 provided significant protection from low-dose WSSV challenge, which suggests that there could be factors other than apoptosis that contribute to the pathogenicity of the virus (Rijiravanich et al. 2008). Both studies provided evidence that a number of caspases are involved in inducing apoptosis in shrimp. Silencing of Rab GTPases, proteins involved in phagosome formation and maturation, resulted in the increase of WSSV copies and a decrease in phagocytic activity against Vibrio parahaemolyticus (Wu et al. 2008; Zong et al. 2008). These findings confirm the role of phagocytosis in the host defense against pathogens. In contrast, silencing of Rab7 effectively inhibited WSSV and YHC infection in P. monodon, suggesting that Rab7 has a role in viral replication (Ongvarrasopone et al. 2008). QM protein, a tumor suppressor in shrimp, was also effectively silenced and shown to regulate proPO activation (Xu et al. 2008). Genes that are key components of the RNAi pathway were also assayed using dsRNAs. Silencing of Dicer-1, which is responsible for the cleavage of dsRNAs, increased the viral loads in shrimp, resulting in sharp increase in mortality demonstrating that Dicer-1 is involved in the antiviral response (Su et al. 2008). Silencing of argonaute, another gene involved in RNAi, resulted in impaired RNAi (Dechklar et al. 2008). Meanwhile, silencing of a novel relish homolog, a gene associated with Rel/NF-κB family that is involved in the Imd pathway, resulted in the suppression of the expression of the AMP penaeidin (Li et al. 2009). This study further implied that an Imd pathway may also be present in shrimp. Table 1 shows the list of genes in shrimp that were analyzed using RNAi.
RNAi as an Antiviral Tool in Shrimp
A number of studies have also shown that injection of dsRNAs or siRNAs specific for viral genes can effectively inhibit the corresponding viral infections (Kim et al. 2007; Tirasophon et al. 2005; Tirasophon et al. 2007; Westenberg et al. 2005; Wu et al. 2007; Xu et al. 2007; Yodmuang et al. 2006). In addition, administration of bacterially synthesized dsRNA specific for WSSV vp28 gene also conferred protective effects against WSSV (Sarathi et al. 2008a, b). These studies suggest that sequence-specific silencing of viral genes may provide a potential therapeutic strategy against viral infections.
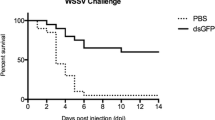
Furthermore, recent studies have demonstrated that non-specific RNAi, RNAi constructs that are not homologous to any gene in shrimp, can protect against viral infection. Introduction of non-specific dsRNA to shrimp resulted in the induction of an antiviral immune response even against unrelated viruses, WSSV, and Taura syndrome virus, suggesting that dsRNA can effectively induce a general antiviral immune response (Robalino et al. 2004). Similar results were observed when shrimp was injected with dsRNA and siRNA specific for green fluorescent protein (GFP), a gene absent in shrimp (Maningas et al. 2008; Westenberg et al. 2005; Yodmuang et al. 2006), indicating that innate antiviral defense in shrimp can be effectively triggered by sequence-independent dsRNA or siRNA. These protective effects, however, were achieved when dsRNAs/siRNAs were given prior to viral challenge. A separate study showed that unrelated GFP-dsRNA failed to elicit protective effects in shrimp with active YHV replication, whereas injection with YHV specific protease dsRNA was effective, suggesting that innate antiviral and sequence-specific mechanisms play distinct roles (Tirasophon et al. 2007).
Conclusions
RNAi have undoubtedly revolutionized the field of biology and emerged as a powerful tool for studying gene function in various organisms. In shrimp, the efficiency of RNAi technology in elucidating gene function in vivo has made it possible to evaluate the complexities of gene regulation in this group. Furthermore, the limitless potential of using RNAi in shrimp opens the door for the discovery of novel genes that might be involved in immune response. RNAi also has the potential of being an excellent therapeutic alternative to combat viral diseases in shrimp. Further studies are needed to explore the prophylactic activity of dsRNAs in order to develop a cheaper and safer substitute to antibiotics. It is equally important to develop means to make these dsRNA feasible for use on a commercial scale.
References
Adachi K, Hirata T, Nagai K, Fujisawa S, Kinoshita M, Sakaguchi M (1999) Purification and characterization of prophenoloxidase from kuruma prawn Penaeus japonicus. Fish Sci 65:919–925
Ai HS, Huang YC, Li SD, Weng SP, Yu XQ, He JG (2008) Characterization of a prophenoloxidase from hemocytes of the shrimp Litopenaeus vannamei that is down-regulated by white spot syndrome virus. Fish Shellfish Immunol 25:28–39
Ai HS, Liao JX, Huang XD, Yin ZX, Weng SP, Zhao ZY, Li SD, Yu XQ, He JG (2009) A novel prophenoloxidase 2 exists in shrimp hemocytes. Dev Comp Immunol 33:59–68
Aladaileh S, Rodney P, Nair SV, Raftos DA (2007) Characterization of phenoloxidase activity in Sydney rock oysters (Saccostrea glomerata). Comp Biochem Physiol B Biochem Mol Biol 148:470–480
Amparyup P, Kondo H, Hirono I, Aoki T, Tassanakajon A (2008) Molecular cloning, genomic organization and recombinant expression of a crustin-like antimicrobial peptide from black tiger shrimp Penaeus monodon. Mol Immunol 45:1085–1093
Amparyup P, Charoensapsri W, Tassanakajon A (2009) Two prophenoloxidases are important for the survival of Vibrio harveyi challenged shrimp Penaeus monodon. Dev Comp Immunol 33:247–256
Aspan A, Huang TS, Cerenius L, Soderhall K (1995) cDNA cloning of prophenoloxidase from the freshwater crayfish Pacifastacus leniusculus and its activation. Proc Natl Acad Sci USA 92:939–943
Assavalapsakul W, Smith DR, Panyim S (2006) Identification and characterization of a Penaeus monodon lymphoid cell-expressed receptor for the yellow head virus. J Virol 80:262–269
Bartlett TC, Cuthbertson BJ, Shepard EF, Chapman RW, Gross PS, Warr GW (2002) Crustins, homologues of an 11.5-kDa antibacterial peptide, from two species of penaeid shrimp, Litopenaeus vannamei and Litopenaeus setiferus. Mar Biotechnol (NY) 4:278–293
Bhuiyan NH, Selvaraj G, Wei Y, King J (2008) Gene expression profiling and silencing reveal that monolignol biosynthesis plays a critical role in penetration defence in wheat against powdery mildew invasion. J Exp Bot 60(2):509–521
Boman HG (1995) Peptide antibiotics and their role in innate immunity. Annu Rev Immunol 13:61–92
Cerenius L, Soderhall K (2004) The prophenoloxidase-activating system in invertebrates. Immunol Rev 198:116–126
Cerenius L, Lee BL, Soderhall K (2008) The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol 29:263–271
Chen MY, Hu KY, Huang CC, Song YL (2005) More than one type of transglutaminase in invertebrates? A second type of transglutaminase is involved in shrimp coagulation. Dev Comp Immunol 29:1003–1016
Cruz J, Nieva C, Mane-Padros D, Martin D, Belles X (2008) Nuclear receptor BgFTZ-F1 regulates molting and the timing of ecdysteroid production during nymphal development in the hemimetabolous insect Blattella germanica. Dev Dyn 237:3179–3191
Da Silva CCA (2002) Activation of prophenoloxidase and removal of Bacillus subtilis from the hemolymph of Acheta domesticus (L.) (Orthoptera: Gryllidae). Neotrop Entomol 31:487–491
De La Vega E, O'leary NA, Shockey JE, Robalino J, Payne C, Browdy CL, Warr GW, Gross PS (2008) Anti-lipopolysaccharide factor in Litopenaeus vannamei (LvALF): a broad spectrum antimicrobial peptide essential for shrimp immunity against bacterial and fungal infection. Mol Immunol 45:1916–1925
De Lorgeril J, Gueguen Y, Goarant C, Goyard E, Mugnier C, Fievet J, Piquemal D, Bachere E (2008) A relationship between antimicrobial peptide gene expression and capacity of a selected shrimp line to survive a Vibrio infection. Mol Immunol 45:3438–3445
Dechklar M, Udomkit A, Panyim S (2008) Characterization of argonaute cDNA from Penaeus monodon and implication of its role in RNA interference. Biochem Biophys Res Commun 367:768–774
Destoumieux D, Bulet P, Loew D, Van Dorsselaer A, Rodriguez J, Bachere E (1997) Penaeidins, a new family of antimicrobial peptides isolated from the shrimp Penaeus vannamei (Decapoda). J Biol Chem 272:28398–28406
Destoumieux-Garzon D, Saulnier D, Garnier J, Jouffrey C, Bulet P, Bachere E (2001) Crustacean immunity. Antifungal peptides are generated from the C terminus of shrimp hemocyanin in response to microbial challenge. J Biol Chem 276:47070–47077
Elbashir SM, Lendeckel W, Tuschl T (2001) RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev 15:188–200
Fagutao FF, Koyama T, Kaizu A, Saito-Taki T, Kondo H, Aoki T, Hirono I (2009) Increased bacterial load in shrimp hemolymph in the absence of prophenoloxidase. FEBS J 276:5298–5306
Fire A, Albertson D, Harrison SW, Moerman DG (1991) Production of antisense RNA leads to effective and specific inhibition of gene expression in C. elegans muscle. Development 113:503–514
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806–811
Golding MC, Long CR, Carmell MA, Hannon GJ, Westhusin ME (2006) Suppression of prion protein in livestock by RNA interference. Proc Natl Acad Sci USA 103:5285–5290
Guo S, Kemphues KJ (1995) par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell 81:611–620
Hall M, Wang R, Van Antwerpen R, Sottrup-Jensen L, Soderhall K (1999) The crayfish plasma clotting protein: a vitellogenin-related protein responsible for clot formation in crustacean blood. Proc Natl Acad Sci USA 96:1965–1970
Hammond SM, Bernstein E, Beach D, Hannon GJ (2000) An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 404:293–296
Hannon GJ (2002) RNA interference. Nature 418:244–251
Huang CC, Sritunyalucksana K, Soderhall K, Song YL (2004) Molecular cloning and characterization of tiger shrimp (Penaeus monodon) transglutaminase. Dev Comp Immunol 28:279–294
Hui JH, Tobe SS, Chan SM (2008) Characterization of the putative farnesoic acid O-methyltransferase (LvFAMeT) cDNA from white shrimp, Litopenaeus vannamei: Evidence for its role in molting. Peptides 29:252–260
Iwanaga S (2002) The molecular basis of innate immunity in the horseshoe crab. Curr Opin Immunol 14:87–95
Iwanaga S, Lee BL (2005) Recent advances in the innate immunity of invertebrate animals. J Biochem Mol Biol 38:128–150
Jorgensen R (1990) Altered gene expression in plants due to trans interactions between homologous genes. Trends Biotechnol 8:340–344
Kim CS, Kosuke Z, Nam YK, Kim SK, Kim KH (2007) Protection of shrimp (Penaeus chinensis) against white spot syndrome virus (WSSV) challenge by double-stranded RNA. Fish Shellfish Immunol 23:242–246
Kopacek P, Hall M, Soderhall K (1993) Characterization of a clotting protein, isolated from plasma of the freshwater crayfish Pacifastacus leniusculus. Eur J Biochem 213:591–597
Lam PB, Burga LN, Wu BP, Hofstatter EW, Lu KP, Wulf GM (2008) Prolyl isomerase Pin1 is highly expressed in Her2-positive breast cancer and regulates erbB2 protein stability. Mol Cancer 7:91
Lee S, Söderhäll K (2002) Early events in crustacean innate immunity. Fish Shellfish Immunol 12:421–437
Li DF, Zhang MC, Yang HJ, Zhu YB, Xu X (2007) Beta-integrin mediates WSSV infection. Virology 368:122–132
Li F, Hui Y, Wang D, Jose Priya TA, Li S, Wang B, Zhang J, Xiang J (2009) Identification of a novel relish homolog in Chinese shrimp Fenneropenaeus chinensis and its function in regulating the transcription of antimicrobial peptides. Dev Comp Immunol 33(10):1093–1101
Liu F, Liu Y, Li F, Dong B, Xiang J (2005) Molecular cloning and expression profile of putative antilipopolysaccharide factor in Chinese shrimp (Fenneropenaeus chinensis). Mar Biotechnol (NY) 7:600–608
Lugo JM, Morera Y, Rodriguez T, Huberman A, Ramos L, Estrada MP (2006) Molecular cloning and characterization of the crustacean hyperglycemic hormone cDNA from Litopenaeus schmitti. Functional analysis by double-stranded RNA interference technique. Febs J 273:5669–5677
Maningas MB, Kondo H, Hirono I, Saito-Taki T, Aoki T (2008) Essential function of transglutaminase and clotting protein in shrimp immunity. Mol Immunol 45:1269–1275
Martinez J, Patkaniowska A, Urlaub H, Luhrmann R, Tuschl T (2002) Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell 110:563–574
Mcgregor AP, Pechmann M, Schwager EE, Feitosa NM, Kruck S, Aranda M, Damen WG (2008) Wnt8 is required for growth-zone establishment and development of opisthosomal segments in a spider. Curr Biol 18:1619–1623
Mori C, Takanami T, Higashitani A (2008) Maintenance of mitochondrial DNA by the Caenorhabditis elegans ATR checkpoint protein ATL-1. Genetics 180:681–686
Ongvarrasopone C, Chanasakulniyom M, Sritunyalucksana K, Panyim S (2008) Suppression of PmRab7 by dsRNA inhibits WSSV or YHV infection in shrimp. Mar Biotechnol 10:374–381
Palakodeti D, Smielewska M, Lu YC, Yeo GW, Graveley BR (2008) The PIWI proteins SMEDWI-2 and SMEDWI-3 are required for stem cell function and piRNA expression in planarians. RNA 14:1174–1186
Patat SA, Carnegie RB, Kingsbury C, Gross PS, Chapman R, Schey KL (2004) Antimicrobial activity of histones from hemocytes of the Pacific white shrimp. Eur J Biochem 271:4825–4833
Perazzolo LM, Lorenzini DM, Daffre S, Barracco MA (2005) Purification and partial characterization of the plasma clotting protein from the pink shrimp Farfantepenaeus paulensis. Comp Biochem Physiol B Biochem Mol Biol 142:302–307
Relf JM, Chisholm JR, Kemp GD, Smith VJ (1999) Purification and characterization of a cysteine-rich 11.5-kDa antibacterial protein from the granular haemocytes of the shore crab, Carcinus maenas. Eur J Biochem 264:350–357
Reyes-Izquierdo T, Vargas-Albores F (2001) Proteinase activity in the white shrimp (Penaeus vannamei) clotting protein. Biochem Biophys Res Commun 287:332–336
Rijiravanich A, Browdy CL, Withyachumnarnkul B (2008) Knocking down caspase-3 by RNAi reduces mortality in Pacific white shrimp Penaeus (Litopenaeus) vannamei challenged with a low dose of white-spot syndrome virus. Fish Shellfish Immunol 24:308–313
Robalino J, Browdy CL, Prior S, Metz A, Parnell P, Gross P, Warr G (2004) Induction of antiviral immunity by double-stranded RNA in a marine invertebrate. J Virol 78:10442–10448
Sappl PG, Carroll AJ, Clifton R, Lister R, Whelan J, Harvey Millar A, Singh K (2008) The Arabidopsis glutathione transferase gene family displays complex stress regulation and co-silencing multiple genes results in altered metabolic sensitivity to oxidative stress. Plant J, 58(1):53–68
Sarathi M, Simon MC, Ahmed VP, Kumar SR, Hameed AS (2008a) Silencing VP28 gene of white spot syndrome virus of shrimp by bacterially expressed dsRNA. Mar Biotechnol (NY) 10:198–206
Sarathi M, Simon MC, Venkatesan C, Hameed AS (2008b) Oral administration of bacterially expressed VP28dsRNA to protect Penaeus monodon from white spot syndrome virus. Mar Biotechnol (NY) 10:242–249
Shockey JE, O'leary NA, De La Vega E, Browdy CL, Baatz JE, Gross PS (2008) The role of crustins in Litopenaeus vannamei in response to infection with shrimp pathogens: An in vivo approach. Dev Comp Immunol 33(5):668–673
Soderhall K, Cerenius L (1998) Role of the prophenoloxidase-activating system in invertebrate immunity. Curr Opin Immunol 10:23–28
Somboonwiwat K, Marcos M, Tassanakajon A, Klinbunga S, Aumelas A, Romestand B, Gueguen Y, Boze H, Moulin G, Bachere E (2005) Recombinant expression and anti-microbial activity of anti-lipopolysaccharide factor (ALF) from the black tiger shrimp Penaeus monodon. Dev Comp Immunol 29:841–851
Su J, Oanh DT, Lyons RE, Leeton L, Van Hulten MC, Tan SH, Song L, Rajendran KV, Walker PJ (2008) A key gene of the RNA interference pathway in the black tiger shrimp, Penaeus monodon: identification and functional characterisation of Dicer-1. Fish Shellfish Immunol 24:223–233
Sun Y, Liu M, Yang B, Lu J, Li B (2008) Inhibition of laryngeal cancer cell invasion and growth with lentiviral-vector delivered short hairpin RNA targeting human mmp-9 gene. Cancer Invest 26:984–989
Supungul P, Klinbunga S, Pichyangkura R, Hirono I, Aoki T, Tassanakajon A (2004) Antimicrobial peptides discovered in the black tiger shrimp Penaeus monodon using the EST approach. Dis Aquat Org 61:123–135
Tanaka S, Nakamura T, Morita T, Iwanaga S (1982) Limulus anti-LPS factor: an anticoagulant which inhibits the endotoxin mediated activation of Limulus coagulation system. Biochem Biophys Res Commun 105:717–723
Tharntada S, Somboonwiwat K, Rimphanitchayakit V, Tassanakajon A (2008) Anti-lipopolysaccharide factors from the black tiger shrimp, Penaeus monodon, are encoded by two genomic loci. Fish Shellfish Immunol 24:46–54
Tincu JA, Taylor SW (2004) Antimicrobial peptides from marine invertebrates. Antimicrob Agents Chemother 48:3645–3654
Tirasophon W, Roshorm Y, Panyim S (2005) Silencing of yellow head virus replication in penaeid shrimp cells by dsRNA. Biochem Biophys Res Commun 334:102–107
Tirasophon W, Yodmuang S, Chinnirunvong W, Plongthongkum N, Panyim S (2007) Therapeutic inhibition of yellow head virus multiplication in infected shrimps by YHV-protease dsRNA. Antiviral Res 74:150–155
Tiu SH-K, Chan S (2007) The use of recombinant protein and RNA interference approaches to study the reproductive functions of a gonad-stimulating hormone from the shrimp Metapenaeus ensis. FEBS J 274:4385–4395
Tiu SH, He JG, Chan SM (2007) The LvCHH-ITP gene of the shrimp (Litopenaeus vannamei) produces a widely expressed putative ion transport peptide (LvITP) for osmo-regulation. Gene 396:226–235
Tiu SH, Benzie J, Chan SM (2008) From hepatopancreas to ovary: molecular characterization of a shrimp vitellogenin receptor involved in the processing of vitellogenin. Biol Reprod 79:66–74
Treerattrakool S, Panyim S, Chan SM, Withyachumnarnkul B, Udomkit A (2008) Molecular characterization of gonad-inhibiting hormone of Penaeus monodon and elucidation of its inhibitory role in vitellogenin expression by RNA interference. FEBS J 275:970–980
Tuschl T, Zamore PD, Lehmann R, Bartel DP, Sharp PA (1999) Targeted mRNA degradation by double-stranded RNA in vitro. Genes Dev 13:3191–3197
Vaucheret H, Beclin C, Fagard M (2001) Post-transcriptional gene silencing in plants. J Cell Sci 114:3083–3091
Wang R, Liang Z, Hal M, Soderhall K (2001) A transglutaminase involved in the coagulation system of the freshwater crayfish. Pacifastacus leniusculus. Tissue localisation and cDNA cloning. Fish Shellfish Immunol 11:623–637
Wang L, Zhi B, Wu W, Zhang X (2008) Requirement for shrimp caspase in apoptosis against virus infection. Dev Comp Immunol 32:706–715
Waterhouse PM, Wang MB, Lough T (2001) Gene silencing as an adaptive defence against viruses. Nature 411:834–842
Westenberg M, Heinhuis B, Zuidema D, Vlak JM (2005) siRNA injection induces sequence-independent protection in Penaeus monodon against white spot syndrome virus. Virus Res 114:133–139
Wu Y, Lü L, Yang L-S, Weng S-P, Chan S-M, He J-G (2007) Inhibition of white spot syndrome virus in Litopenaeus vannamei shrimp by sequence-specific siRNA. Aquaculture 271:21–30
Wu W, Zong R, Xu J, Zhang X (2008) Antiviral phagocytosis is regulated by a novel Rab-dependent complex in shrimp Penaeus japonicus. J Proteome Res 7:424–431
Xu J, Han F, Zhang X (2007) Silencing shrimp white spot syndrome virus (WSSV) genes by siRNA. Antiviral Res 73:126–131
Xu J, Wu S, Zhang X (2008) Novel function of QM protein of shrimp (Penaeus japonicus) in regulation of phenol oxidase activity by interaction with hemocyanin. Cell Physiol Biochem 21:473–480
Yeh MS, Huang CJ, Leu JH, Lee YC, Tsai IH (1999) Molecular cloning and characterization of a hemolymph clottable protein from tiger shrimp (Penaeus monodon). Eur J Biochem 266:624–633
Yodmuang S, Tirasophon W, Roshorm Y, Chinnirunvong W, Panyim S (2006) YHV-protease dsRNA inhibits YHV replication in Penaeus monodon and prevents mortality. Biochem Biophys Res Commun 341:351–356
Zasloff M (1992) Antibiotic peptides as mediators of innate immunity. Curr Opin Immunol 4:3–7
Zong R, Wu W, Xu J, Zhang X (2008) Regulation of phagocytosis against bacterium by Rab GTPase in shrimp Marsupenaeus japonicus. Fish Shellfish Immunol 25:258–263
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hirono, I., Fagutao, F.F., Kondo, H. et al. Uncovering the Mechanisms of Shrimp Innate Immune Response by RNA Interference. Mar Biotechnol 13, 622–628 (2011). https://doi.org/10.1007/s10126-010-9292-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-010-9292-0