Abstract
Hemocyanins are multi-functional proteins, although they are well known to be respiratory proteins of invertebrate to date. In the present study, the agglutination ability of two oligomers of hemocyanin, hexamer and dodecamer, with pathogenic bacteria and red blood cells (RBCs) is investigated in pacific white shrimp, Litopenaeus vannamei. Hexameric hemocyanin exhibits an extremely high stability even in the absence of Ca2+ and in alkaline pH. Dodecamer (di-hexamer) is easily dissociated into hexamers in unphysiological conditions. Hexamer and dodecamer are interchanged reciprocally with environmental conditions. Both oligomers can bind to bacteria and RBCs, but agglutination is observed only using dodecamer but not using hexamer in agglutination assay. However, the agglutination is detected when hexamer is utilized in the presence of antiserum against hemocyanin. These results indicate that dodecamer of hemocyanin is required for agglutination with bacteria and RBCs. It can be logically inferred that there is only one carbohydrate-binding site to bacterial cells and RBCs in the hexamer, while at least two sites in the dodecamer. Our finding has provided new insights into structural–functional relationship of hemocyanin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hemocyanins are copper-containing respiratory proteins that serve for the transport of oxygen. They appear blue when oxygenated but colorless when de-oxygenated in the body fluid of many arthropod and mollusks species. As the main protein component of hemolymph, hemocyanin typically represents up to 95% of the total amount of proteins (Sellos et al. 1997). In the hemolymph of most crustaceans, hemocyanins occur commonly as aggregates of both hexamers and dodecamers with species-dependent ratios (Dainese et al. 1998; Hagner-Holler et al. 2005; Podda et al. 2008; Terwilliger and Dumler 2001).
Hemocyanins are multifunctional proteins, though they have been extensively studied as respiratory proteins of invertebrate at all times. At present, many reports uncover their antimicrobial activity as phenoloxidase activated by sodium dodecylsulfate (SDS) in vitro (Decker et al. 2001; Jaenicke and Decker 2004; Nagai and Kawabata 2000; Nagai et al. 2001; Pless et al. 2003) or by a specific serine protease in vivo (Decker and Jaenicke 2004; Jiang et al. 1998, 2007; Lee et al. 1998; Satoh et al. 1999; Wang et al. 2001) and antiviral activity (Zhang et al. 2004a). Reports also reveal that arthropod hemocyanins function as osmolytes (Paul and Pirow 1998), carriers of ecdysones (Jaenicke et al. 1999), and parts of the cuticle (Paul et al. 1994), and an antigen reacted with anti-human IgG and IgA (Zhang et al. 2004b, 2006).
A previous report has indicated that oxygen affinity of hemocyanin is conformation-dependent. Hexameric hemocyanin from the genus of monodon exhibits higher stability and lower oxygen affinity than dodecameric oligomer, while usually, the higher aggregate forms are more stable and have lower affinity in other crustacean hemocyanin (Beltramini et al. 2005). Recently, the functional difference was also found between hemocyanin subunits. Hemocyanin subunits from shrimp Penaeus japonicus play different roles in shrimp anti-white-spot-syndrome virus (WSSV) defense, a subunit PjHcL more sensitive to WSSV infection than subunit PjHcY (Lei et al. 2008). In addition, conformation- or oligomer-dependent immune function has been highly concerned in other proteins. Pulmonary surfactant protein-D (SP-D) is a C-type lectin synthesized in many tissues including respiratory epithelial cells in the lung. The assembly of SP-D trimers into dodecamers is required for the proper regulation of surfactant phospholipid homeostasis and the prevention of emphysema and foamy macrophages in vivo (Zhang et al. 2001). Mutations of the human mannose-binding lectin that compromises an assembly of higher-order oligomers result in reduced legend-binding capacity and thus reduce capability to activate complement system (Larsen et al. 2004). Thus, clarifying the function of hemocyanin oligomers may be of importance in understanding the multi-function protein.
Recently, we have shown that hemocyanin from Litopenaeus vannamei (P. vannamei) is a lectin-like protein. It agglutinates with eight species of shrimp pathogenic bacteria and four types of animal erythrocytes. The agglutination can be inhibited by saccharides (Zhang et al. 2006). However, little is known about the agglutination ability of hemocyanin oligomers with bacteria and RBCs.
In this paper, we report that hexameric hemocyanin from pacific white shrimp L. vannamei exhibits an unusually high stability, while the dodecamer (di-hexamer) is unstable under unphysiological conditions. Hexameric and dodecameric hemocyanins are interchanged reciprocally with environmental conditions. Dodecamer of hemocyanin is required for agglutination with pathogenic bacteria and RBCs. It is logically inferred that there is only a single high-affinity carbohydrate-binding site in the hexamer, while at least two in the dodecamer, which shows a typical modal of a structural–functional relationship. These results have provided new insights into the functional characterization of hemocyanin.
Materials and Methods
Animal Collection and Shrimp Serum Preparation
L. vannamei shrimps from natural source, weighing 15–20 g and irrespective of sex, were purchased from a local market and kept in well-aerated seawater. Only apparently healthy shrimps were selected for hemolymph extraction as described previously (Zhang et al. 2006). Hemolymph was drawn directly from the pericardial sinus using a 1.0-ml disposable syringe, and then allowed to clot overnight at 4°C. The hemolymph were collected after centrifuging at 5,000×g for 10 min at 4°C to remove debris and stored at −80°C until analysis.
Hemocyanin Purification and Molecular Weight Determination
Hemocyanin purification was performed by anion-exchange chromatography on a prepacked UNO Q6 column (Bio-Rad), equilibrated with 50 mM Tris–HCl buffer at pH 7.6 and 10 mM CaCl2. Elution was performed with a linear gradient from 0 to 0.6 M NaCl in 30 min at a flow rate of 1 ml/min. Molecular weights of hemocyanin oligomers were determined by gel-filtration chromatography that was carried out by a sephadex G-75 column, and elution was performed with 50 mM Tris–HCl buffer at pH 7.6 and 10 mM CaCl2 at a flow rate of 0.2 ml/min, 1.5 ml per tube. The column was calibrated firstly by using the Amersham Biosciences gel filtration kits: catalase (232 kDa), ferritin (440 kDa), thyroglobulin (699 kDa), and blue dextran (2000 kDa). Both anion-exchange chromatography and gel-filtration chromatography elutions were monitored by using a spectrophotometer at 280 nm.
Gel Electrophoresis and Immunoblotting Assays
SDS polyacrylamide gel electrophoresis (SDS-PAGE) was performed under reducing conditions according to the classical procedure on 7.5% slab polyacrylamide gels. Native–PAGE was performed in 5% slab polyacrylamide gels, with the absence of SDS in gels and the absence of both SDS and β-mercaptoethanol in sample loading buffer. SDS and native gels were stained with Coomassie Brilliant Blue R-250. Immunoblotting assay was performed using rabbit anti-shrimp hemocyanin (1:2,000) as the primary antibody following the standard procedure.
Oligomer Dissociation and Reassembly Experiments
Oligomer dissociation and reassembly experiments were carried out as described previously with minor modification (Beltramini et al. 2005; Decker et al. 2001; Olianas et al. 2006). Dodecameric hemocyanin was dissociated into lower aggregation by dialysis at 4°C in 50 mM glycine/OH− buffer at pH 9.5, 10 mM in ethylenediaminetetraacetic acid (EDTA), with a protein concentration of 5 mg/ml. During dialysis, the buffer solution was replaced six times in 72 h. Reassembly experiment was performed by dialysis against 50 mM Tris–HCl buffer at pH 7.4, 150 mM NaCl, and 10 mM CaCl2 at a protein concentration of 5 mg/ml. The oligomers of hemocyanin after the dissociation and reassembly were investigated using native–PAGE described as above.
Assays for Binding of Hemocyanin to Bacterial Cells and RBCs
Healthy human RBC types A, B, AB, and O, and pathogenic bacteria Vibrio parahaemolyticus were used for the assay. Healthy individuals were from healthy physical examination in Zhongshan Hospital, Xiamen. Informed consent was obtained from each subject; the protocol for this study was approved by the Institutional Review Board of Xiamen University. Human blood were collected in heparinized tubes, centrifuged at 3,000×g for 10 min at 4°C. After the removal of plasma, RBCs were washed three times with 0.85% NaCl and diluted to 2% suspension in TBS–Ca2+ (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 10 mM CaCl2). Bacteria were diluted to 108 CFU/ml in Tris-buffered saline (TBS)–Ca2+ buffer after washed three times with 0.85% NaCl. Purified hexamer and dodecamer were separately incubated with RBCs and bacteria for 1 h with a gentle shaking. The RBCs and bacterial cells were washed with 0.85% NaCl at least three times. The resulting pellets were boiled in sample loading buffer (50 mM Tris–HCl at pH 6.8, 10% glycerol, 2% SDS, 1% β-mercaptoethanol, and 0.01% bromophenol blue) and submitted to SDS-PAGE. The gels were either stained with Coomassie Brilliant Blue R-250 or transferred to nitrocellulose membranes for immunoblotting analysis.
Agglutination Activity Assay
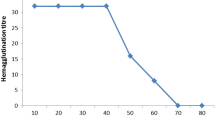
Agglutination activity of hemocyanin separately with rabbit RBCs, pooled human RBCs, and RBC types A, B, AB, and O, V. parahaemolyticus and Staphylococcus aureus were investigated using direct agglutination assay as described previously (Zhang et al. 2006). Blood were collected as described above and diluted to 0.5% suspension in TBS–Ca2+ (50 mM Tris–HCl at pH 7.4, 150 mM NaCl, and 50 mM CaCl2) or TBS (50 mM Tris–HCl at pH 7.4 and 150 mM NaCl), and bacteria were diluted to 108 CFU/ml in TBS–Ca2+ or TBS buffer. Twenty-microliter serial twofold dilutions of shrimp hemolymph and purified oligomers in TBS–Ca2+ or TBS were added on glass slides, and an equal volume of 0.5% RBC or bacterial suspension was then added. After incubation for 30 min at 37°C, agglutination was observed under light microscope. Agglutinative titer was defined as the highest dilution of the identical volume samples when the agglutination was detected.
Antibody-Dependent Agglutination of Hexameric Hemocyanin with RBCs
Both purified and dissociated hexameric hemocyanins were used for this analysis. The mixture of each of the hexameric hemocyanins with rabbit antiserum to hemocyanin was added into series of dilutions of human RBCs. The other procedures for haemagglutination were the same to the description above.
Results
Hexameric and Dodecameric Hemocyanins are Interchanged Reciprocally and the Dodecamer is the Predominant One in L. vannamei
Freshly pooled hemolymph were collected from L. vannamei and then directly utilized for isolation of hemocyanin by anion-exchange chromatography. Two protein peaks (1 and 2) were detected (Fig. 1a, 1–3). They appeared in 5 and 20 min, respectively. Fractions of the two peaks and freshly pooled shrimp hemolymph were isolated with the use of SDS-PAGE. Two bands at molecular weights around 73 and 75 kDa were observed (Fig. 1b-1). The percentages between the two bands were similar in all of the three samples (Fig. 1b-2). Both bands were identified as hemocyanin using mass spectrometry analysis (data not shown). These results indicated that the proteins from the two fractions of peaks were two different aggregation states of hemocyanin. Furthermore, gel-filtration chromatography was applied to investigate the aggregation forms of hemocyanin. Two peaks were detected (Fig. 1c). Their molecular weights were approximately 900 and 450 kDa based on prior calibration of the column with protein standards. Thus, peaks 1 and 2 were attributed respectively to the dodecamer and the hexamer. The results were in agreement with previous reports that there are two main structure forms of hexameric and dodecameric hemocyanins in the shrimps including P. monodon isolated by using gel-filtration chromatography (Beltramini et al. 2005).
Characterization of two oligomers, hexamer and dodecamer, of shrimp hemocyanin by anion-exchange and gel-filtration chromatography and SDS-PAGE. a Typical elution profiles of anion-exchange chromatography, 1 freshly pooled shrimp hemolymph, 2 peaks 1 in a (1) was re-chromatographed in the same condition, 3 and 4 freshly pooled shrimp hemolymph were exposed at 4°C for 1 and 3 days, respectively. b ( 1) The 7.5% SDS-PAGE of freshly pooled shrimp hemolymph (line 1) and two ion-exchange fractions peak 1 (line 2) and 2 (line 3); gels were stained with Coomassie Brilliant Blue R-250. b (2) Histogram displays the intensity of bands from b (1) analyzed by Phoretix 2D software. c Gel-filtration chromatography of shrimp hemolymph suggests apparent molecular weights of two oligomeric forms, hexamer and dodecamer, of hemacyanin
Very interestingly, the proteins obtained were mainly the dodecamer with small portion of the hexamer from freshly pooled hemolymph (Fig. 1a-1). A similar result may be achieved when dodecameric fraction (peak 1) was isolated again by anion-exchange chromatography (Fig. 1a-2). However, when the hemolymph was exposed at 4°C for 3 days, almost of all hemocyanin was hexamer (Fig. 1a-4). Figure 1a-3 showed the result when the hemolymph was exposed at 4°C for 1 day. The profiles with decrease of peak 1 and elevation of peak 2 were repeatedly achieved in several times of ion-exchange chromatography, indicating gradually decreased dodecamer and elevated hexamer with time going. These results indicate that hexameric and dodecameric hemocyanins are reciprocally interchanged with environmental conditions. Dodecamer hemocyanin is a predominant and functional structure form in L. vannamei hemolymph.
The Hexamer was Stable While the Docecamer was Not
Furthermore, we compared the stability of the two forms of hemocyanins. As we mentioned above, hemocyanin was transformed from the docecamer into the hexamer when it was kept longer at unphysiological conditions. The two fractions were further investigated by using native–PAGE, and freshly pooled hemolymph was used as a control. Two bands, corresponding to docecameric and hexameric hemocyanins, were observed in the control hemolymph (Fig. 2a, line 1) and the fraction of peak 1 (Fig. 2a, line 2), whereas only a band at lower molecular weight, corresponding to the hexamer, was detected in the fraction of peak 2 (Fig. 2a, line 3). The hexameric band was much larger than the dodecameric one, and their ratio was similar between the peak 1 fraction and the control group. The reason why predominant hexamer band was detected in the two samples may be related to electrophoresis conditions.
Native–PAGE analysis of L. vannamei hemocyanin. a The 5% native–PAGE of shrimp serum (line 1) and two ion-exchange fractions peak 1 (line 2) and peak 2 (line 3). b The 5% native–PAGE of shrimp hemolymph (line 1), dodecameric hemocyanin after dialysis of 72 h with 50 mM glycine/OH− buffer at pH 9.5, 10 mM EDTA (line 2), and after subsequent dialysis of 72 h in 50 mM Tris–HCl buffer at pH 7.4, 150 mM NaCl, 10 mM CaCl2 (line 3). All gels were stained with Coomassie Brilliant Blue R-250
Arthropod hemocyanins are dissociated into subunits under nondenaturating conditions by removing divalent cations with EDTA and increasing the pH (Beltramini et al. 2005; Decker et al. 2001; Olianas et al. 2006). We applied this method to obtain hemocyanin oligomers of L. vannamei. As a result, the dodecamer was dissociated into the hexamer in the conditions (Fig. 2b, line 2), whereas the hexamer was rather stable even if the dialysis was kept for 72 h. The result that the stability of hexamer was unusually high was consistent with the previous reports of other species, P. monodon (Beltramini et al. 2005) and P. setiferus (Brouwer et al. 1978). However, the hexamer could be re-aggregated into the dodecamer after subsequent dialysis of 72 h in 50 mM Tris–HCl buffer, 150 mM NaCl, and 10 mM CaCl2 at pH 7.4. This successful reassembly was identified by native–PAGE as shown in Fig. 2b, line 3.
Both Hexameric and Docecameric Hemocyanins Could Bind to Bacteria and RBCs
Our previous report has indicated that hemocyanin agglutinates with pathogenic bacteria and animal RBCs (Zhang et al. 2006). In the present study, we investigate whether the two structural forms of hemocyanin bind with these cells. For this regard, docecameric and hexameric hemocyanins respectively obtained from peaks 1 and 2 were incubated with the major pathogenic bacteria V. parahaemolyticus or pooled human RBCs. The resulting bacterial and RBC pellets were submitted to SDS-PAGE and then transferred to nitrocellulose membranes for immunoblotting analysis using rabbit anti-hemocyanin serum as the primary antibody. Immunoblotting results showed that a positive band was detected in both samples including hexameric and docecameric hemocyanins (Fig. 3, lines 6 and 7, respectively). The staining of two bands from the two samples was similar. Moreover, the similar results were obtained for the four types of human RBCs (data not shown). These results indicate that both hexameric and docecameric hemocyanins have the same ability in binding to bacteria and human BRCs, suggesting that they have the same binding sites to these cells.
A bacterial or RBC whole-cell binding to hemocyanin assay after SDS-PAGE (left panel) and Western-blotting analysis using rabbit anti-hemocyanin antiserum as the primary antibody (right panel). Line 1/5 Hemocyanin was loaded as a positive control; line 2/6 and 3/7 hexamer and docecamer hemocyanins were separately interacted with bacterial or RBCs; line 4/8 only bacterial or RBCs was loaded as a negative control
The Docecamer was Required for Agglutination with RBCs and Bacteria
Furthermore, we investigated the agglutination of hexameric and docecameric hemocyanins separately with bacteria and RBCs. When the bacteria and RBCs were separately incubated with freshly pooled hemolymph and purified dodecamer hemocyanin, distinct agglutination of RBCs and bacteria were observed, whereas no agglutination was identified using purified hexameric hemocyanin. Figure 4a showed the representative agglutination maps of human RBCs and V. parahaemolyticus, and Table 1 summarized the agglutination titers. Equal results were appreciated when the hexamer was reassociated to the dodecamer, and the dodecamer was dissolved into the hexamer by dialysis. Further investigation indicated that there was no difference of agglutination activity among the four types of human RBCs, suggesting that the agglutination was insusceptible to glycophorin of human RBCs. Interestingly, when antiserum against hemocyanin was added into the mixture of human RBCs and the hexamer, significant agglutination appeared (Fig. 4b, and Table 1). These results indicate that the dodecamer was required for agglutination with bacteria and RBCs. In addition, agglutination reaction did not occur in a buffer without Ca2+ (TBS), which suggested that calcium is indispensable to it.
Typical profiles of L. vannamei hemocyanin oligomers agglutination with RBCs and bacteria. a Representative agglutination maps of pooled human RBCs and V. parahaemolyticus by dodecameric hemocyanin. b Purified hexamer incubated with human RBCs with or without addition of antiserum to hemocyanin for 30 min at 37°C. Control indicates the incubation of human RBCs only with TBS–Ca2+. Agglutination was examined using light microscopy
Discussion
Lectins are carbohydrate-binding proteins that existed widely in animals, plants, and microorganisms. A common feature of lectins is their multivalent binding to a variety of carbohydrates expressed on cell surfaces (Cominetti et al. 2002; Minamikawa et al. 2004). Each lectin molecule contains two or more typical carbohydrate-combining sites that cause cross-linking of cells, and the cells therefore agglutinated (Lis and Sharon 1998). At least seven distinct structurally families of carbohydrate-recognition domains are determined in lectins that are involved in cell adhesion, intracellular trafficking, cell–cell signaling, glycoprotein turnover, and innate immunity (Drickamer and Fadden 2002). Until now, a few lectins have been purified and characterized in shrimps (Alpuche et al. 2005; Cominetti et al. 2002; Liu et al. 2007; Luo et al. 2006; Ma et al. 2007; Maheswari et al. 2002; Ratanapo and Chulavatnatol 1990), and they all belong to C-type lectins, in which calcium (Ca2+) is required for carbohydrate recognition domains (CRDs) to bind carbohydrates. Our previous report has also indicated that hemocyanin of L. vannamei is a lectin-like protein (Zhang et al. 2006). However, the agglutination characteristics of hemocyanin oligomers still remained unknown to date.
In the present study, we clearly demonstrate that there are two oligomeric forms of hemocyanin, dodecamer and hexamer, in L. vannamei. Dodecameric hemocyanin is predominant in shrimp hemolymph and is required for agglutination with RBCs and bacteria. It is a very interesting finding that both dodecamer and hexameric hemocyanins bound to bacterial cells and RBCs, but significant agglutination was observed only using the dodecamer but not the hexamer in agglutination assay. It is logically inferred that there is only one carbohydrate-binding site to bacterial cells and RBCs in hexameric hemocyanin, while at least two sites in the dodecamer. The conclusion is supported by the fact that the hexamer acquired high agglutination activity in the presence of antiserum to hemocyanin. This is because the antibodies that have two antigen-binding sites can cross-link cells by the combination of two of hemocyanins on cells, each from a cell. The reason why the hexamer has only one site should owe to a unique three-dimensional-fold adopted together by the six subunits of the oligomer based on its high stability. Indeed, there is no significant sequence of the hemocyanin similarity to other known carbohydrate recognition domains (CRDs) including lectins. Its amino acids involved in the pairwise interactions of tight contact between dimers differ from other known hemocyanin sequence of crustacean (Beltramini et al. 2005). The sporadic substitutes of the amino acid that are likely to be involved in the formation of the quaternary structure may not only lead to an increased stability but also contribute to the formation of the distinguished fold pattern of the hexamer. The feature was confirmed by our results that the hexamer was rather stable even in absence of Ca2+ and in alkaline pH. Therefore, the hexameric hemocyanin cannot agglutinate with these cells but the dodecamer can. The functional differences for the two oligomers exist not only in cell adhesion but also in oxygen-binding properties, a primary function of it (Beltramini et al. 2005). Our findings not only indicate oligomer-dependent agglutination ability of L. vannamei hemocyanin but also provide a novel, typical model of structural and functional relationship.
In summary, hexameric hemocyanin from L. vannamei with very high stability could not agglutinate with the pathogenic bacteria and RBCs because it has only a single high affinity or functional carbohydrate-binding site so that its lacks the ability of cross-linking cells, while dodecamer, two hexamers clustering, is required for cell aggregates. These results have provided new insights into the characteristics of L. vannamei hemocyanin. Apparently, a novel discovery is achieved in the present study that the agglutination of hemocyanin with bacteria and RBCs is conformation- and oligomer-dependent. Determination of the three-dimensional conformation will be needed to provide a direct evidence for the unique fold pattern and a carbohydrate-binding mechanism.
References
Alpuche J, Pereyra A, Agundis C, Rosas C, Pascual C, Slomianny MC, Vázquez L, Zenteno E (2005) Purification and characterization of a lectin from the white shrimp Litopenaeus setiferus (Crustacea decapoda) hemolymph. Biochim Biophys Acta 1724:86–93
Beltramini M, Colangelo N, Giomi F, Bubacco L, Di Muro P, Hellmann N, Jaenicke E, Decker H (2005) Quaternary structure and functional properties of Penaeus monodon hemocyanin. FEBS J 272:2060–2075
Brouwer M, Bonaventura C, Bonaventura J (1978) Analysis of the effect of three different allosteric ligands on oxygen binding by hemocyanin of the shrimp, Penaeus setiferus. Biochemistry 17:2148–2154
Cominetti MR, Marques MR, Lorenzini DM, Löfgren SE, Daffre S, Barracco MA (2002) Characterization and partial purification of a lectin from the hemolymph of the white shrimp Litopenaeus schmitti. Dev Comp Immunol 26:715–721
Dainese E, Di Muro P, Beltramini M, Salvato B, Decker H (1998) Subunits composition and allosteric control in Carcinus aestuarii hemocyanin. Eur J Biochem 256:350–358
Decker H, Jaenicke E (2004) Recent findings on phenoloxidase activity and antimicrobial activity of hemocyanins. Dev Comp Immunol 28:673–687
Decker H, Ryan M, Jaenicke E, Terwilliger N (2001) SDS-induced phenoloxidase activity of hemocyanins from Limulus polyphemus, Eurypelma californicum, and Cancer magister. J Biol Chem 276:17796–17799
Drickamer K, Fadden AJ (2002) Genomic analysis of C-type lectins. Biochem Soc Symp 69:59–72
Hagner-Holler S, Kusche K, Hembach A, Burmester T (2005) Biochemical and molecular characterisation of hemocyanin from the amphipod Gammarus roeseli: complex pattern of hemocyanin subunit evolution in Crustacea. J Comp Physiol B 175:445–452
Jaenicke E, Decker H (2004) Conversion of hemocyanin to tyrosinase and catecholoxidase. Micron 35:89–90
Jaenicke E, Foll R, Decker H (1999) Spider hemocyanin binds ecdysone and 20-OH-ecdysone. J Biol Chem 274:34267–34271
Jiang H, Wang Y, Kanost M (1998) Pro-phenol oxidase activating proteinase from an insect, Manduca sexta: a bacterialinducible protein similiar to Drosophila easter. Proc Natl Acad Sci USA 95:12220–12225
Jiang N, Tan NS, Ho B, Ding JL (2007) Respiratory protein-generated reactive oxygen species as an antimicrobial strategy. Nat Immunol 8:1114–1122
Larsen F, Madsen HO, Sim RB, Koch C, Garred P (2004) Disease-associated mutations in human mannose-binding lectin compromise oligomerization and activity of the final protein. J Biol Chem 279:21302–21311
Lee S, Kwon T, Hyun J, Choi J, Kawabata S, Iwanaga S, Lee B (1998) In vitro activation of prophenoloxidase by two kinds of prophenoloxidase-activating factors isolated from hemolymph of coleopteran, Holotrichia diomphalia larvae. Eur J Biochem 254:50–57
Lei K, Li F, Zhang M, Yang H, Luo T, Xu X (2008) Difference between hemocyanin subunits from shrimp Penaeus japonicus in anti-WSSV defense. Dev Comp Immunol 3:808–813
Lis H, Sharon N (1998) Lectins: Carbohydrate-specific proteins that mediate cellular recognition. Chem Rev 98:637–674
Liu YC, Li FH, Dong B, Wang B, Luan W, Zhang XJ, Zhang LS, Xiang JH (2007) Molecular cloning, characterization and expression analysis of a putative C-type lectin (Fclectin) gene in Chinese shrimp Fenneropenaeus chinensis. Mol Immunol 44:598–607
Luo T, Yang H, Li F, Zhang X, Xu X (2006) Purification, characterization and cDNA cloning of a novel lipopolysaccharide-binding lectin from the shrimp Penaeus monodon. Dev Comp Immunol 30:607–617
Ma TH, Tiu SH, He JG, Chan SM (2007) Molecular cloning of a C-type lectin (LvLT) from the shrimp Litopenaeus vannamei: early gene down-regulation after WSSV infection. Fish Shellfish Immunol 23:430–437
Maheswari R, Mullainadhan P, Arumugam M (2002) Isolation and characterization of an acetyl group-recognizing agglutinin from the serum of the Indian white shrimp Fenneropenaeus indicus. Arch Biochem Biophys 402:65–76
Minamikawa M, Hine M, Russell S, Huber P, Duignan P, Lumsden JS (2004) Isolation and partial characterization of a calcium-dependent lectin (chiletin) from the haemolymph of the flat oyster, Ostrea chilensis. Fish Shellfish Immunol 17:463–476
Nagai T, Kawabata S (2000) A link between blood coagulation and prophenoloxidase activation in arthropod host defense. J Biol Chem 275:29264–29267
Nagai T, Osaki T, Kawabata S (2001) Functional conversion of hemocyanin to phenoloxidase by horseshoe crab antimicrobial peptides. J Biol Chem 276:27166–27170
Olianas A, Sanna MT, Messana I, Castagnola M, Masia D, Manconi B, Cau A, Giardina B, Pellegrini M (2006) The hemocyanin of the shamefaced crab Calappa granulata: structural–functional characterization. J Biochem (Tokyo) 139:957–966
Paul R, Bergner B, Pfeffer-Seidl A, Decker H, Efinger R, Storz H (1994) Gas transport in the haemolymph of arachnids I. Oxygen transport and the physiological role of haemocyanin. J Exp Biol 188:25–46
Paul R, Pirow R (1998) The physiological significance of respiratory proteins in invertebrates. Zoology 100:319–327
Pless D, Aguilar M, Falcon A, Lozano-Alvarez E, Heimer dela Cotera E (2003) Latent phenoloxidase activity and N-terminal amino acid sequence of hemocyanin from Bathynomus giganteus, a primitive crustacean. Arch Biochem Biophys 409:402–410
Podda G, Manconi B, Olianas A, Pellegrini M, Messana I, Mura M, Castagnola M, Giardina B, Sanna MT (2008) Structural and functional characterization of hemocyanin from the anemone hermit crab Dardanus calidus. J Biochem (Tokyo) 143:207–216
Ratanapo S, Chulavatnatol M (1990) Monodin, a new sialic acid specific lectin from black tiger prawn (Penaeus monodon). Comp Biochem Physiol 97:515–520
Satoh D, Horii A, Ochiai M, Ashida M (1999) Prophenoloxidase-activating enzyme of the silkworm, Bombyx mori: purification, characterization and cDNA cloning. J Biol Chem 274:7441–7453
Sellos D, Lemoine S, Van Wormhoudt A (1997) Molecular cloning of hemocyanin cDNA from Penaeus vannamei (Crustacea, Decapoda): structure, evolution and physiological aspects. FEBS Lett 407:153–158
Terwilliger N, Dumler K (2001) Ontogeny of decapod crustacean hemocyanin: effects of temperature and nutrition. J Exp Biol 204:1013–1020
Wang R, Lee S, Cerenius L, Söderhäll K (2001) Properties of the prophenoloxidase activating enzyme of the freshwater crayfish, Pacifastacus leniusculus. Eur J Biochem 268:895–902
Zhang L, Ikegami M, Crouch EC, Korfhagen TR, Whitsett JA (2001) Activity of pulmonary surfactant protein-D (SP-D) in vivo is dependent on oligomeric structure. J Biol Chem 276:19214–19219
Zhang X, Huang C, Qin Q (2004a) Antiviral properties of hemocyanin isolated from shrimp Penaeus monodon. Antiviral Res 61:93–99
Zhang YL, Wang SY, Peng XX (2004b) Identification of a type of human IgG-like protein in shrimp Penaeus vannamei by mass spectrometry. J Exp Biol Ecol 301:39–54
Zhang YL, Wang SY, Xu AL, Chen J, Lin BK, Peng XX (2006) Affinity proteomic approach for identification of an IgA-like protein in Litopenaeus vannamei and study on its agglutination characterization. J Proteome Res 5:815–821
Acknowledgment
This work was sponsored by grants from the National Basic Research Program of China (2006CB101807), Guangdong Natural Science Foundation (7117645), Science and Technology Program of Guangdong (2007B020701006) and Shantou City (no. 2006-148).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pan, Jy., Zhang, Yl., Wang, Sy. et al. Dodecamer is Required for Agglutination of Litopenaeus vannamei Hemocyanin with Bacterial Cells and Red Blood Cells. Mar Biotechnol 10, 645–652 (2008). https://doi.org/10.1007/s10126-008-9115-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-008-9115-8