Abstract
The pacific white shrimp (Litopenaeus vannamei) is one of the most economically important marine aquaculture species in the world. To facilitate gene cloning and characterization, genome analysis, physical mapping, and molecular selection breeding of marine shrimp, we have developed the techniques to isolate high-quality megabase-sized DNA from hemocyte nuclear DNA of female shrimp and constructed a bacterial artificial chromosome (BAC) genomic library for the species. The library was constructed in the Hind III site of the vector pECBAC1, consisting of 101,760 clones arrayed in 265 384-well microtiter plates, with an average insert size of 101 kb, and covering the genome approximately fivefold. To characterize the library, 92,160 clones were spotted onto high-density nylon filters for hybridization screening. A set of 18 pairs of overgo probes designed from eight cDNA sequences of L. vannamei genes were used in hybridization screening, and 35 positive clones were identified. These results suggest that the shrimp BAC libraries will provide a useful resource for screening of genomic regions of interest candidate genes, gene families, or large-sized synthetic DNA region and promote future works on comparative genomics, physical mapping, and large-scale genome sequencing in the species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pacific white shrimp (Litopenaeus vannamei) is one of the most economically important marine aquaculture species in the world. Recently, the shrimp aquaculture industry has been threatened by depletion of the wild broodstrock and outbreaks of viral and bacterial disease. Genetic improvement programs are urgently needed for the sustainability of the industry.
Genome sequence, as a basic resource, provides greater understanding of the fundamental biology of shrimp as well as immediate applications in their selective breeding (Benzie 2005). In the past several years, rapid progresses have been made in shrimp genomics research. Large numbers of AFLP, SSR, and SNP have been developed and evaluated; framework genetic linkage maps have been constructed for Penaeus monodon (Wilson et al. 2002; Maneeruttanarungroj et al. 2006), Marsupenaeus japonicus (Li et al. 2003), L. vannamei (Zhang et al. 2007), and Fenneropenaeus chinensis (Li et al. 2006; Tian et al. 2008); a large collection of expressed sequence tags (ESTs) have been generated, and more than 186,000 ESTs are available (http://www.marinegenomics.org/organisms); several large-scale EST projects are ongoing, such as the P. monodon EST project (http://pmonodon.biotec.or.th) and the ShEST—Shrimp EST Genome Project (http://www.shrimp.ufscar.br); and hundreds of genes were cloned, especially for immune- and sex-related genes (Sellos et al. 1997; Luo et al. 2003; He et al. 2004, 2005; O'Leary and Gross 2006; Wang et al. 2006, 2007a, 2008a; Zhang et al. 2006; Liu et al. 2007; Yang et al. 2007; Chaivisuthangkura et al. 2008; Tharntada et al. 2008; Fagutao et al. 2008; Pan et al. 2008; Leelatanawit et al. 2009); microarrays and RNAi technology were used to study genome-wide expression in shrimp (Wang et al. 2006, 2008b; Robalino et al. 2007; Shekhar and Lu 2009).
However, the research on shrimp genome is still limited and fragmented. There are no effective DNA markers for functional genes and quantitative trait loci (QTLs), physical map construction, and large-scale genome sequencing. While large-insert shrimp bacterial artificial chromosome (BAC) libraries, which are required for genome analysis and positional cloning of genes, physical mapping, and genome sequencing had been proposed a decade ago (Alcivar-Warren 1997), no high-quality large-insert BAC library for shrimp species have been reported and made available to the public.
Several factors have contributed to this lack of progress in developing a shrimp BAC library. First, shrimp have relatively large genomes (e.g., for L. vannamei, 2n = 88, C-value = 2.50 pg; Animal Genome Size Database, http://www.genomesize.com). The size of the L. vannamei genome is approximately two-thirds of the human genome, or nearly 2 billion base pairs (http://www.shrimp.ufscar.br). On the other hand, it is very difficult to construct a large-insert library in shrimp for some unknown reasons. Despite the extensive efforts by several groups to develop large-insert library for shrimp, an effective BAC library is not available. A 55-kb-insert BAC library (M. japonicus) and two fosmid libraries (P. monodon and L. vannamei) had been constructed (Takashi et al. 2006; Huang et al. 2007; Saski et al. 2009). Basing on the larger clone inserts of the library, fewer clones are needed to represent the entire genome sequence and fewer contigs will be required to span the genome in the physical map (Ren et al. 2005); these smaller insert libraries are not suitable for physical mapping and positional cloning of genes . Therefore, the development of novel and more efficient methods for shrimp BAC library construction is an urgent requirement.
Here, we report construction and characterization of a large-insert, arrayed BAC library for L. vannamei, and the utilization of the library for isolating a set of clones containing the immune- and sex-related genes in the shrimp. The BAC library will provide powerful tools for advanced genetics and genomics research of shrimp and related species.
Materials and Methods
Animal Materials
Adult pacific white shrimps (L. vannamei) with lengths of 11–13 cm and weights of 21–29 g were sampled from a family in our breeding base of Hainan Province, China. The blood of about 120 female shrimps was collected from the ventral sinus at the first abdominal segment by using an equal volume of modified Alsever's solution (MAS) as anticoagulant (Bachere et al. 1988); then the hemocytes were collected by centrifugation at 4°C, 800×g for 5 min, washed twice in MAS, and resuspended in 1× HB buffer (10 mmol/L Tris, 80 mmol/L KCl, 10 mmol/L EDTA, 1 mmol/L spermidine, 1 mmol/L spermine, 0.5 mol/L sucrose, pH 9.4–9.5) (Zhang 2000). Samples were immediately preserved in liquid nitrogen, and stored in −80°C until use.
BAC Vector Preparation
The pECBAC1 vector (Frijters et al. 1997; for the restriction map, see Ren et al. 2005; He et al. 2007; or http://hbz7.tamu.edu) was used in the library construction. Vector DNA was isolated by the alkaline lysis method, purified by two rounds of cesium chloride gradient centrifugation (Sambrook and Russell 2001; Ren et al. 2005; He et al. 2007), digested completely with Hind III (Invitrogen, Carlsbad, CA), and dephosphorylated with calf intestinal alkaline phosphatase (New England Biolabs, Ipswich, MA; Zhang 2000; Wu et al. 2004). The digested vector DNA was precipitated, dissolved in TE (10 mmol/L Tris–HCl, 1 mmol/L EDTA, pH 8.0) to a final concentration of 10 ng/μl, and stored at −20°C.
High-Molecular-Weight DNA Preparation
High-molecular-weight (HMW) DNA was prepared according to the methods described by Zhang (2000) with some modifications (Wu et al. 2004; Ren et al. 2005; He et al. 2007, Zhang et al. 2008). Frozen samples were thawed at room temperature, and the cell density of the hemocyte was adjusted to approximately 107 cells/ml. The resuspended hemocyte nuclei were incubated at 45°C for 5 min, and mixed with an equal volume of 1% (w/v) molten low-melting-point (LMP) agarose. The mixture was then aliquoted with a cut pipette tip into 100-μl plug molds (Bio-Rad Laboratories, Hercules, CA) on ice. The nuclei embedded in LMP agarose plugs were lyzed by incubating in lysis buffer (0.25 mol/L EDTA; 1% sodium lauryl sarcosine, and 0.5 mg/ml proteinase K, pH 9.0–9.3) for 24 h at 50°C with gentle shaking. The plugs were washed once in 0.5 mol/L EDTA (pH 9.0–9.3) for 1 h at 50°C and incubated in 0.05 mol/L EDTA (pH 8.0) for 1 h on ice. The plugs were then incubated three times in 10–20 volumes of ice-cold TE containing 0.1 m mol/L phenylmethyl sulfonyl fluoride (PMSF) on ice for 1 h each, and then the plugs were washed three times in 10–20 volumes of ice-cold TE on ice and stored in TE at 4°C.
Before use, plugs were subjected to pre-electrophoresis to remove smaller DNA fragments and possible substances that might inhibit the activity of DNA restriction enzymes and DNA ligase. The pre-electrophoresis was conducted by pulsed-field gel electrophoresis (PFGE) in a 1.0% (w/v) agarose gel on a CHEF DRIII (Bio-Rad, USA) at 4 V/cm, 12.5°C, 5-s pulse time for 8 h in 0.5× TBE (Sambrook and Russell 2001). After pre-electrophoresis, the plugs were collected, dialyzed against ice-cold TE on ice, and then stored at 4°C.
BAC Library Construction
To determine the optimal partial digestion condition, HMW DNA was partially digested by varying the concentration of Hind III as described by Zhang et al. (2008). The partial digestion condition resulting in a majority of restricted fragments ranging from 100 to 200 kb was selected for large-scale partial digestion.
The partial-digested DNA was fractionated by PFGE on a 1% (w/v) agarose gel in 0.5× TBE at 6 V/cm, 12.5°C with 90 s switch time for 10 h, followed by 4 V/cm, 12.5°C with 5 s switch time for 8 h. DNA fragments ranging from 100 to 200 kb were excised and recovered from the gel slices by electroelution in dialysis tubing (Spectra/Pro Dialysis 7, Spectrum Laboratories Inc.) at 6 V/cm, 12.5°C, and a 35 s switch time in 0.5× TBE for 4 h, followed by reversing the polarity of the current for 60 s. The DNA was gently collected with a cut pipette tip and then subjected to a second size selection, excised, recovered, and dialyzed as described by Zhang et al. (2008).
Purified shrimp DNA fragments were ligated into the cloning-ready vector pECBAC1 at a 1:4 molar ratio of insert to vector molecules. One unit of T4 DNA ligase (Invitrogen Carlsbad, CA) for every 50 μl of reaction volume was used for ligation at 16°C for 8–12 h. Then 1.5 μl of the ligation mixture were transformed into 20 μl Escherichia coli strain ElectroMAX DH10B competent cells using the BRL Cell Porator and Voltage Booster System (Invitrogen Carlsbad, CA). Transformed cells were immediately resuspended in 1 ml of SOC medium (Sambrook and Russell 2001) and incubated at 37°C for 1 h with shaking at 200 rpm. Transformants containing recombinant DNA clones were selected on LB medium supplemented with chloramphenicol (12.5 μg/ml), X-Gal (40 μg/ml), and IPTG (100 μg/ml), then incubated at 37°C for 32 h. The average insert size of BAC clones derived from each ligation was determined by analyzing 28–100 random BAC clones. The ligations that gave >200 clones/μl ligation with the largest insert sizes were chosen for the library construction. The recombinant white clones were arrayed into 384-well microtiter plates containing 60 μl LB freezing media plus 12.5 μg/ml chloramphenicol and stored at −80°C.
BAC Library Characterization
One hundred randomly selected BAC clones were analyzed for insert size determination. Each clone was inoculated into 5 ml LB medium containing 12.5 μg/ml of chloramphenicol and incubated at 37°C overnight with shaking at 250 rpm. BAC DNA was isolated according to the modified standard alkaline lysis method (Zhang 2000; Wu et al. 2004; Ren et al. 2005; He et al. 2007). The BAC DNA was digested with 0.1 units of Not I (New England Biolabs, Ipswich, MA) at 37°C for 3 h to release the insert DNA from the vector. The digested BAC DNA was separated on a 1% agarose CHEF gel. The insert size of each clone was estimated by summing the sizes of all insert DNA fragment bands.
To examine the stability of inserted shrimp DNA in the library, ten BAC clones with insert sizes larger than 150 kb were each inoculated into 5 ml LB medium containing 12.5 μg/ml chloramphenicol and cultured at 37°C with 250 rpm shaking overnight. Then 1 μl of the overnight culture was inoculated in another 5 ml LB medium overnight at same growth condition. Serial cultures were made continuously for 5 days, approximately 100 generations for E. coli. BAC DNAs were isolated from day 1 and day 5 cultures, digested by BamH I and analyzed by electrophoresis on 1% CHEF gel as above (Tao and Zhang 1998; Zhang et al. 2008).
BAC Library Screening with Overgo Probes
Clones from the shrimp BAC library arrayed in 240 384-well microtiter plates were gridded robotically on five 22.5 × 22.5 cm Hybond XL membranes (Amersham Biosciences, USA ) in a 4 × 4 format using the GeneTAC™ G3 Robotic Workstation (Genomic Solutions, Inc., USA). Each of the high-density nylon filters contained double-spotted clones from 48 384-well microtiter plates (18,432 × 2 spots). The high-density colony filters were processed according to a procedure described previously (Zhang et al. 1996; Zhang 2000; Wu et al. 2004; Ren et al. 2005; He et al. 2007).
A set of 18 pairs overgo probes were designed for eight genes (anti-lipopolysaccharides, lysozyme, prophenoloxidase, toll protein, hemocyte transglutaminase, hemocyanin, penaeidin, vasa-like protein; sequences as Table 1). All overgos were labeled with [32P]-dCTP (Amersham Biosciences, USA) and hybridized to the library filters according to Feng et al. (2006). After 2 h of pre-hybridization with salmon sperm DNA in hybridization buffer, hybridizations were carried out at 40°C overnight. Filters were then washed in 2× SSC, 0.1% SDS at 40°C twice to four times, each for 20 min. Hybridization signals were detected autoradiographically using Kodak XAR film. Positive clones were picked up and gridded on filters and hybridized as before with the overgo probes from individual gene to identify every clone to gene.
Results
BAC Library Construction and Characterization
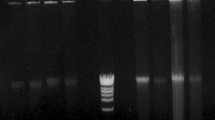
We constructed a BAC library from hemocyte nuclear DNA of female L. vannamei in the F-factor-based BAC vector pECBAC1. The library was constructed from the shrimp DNA partially digested with Hind III. The library consisted of a total of 101,760 recombinant clones arrayed in 265 384-well microtiter plates. A random sampling of 100 clones from the library was analyzed on pulsed-field gels showed that approximately 95% of the clones contained DNA inserts with an average size of 101 kb (Fig. 1), ranging from 70 to 160 kb; and 80% of the clones had inserts larger than 90 kb (Fig. 2). Based on an approximate genome size of 2,000 Mb for L. vannamei, the library is theoretically equivalent to five haploid genomes of the species. Reflecting the use of vector pECBAC1 and restriction enzyme Hind III to construct the L. vannamei BAC library, this BAC library is designated LvHE.
Analysis of insert sizes of the shrimp BAC library. A random sample of white colonies (BACs) selected from the BAC library and analyzed on a pulsed-field gel. BAC DNA was digested with Not I to release the shrimp DNA insert from the vector (the 7.5-kb band), and subjected to PFGE with a CHEF DRIII. M is for Lambda Ladder PFG Marker; the lanes between the markers are the Not I digested BAC DNAs, the 7.5-kb band in the sample lanes is from the pECBAC1 vector
The stability of the BAC clones were tested by continuously growing ten random clones for 5 days, followed by analyzing the restriction patterns of the DNA of the culture clones on a pulsed-field agarose gel (Fig. 3). Identical restriction patterns were observed between the DNAs of all ten clones isolated from the day 1 and day 5 cultures, indicating that the clones of the library are stable in the bacterial host for at least 100 generations.
Stability detection of the shrimp BAC clones. Ten clones having an insert size of 150 kb or larger were grown continuously for 5 days and analyzed by restriction digestion with BamH I. M Lambda Ladder PFG Marker, 1–10 restriction patterns of the DNA isolated from Day 1 cells, and 1′–10′ restriction patterns of the DNA isolated from day 5 cells
Library Screening with Overgo Probes
To validate the genome coverage of the libraries and test their utility for gene characterization, we screened 240 384-well microtiter plates (92,160 clones) representing 4.65× shrimp haploid genomes gridded onto five high-density nylon filters using the 18 overgos designed from eight genes involved in the shrimp innate immune system (anti-lipopolysaccharides, lysozyme, prophenoloxidase, toll protein, hemocyte transglutaminase, hemocyanin, penaeidin) and sex determination (vasa-like protein; Table 1). Positive clones (Fig. 4) were identified for each gene probe, with a total of 35 positive BAC clones for the eight gene probes with an average of 4.4 positive clones per gene. Of these clones, one clone was positive for toll protein; two clones were positive for anti-lipopolysaccharides, lysozyme, and vasa-like protein; six clones were positive for hemocyte transglutaminase; seven clones were positive for prophenoloxidase and hemocyanin; and eight clones were positive for penaeidin, respectively (Table 1).
Discussion
Despite penaeid shrimp playing a very important role in aquaculture, structural and functional genomic research of the shrimp species lags well behind that of other aquaculture species, such as salmon, catfish, and oyster. The shortage of basic knowledge of shrimp genetics, biochemistry, cell biology, and reproduction biology makes it difficult to find the essential reasons leading to failure in shrimp BAC library construction experiments.
Library Construction
We have attempted to construct BAC libraries with muscle and sperm tissue of shrimp. However, approximately 30–70% of the putative transformed clones have no insert. A high fraction of non-insert clones will be troublesome if the library is used for random end sequencing and BAC DNA fingerprinting. When hemocyte was used as the starting material, about 95% transformed clones have inserts, indicating that the hemocyte has some advantage for shrimp BAC library construction. While there must be some substance that interferes with the ligation between shrimp DNA and vector, it seems that it has less of an effect in hemocyte tissue than in muscle or sperm. The abundance of mucopolysaccharides and alkaline phosphatase and other secondary metabolites in shrimp were speculated to restrain megabase-sized DNA manipulation and thus, construction of a large-insert BAC library for the species.
Mucopolysaccharides are complex sugar molecules; it is shown that all the invertebrate species contain variable amounts of one or more types. There is a possible biological role of the sulfated mucopolysaccharides in cell recognition or aggregation or both (Cassaro and Dietrich 1977). There may be more types and more complex mucopolysaccharides in marine invertebrates due to the special marine environment, but little is known about mucopolysaccharides in shrimp. Since mucopolysaccharides have similar chemical properties to those of nucleic acids, it is difficult to separate them from DNA in our extensive pre-experiments.
The shrimp alkaline phosphatase (SAP) is found extensively in shrimp tissue, and even in the waters and sediments of shrimp culture ponds (Su et al. 2005; Wang and He 2009). SAP is used in vitro to dephosphorylate DNA or dNTPs, and it has the properties more suited for dephosphorylation of DNA than the traditionally used phosphatase from calf intestine. The enzyme shows high stability towards SDS and 2-mercaptoethanol; it is completely inhibited by EDTA, but the activity can be restored to a large degree by Zn2+ and Mn2+ (Olsen et al. 1991; Chander and Thomas 2001). Most SAP can be inactivated at 65°C, but some forms of SAP purified from the hepatopancreas of P. japonicus are stable to heating at 65°C for 5 min (Chuang and Shih 1990). It is possible that remnants of SAP in shrimp tissue dephosphorylize DNA during the isolation procedure to prevent ligation with the vector, or dephosphorylation prior to end-labeling using T4 Polynucleotide Kinase.
Previous experience has shown that the DNA extraction, size selection, and ligation with vector must be performed very gently and carefully to obtain optimal BAC libraries. The small DNA fragments resulting from physical shearing can be an inhibitor for construction of a large-insert library. The integrity of cohesive cloning ends and the absence of undigested vector molecules are a critical requirement for preparing high-quality BAC libraries.
It is preferable to construct a library from a single animal to avoid sequence assembly problems owing to polymorphisms. However, it was proved to be very difficult to obtain enough hemocytes from one shrimp, and thus we have to mix the hemocytes from several shrimps. While no sex chromosome has been observed in penaeid shrimps up to the present, the current sex-linked linkage map imply that the female may have the heterogametic sex chromosome in L. vannamei(Zhang et al. 2007). Presumably, female shrimp may provide maximum genetic material; we used female shrimp for BAC library construction in this study.
Furthermore, the construction of the BAC libraries with two different restriction enzymes increases the balanced distribution of the clones along the genome, improving the realized genome coverage of the libraries (Zhang et al. 1996; Tao et al. 2001; Ren et al. 2005). We had even tried the partial digestion of shrimp DNA from hemocyte, muscle, and sperm tissues with BamH I and EcoR I for BAC library construction, respectively, but no satisfactory ligation was obtained; a higher percentage (20–80%) of putative transformed clones having no insert is still the main problem. Coincidentally, the BAC library of kuruma shrimp (M. japonicus) previously constructed (Takashi et al. 2006), also was partially digested with Hind III. Thus, it seems that Hind III is suitable for digesting shrimp DNA for BAC library construction, but no reason was found yet.
Compared with the BAC libraries constructed for other marine economically important species(Cunningham et al. 2006; Wang et al. 2007b), the shrimp BAC libraries (LvHE) have relatively small insert size and genome coverage; however, to our knowledge it is currently the best quality BAC library available for shrimp.
Characterization and Utilization of LvHE
This BAC library can be used for BAC end sequencing and functional analysis of shrimp genome sequences, though it is not sufficient for extensive analysis of the shrimp genome physical mapping or clone-based genome sequencing. The LvHE BAC library contains a total of 101,760 clones with an average insert size of 101 kb and represents approximate fivefold genome coverage. According to the formula of estimates, the probability that a unique DNA sequence will be found on a library, N = ln(1 − P) / ln(1 − I/GS) (Clarke and Carbon 1976), where N=number of clones, P=probability, I=insert size, GS=genome size (2,000 Mb for L. vannamei), theoretically provides more than a 99% probability of obtaining at least one clone using a single-copy probe. This is consistent with the result from overgos hybridization in which 18 gene-specific overgo probes were used and positive clones were obtained for every gene probe.
One to eight positive clones have been obtained for each of the eight gene-specific probes (anti-lipopolysaccharide, lysozyme, prophenoloxidase, toll protein, hemocyte transglutaminase, hemocyanin, penaeidin, vasa-like protein) by screening a subset of the library clones with the overgos hybridization. Shrimps have no adaptive immune systems and its defense system is believed to rest entirely with the innate immune system to resist against the invasion of a pathogen. Previous studies showed that the eight genes play important roles in the innate immune system and sex determination in shrimp (Jimenez-Vega and Vargas-Albores 2007; Tharntada et al. 2008; Burge et al. 2007; Wang et al. 2007a, 2008a; Yang et al. 2003, 2007; Cheng et al. 2008; Sellos et al. 1997; Zhang et al. 2006; O'Leary and Gross 2006; Aflalo et al. 2007). Further analysis of the positive clones of the genes will lead to a better understanding of how these genes participate in the invertebrate defense to pathogens, thus providing knowledge to design strategies and tools for effectively controlling diseases in shrimp.
The construction of a large-insert BAC library enabled us to begin developing a well-characterized genomic resource for the shrimp. Deciphering the genomic content and structure of L. vannamei will greatly facilitate genome-wide searches for genes of interest in shrimp. Identification of genes involved in immune defenses in L. vannamei against pathogens may help in the development of genetic improvement strategies, and rapid identification of genes involved in disease resistance may help guide the marker-assisted selection (MAS) in shrimp. In 2007, a genetic linkage map of L. vannamei was constructed using amplified fragment-length polymorphism (AFLP) and microsatellite markers in our lab (Zhang et al. 2007). The map will create a high-density framework for anchoring more codominant markers such as microsatellite, SNPs, and expressed sequence tags, all of which can be identified by sequencing BAC clones. These loci are currently being utilized as markers in penaeid shrimp genome programs worldwide.
The need to identify the genomic mechanisms for specific traits is becoming more important in breeding and management of shrimp. Traits of importance might be related to growth, disease resistance, food conversion efficiency, or taste. When placed on genetic maps, these markers will facilitate alignment of these maps with physical map contigs from the BAC library described in future study. Likewise, a range of shrimp cDNA, RFLP, and AFLP markers, many already localized on genetic maps, will be readily assigned to physical map contigs. These markers can serve as anchors for integrating genetic and physical genomic resources.
In summary, shrimp BAC libraries can be used to further characterize the genomes of penaeid shrimp species, increasing our ability to wisely utilize their genomic resources for shrimp genetic improvement.
References
Aflalo ED, Bakhrat A, Raviv S, Harari D, Sagi A, Abdu U (2007) Characterization of a vasa-like gene from the pacific white shrimp Litopenaeus vannamei and its expression during oogenesis. Mol Reprod Dev 74:172–177
Alcivar-Warren A (1997) First aquaculture species genome mapping workshop (meeting report). Anim Genet 28:451–452
Bachere E, Chagot D, Grizel H (1988) Separation of Crassostrea gigas hemocytes by density gradient centrifugation and counterflow centrifugal elutriation. Dev Comp Immunol 12:549–559
Benzie JAH (2005) Marine Shrimp Genomics. Symposium of the 21st COE Marine Bio-manipulation Frontier for Food Production “Potential and Perspective of Marine Bio-Manipulation” in Hokkaido, Japan
Burge EJ, Madigan DJ, Burnett LE, Burnett KG (2007) Lysozyme gene expression by hemocytes of Pacific white shrimp, Litopenaeus vannamei, after injection with Vibrio. Fish Shellfish Immunol 22:327–339
Cassaro CMF, Dietrich CP (1977) Distribution of sulfated mucopolysaccharides in invertebrates. J Biol Chem 252:2254–2261
Chaivisuthangkura P, Tawilert C, Tejangkura T, Rukpratanporn S, Longyant S, Sithigorngul W, Sithigorngul P (2008) Molecular isolation and characterization of a novel occlusion body protein gene from Penaeus monodon nucleopolyhedro virus. Virology 381:261–267
Chander R, Thomas P (2001) Alkaline phosphatase from jawala shrimp (Acetes indicus). J Food Biochem 25:91–103
Cheng W, Tsai IH, Huang CJ, Chiang PC, Cheng CH, Yeh MS (2008) Cloning and characterization of hemolymph clottable proteins of kuruma prawn (Marsupenaeus japonicus) and white shrimp (Litopenaeus vannamei). Dev Comp Immunol 32:265–274
Chuang NN, Shih SL (1990) Purification and some properties of alkaline phosphatase from the hepatopancreas of the shrimp Penaeus japonicus (Crustacea: Decapoda). J Exp Zool 256:1–7
Clarke L, Carbon J (1976) A colony bank containing synthetic ColE1 hybrid plasmids representative of the entire E. coli genome. Cell 9:91–99
Cunningham C, Hikima J, Jenny MJ, Chapman RW, Fang GC, Saski C, Lundqvist ML, Wing RA, Cupit PM, Gross PS, Warr GW, Tomkins JP (2006) New resources for marine genomics: bacterial artificial chromosome libraries for the Eastern and Pacific oysters (Crassostrea virginica and C. gigas). Mar Biotechnol 8:521–533
Fagutao FF, Yasuike M, Caipang CM, Kondo H, Hirono I, Takahashi Y, Aoki T (2008) Gene expression profile of hemocytes of kuruma shrimp, Marsupenaeus japonicus following peptidoglycan stimulation. Mar Biotechnol 10:731–740
Feng J, Vick BA, Lee MK, Zhang HB, Jan CC (2006) Construction of BAC and BIBAC libraries from sunflower and identification of linkage group-specific clones by overgo hybridization. Theor Appl Genet 113:23–32
Frijters ACJ, Zhang Z, van Damme M, Wang G, Ronald PC, Michelmore RW (1997) Construction of a bacterial artificial chromosome library containing large EcoR I and Hind III genomic fragments of lettuce. Theor Appl Genet 94:390–399
He N, Liu H, Xu X (2004) Identification of genes involved in the response of haemocytes of Penaeus japonicus by suppression subtractive hybridization (SSH) following microbial challenge. Fish Shellfish Immunol 17:121–128
He N, Qin Q, Xu X (2005) Differential profile of genes expressed in hemocytes of White Spot Syndrome Virus-resistant shrimp (Penaeus japonicus) by combining suppression subtractive hybridization and differential hybridization. Antivir Res 66:39–45
He L, Du C, Li Y, Scheuring C, Zhang HB (2007) Large-insert bacterial clone libraries and their applications. In: Liu Z (ed) Aquaculture genome technologies. Blackwell, Ames, pp 215–244
Huang S, Liu T, Shu H, Wu K, Tsai S, Lin Y, Yu H (2007) A first glimpse into the genome of a marine shrimp Penaeus monodon by way of fosmid end sequencing(abstract). Plant & Animal Genomes XIV Conference P632, San Diego, California, USA
Jimenez-Vega F, Vargas-Albores F (2007) Isoforms of Litopenaeus vannamei anti-lipopolysaccharide and its expression by bacterial challenge. J Shellfish Res 26:1169–1175
Leelatanawit R, Sittikankeaw K, Yocawibun P, Klinbunga S, Roytrakul S, Aoki T, Hirono I, Menasveta P (2009) Identification, characterization and expression of sex-related genes in testes of the giant tiger shrimp Penaeus monodon. Comp Biochem Physiol A Mol Integr Physiol 152:66–76
Li Y, Byrne K, Miggiano E, Whan V, Moore S, Keys S, Crocos P, Preston N, Lehnert S (2003) Genetic mapping of the Kuruma prawn Penaeus japonicus using AFLP markers. Aquaculture 219:143–156
Li Z, Li J, Wang Q, He Y, Liu P (2006) AFLP-based genetic linkage map of marine shrimp Penaeus (Fenneropenaeus) chinensis. Aquaculture 261:463–472
Liu YC, Li FH, Dong B, Wang B, Luan W, Zhang XJ, Zhang LS, Xiang JH (2007) Molecular cloning, characterization and expression analysis of a putative C-type lectin (Fclectin) gene in Chinese shrimp Fenneropenaeus chinensis. Mol Immunol 44:598–607
Luo T, Zhang X, Shao Z, Xu X (2003) PmAV, a novel gene involved in virus resistance of shrimp Penaeus monodon. FEBS Lett 551:53–57
Maneeruttanarungroj C, Pongsomboon S, Wuthisuthimethavee S, Klinbunga S, Wilson KJ, Swan J, Li Y, Whan V, Chu K-H, Li CP, Tong J, Glenn K, Rothschild M, Jerry D, Tassanakajon A (2006) Development of polymorphic expressed sequence tag-derived microsatellites for the extension of the genetic linkagemap of the black tiger shrimp (Penaeus monodon). Anim Genet 37:363–368
O'Leary NA, Gross PS (2006) Genomic structure and transcriptional regulation of the penaeidin gene family from Litopenaeus vannamei. Gene 371:75–83
Olsen RL, Øverbø K, Myrnes B (1991) Alkaline phosphatase from the hepatopancreas of shrimp (Pandalus borealis): a dimeric enzyme with catalytically active subunits. Comp Biochem Physiol B 99:755–761
Pan JY, Zhang YL, Wang SY, Peng XX (2008) Dodecamer is required for agglutination of Litopenaeus vannamei hemocyanin with bacterial cells and red blood cells. Mar Biotechnol 10:645–652
Ren C, Xu ZY, Sun S, Lee MK, Wu C, Zhang HB (2005) Genomic libraries for physical mapping. In: Meksem K, Kahl G (eds) The handbook of plant genome mapping: genetic and physical mapping. Wiley-VCH, Weinheim, pp 173–213
Robalino J, Almeida JS, McKillen D, Colglazier J, Trent HF 3rd, Chen YA, Peck ME, Browdy CL, Chapman RW, Warr GW, Gross PS (2007) Insights into the immune transcriptome of the shrimp Litopenaeus vannamei: tissue-specific expression profiles and transcriptomic responses to immune challenge. Physiol Genomics 229:44–56
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 1.1–68
Saski C, Hilderman R, Chapman R, Benzie J (2009) Advancing shrimp genomics (abstract). Plant & Animal Genomes XIV Conference W021 San Diego, California, USA
Sellos D, Lemoine S, Van Wormhoudt A (1997) Molecular cloning of hemocyanin cDNA from Penaeus vannamei (Crustacea, Decapoda): structure, evolution and physiological aspects. FEBS Lett 407:153–158
Shekhar MS, Lu Y (2009) Application of nucleic-acid-based therapeutics for viral infections in shrimp aquaculture. Mar Biotechnol 11:1–9
Su Y, Ma S, Dong S (2005) Variation of alkaline phosphatase activity in sediments of shrimp culture ponds and its relationship with the contents of C, N and P. J Ocean U China 4:75–79
Takashi K, Takayuki K, Hidehiro K, Ikuo H, Takashi A (2006) genomic bacterial artificial chromosome library of the Kuruma shrimp Marsupenaeus japonicus (abstract). Plant & Animal Genomes XIV Conference W191 San Diego, California, USA
Tao Q, Zhang HB (1998) Cloning and stable maintenance of DNA fragments over 300 kb in Escherichia coli with conventional plasmid-based vectors. Nucleic Acids Res 26:4901–4909
Tao Q, Chang YL, Wang J, Chen H, Islam-Faridi MN, Scheuring C, Wang B, Stelly DM, Zhang HB (2001) Bacterial artificial chromosome-based physical map of the rice genome constructed by restriction fingerprint analysis. Genetics 158:1711–1724
Tharntada S, Somboonwiwat K, Rimphanitchayakit V, Tassanakajon A (2008) Anti-lipopolysaccharide factors from the black tiger shrimp, Penaeus monodon, are encoded by two genomic loci. Fish Shellfish Immunol 24:46–54
Tian Y, Kong J, Wang WJ (2008) Construction of AFLP-based genetic linkage maps for the Chinese shrimp Fenneropaeneus chinensis. Chin Sci Bull 53:1205–1216
Wang Y, He Z (2009) Effect of probiotics on alkaline phosphatase activity and nutrient level in sediment of shrimp, Penaeus vannamei, ponds. Aquaculture 287:94–97
Wang B, Li F, Dong B, Zhang X, Zhang C, Xiang J (2006) Discovery of genes in response to white spot syndrome virus (WSSV) infection in Fenneropenaeus chinensis through cDNA microarray. Mar Biotechnol 8:491–500
Wang S, Xu P, Thorsen J, Zhu B, de Jong PJ, Waldbieser G, Kucuktas H, Liu Z (2007a) Characterization of a BAC library from channel catfish Ictalurus punctatus: indications of high levels of chromosomal reshuffling among teleost genomes. Mar Biotechnol 9:701–711
Wang YC, Chang PS, Chen HY (2007b) Tissue expressions of nine genes important to immune defence of the Pacific white shrimp Litopenaeus vannamei. Fish Shellfish Immunol 23:1161–1177
Wang B, Li F, Luan W, Xie Y, Zhang C, Luo Z, Gui L, Yan H, Xiang J (2008a) Comparison of gene expression profiles of Fenneropenaeus chinensis challenged with WSSV and Vibrio. Mar Biotechnol 10:664–675
Wang YC, Chang PS, Chen HY (2008b) Differential time-series expression of immune-related genes of Pacific white shrimp Litopenaeus vannamei in response to dietary inclusion of beta-1, 3-glucan. Fish Shellfish Immunol 24:113–121
Wilson K, Li Y, Whan V, Lehnert S, Byrne K, Moore S, Pongsomboon S, Tassanakajon A, Rosenberg G, Ballment E, Fayazi Z, Swan J, Kenway M, Benzie J (2002) Genetic mapping of the black tiger shrimp Penaeus monodon with amplified fragment length polymorphism. Aquaculture 204:297–309
Wu C, Sun S, Padmavathi N, Santos FA, Springman R, Ding K, Meksem K, Lightfoot DA, Zhang HB (2004) A BAC and BIBAC-based physical map of the soybean genome. Genome Res 14:319–326
Yang Y, Poncet J, Garnier J, Zatylny C, Bache’re E, Aumelas A (2003) Solution structure of the recombinant penaeidin-3, a shrimp antimicrobial peptide. J Biol Chem 278:36859–36867
Yang LS, Yin ZX, Liao JX, Huang XD, Guo CJ, Weng SP, Chan SM, Yu XQ, He JG (2007) A Toll receptor in shrimp. Mol Immunol 44:2009–2018
Zhang HB (2000) Construction and manipulation of large-insert bacterial clone libraries—manual. Texas A&M University, Texas. http://hbz.tamu.edu
Zhang HB, Woo S, Wing RA (1996) BAC, YAC and cosmid library construction. In: Foster G, Twell D (eds) Plant gene isolation: principles and practice. Wiley, England, pp 75–99
Zhang Y, Wang S, Xu A, Chen J, Lin B, Peng X (2006) Affinity proteomic approach for identification of an IgA-like protein in Litopenaeus vannamei and study on its agglutination characterization. J Proteome Res 5:815–821
Zhang L, Yang C, Zhang Y, Li L, Zhang X, Zhang Q, Xiang J (2007) A genetic linkage map of Pacific white shrimp (Litopenaeus vannamei): sex-linked microsatellite markers and high recombination rates. Genetica 131:37–49
Zhang Y, Zhang X, Scheuring CF, Zhang HB, Huan P, Li F, Xiang J (2008) Construction and characterization of two bacterial artificial chromosome libraries of Zhikong scallop, Chlamys farreri Jones et Preston, and identification of BAC clones containing the genes involved in its innate immune system. Mar Biotechnol 10:358–365
Acknowledgments
This project is supported by National High-Tech Research and Development Program of China (2007AA09Z430), Key Program of National Natural Science Foundation of China (No. 30730071), Major State Basic Research Development Program of China (2006CB101804) and Public Industry (Agriculture) Specific Research Program (No.200803012). The authors thank Prof. Linsheng Song, Dr. Baozhong Liu, Dr. Jiquan Zhang, Hao Jiang, Cui Zhao, Fan He, and other laboratory members for technical advice and assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiaojun Zhang and Yang Zhang contributed equally to the article.
Rights and permissions
About this article
Cite this article
Zhang, X., Zhang, Y., Scheuring, C. et al. Construction and Characterization of a Bacterial Artificial Chromosome (BAC) Library of Pacific White Shrimp, Litopenaeus vannamei . Mar Biotechnol 12, 141–149 (2010). https://doi.org/10.1007/s10126-009-9209-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-009-9209-y