Abstract
Hemocyanin is a copper-containing respiratory protein that is widespread within the arthropod phylum. Among the Crustacea, hemocyanins are apparently restricted to the Malacostraca. While well-studied in Decapoda, no hemocyanin sequence has been known from the ’lower’ Malacostraca. The hemocyanin of the amphipod Gammarus roeseli is a hexamer that consists of at least five distinct subunits. The complete cDNA sequence of one subunit and a tentative partial sequence of another subunit have been determined. The complete G. roeseli hemocyanin subunit comprises 2,150 bp, which translates in a protein of 672 amino acids with a molecular mass of 76.3 kDa. Phylogenetic analyses show that, in contrast to previous assumptions, the amphipod hemocyanins do not belong to the α-type of crustacean hemocyanin subunits. Rather, amphipod hemocyanins split from the clade leading to α and γ-subunits most likely at the time of separation of peracarid and eucarid Crustacea about 300 million years ago. Molecular clock analyses further suggest that the divergence of β-type subunits and other crustacean hemocyanins occurred around 315 million years ago (MYA) in the malacostracan stemline, while α- and γ-type subunits separated 258 MYA, and pseudohemocyanins and γ-subunits 210 million years ago.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hemocyanins are copper-based respiratory proteins that serve for the transport of oxygen in the body fluid of many arthropod species. Their structure and evolution have been thoroughly studied within the Chelicerata and Crustacea (van Holde and Miller 1995; Markl 1986; Burmester 2001; 2002), although hemocyanins also occur in some myriapod and insect taxa (Mangum et al. 1985; Jaenicke et al. 1999; Hagner-Holler et al. 2004). The basic structure of arthropod hemocyanins is a hexamer or an oligomer of hexamers, each consisting of six similar or identical subunits. The subunits, which have a molecular mass in the range of 75–80 kDa, can carry one O2 molecule bound between two copper-ions that are co-ordinated by six histidines of the polypeptide chain. The arthropod hemocyanins do not share a common ancestry with the molluscan hemocyanins (Burmester 2001), but evolved from phenoloxidases in the stemline of the Arthropoda (Immesberger and Burmester 2004). Both arthropod hemocyanins and phenoloxidases are members of a large protein superfamily that also includes at least three types of copper-less, non-respiratory proteins: decapod pseudohemocyanins (cryptocyanins), insect hexamerins and hexamerin receptors (Burmester and Scheller 1996; Terwilliger et al. 1999; Burmester 1999, 2001, 2002).
Within the Crustacea, haemoglobins have occasionally been observed among the entomostracan taxa (Mangum 1983; Weber and Vinogradov 2001). Hemocyanins appear to be restricted to the Malacostraca, and it has been postulated that the possession of hemocyanin is in fact a synapomorphic character of this monophyletic taxon (Mangum 1983; Markl 1986). The crustacean hemocyanin structure and subunit composition have been investigated by Markl and co-workers employing protein-biochemical and immunological techniques (Markl 1986; Markl et al. 1979, 1986). These data have shown that, in contrast to the rather conservative hemocyanin oligomers of Chelicerata and Myriapoda, quaternary structures and subunit compositions are highly variable among the hemocyanin of malacostracan Crustacea. Malacostracan hemocyanins usually form hexamers (1×6) or dodecamers (2×6), although a 4×6 structure has also been reported (Miller et al. 1977; Markl 1986; Markl et al. 1986; Markl and Decker 1992). By immunological means, malacostracan hemocyanin subunits classified in three types referred to as α, β and γ (Markl 1986; Markl et al. 1986; Burmester 2002).
While the hemocyanin structure, sequences and physiological characteristics have been investigated in detail in the Decapoda (for review, see: Markl 1986; Markl et al. 1979, 1986; Stöcker et al. 1988), there is much less information from other malacostracan taxa. In fact, only four studies so far have focused on hemocyanins from amphipod species (Spicer and Taylor 1994; Chan and Weeks 1992; Hodgson and Spicer 2001; Spicer and Hodgson 2003). Here we investigate the structure, subunit composition and subunit sequences for the amphipod Gammarus roeseli . The phylogenetic investigation of this protein allows an assessment of the emergence of crustacean hemocyanin subunit diversity.
Materials and methods
Analyses of hemolymph proteins
Gammarus roeseli (Crustacea; Malacostraca; Amphipoda; Gammaridae) were captured in the Appelbach near Wöllstein, Germany. Animals were kept in the laboratory for up to 24 h at 8°C. The hemolymph was withdrawn from individual specimens by the aid of a syringe and immediately diluted with 10μl 100 mM Tris–HCl, 10 mM CaCl2, 10 mM MgCl2, pH 7.8. The samples were centrifuged for 10 min at 13,000×g at 4°C and either immediately used or stored at −20°C. Protein concentrations were determined by the method of Bradford (1976). Electron microscopic analysis of the hemolymph samples was performed by negative staining with uranyl-acetate (Harris and Agutter 1970).
Gelelectrophoresis and hemocyanin purification
Denaturating SDS-PAGE was carried out on 7.5% gels under standard conditions (Sambrook and Russell 2000). The G. roeseli hemocyanin subunit bands were located by staining the gel margins with Coomassie Brilliant Blue R-250 and excised. Specific antibodies against hemocyanin were obtained from samples that had been separated by SDS-PAGE. The proteins were electro-eluted overnight at 50 V in 25 mM Tris, 380 mM glycine. About 300 μg purified hemocyanin was used to raise polyclonal antibodies in a rabbit.
Western Blotting
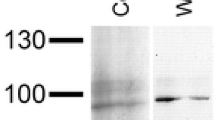
For Western blotting, proteins were transferred onto nitrocellulose at about 0.8 mA/cm2 . Non-specific binding sites were blocked by incubating the membrane 2 h at room temperature in 5% non-fat dry milk in TBST (10 mM Tris–HCl, pH 7.4, 140 mM NaCl, 0.25% Tween-20). The filters were incubated over night at 4°C with various anti-hemocyanin antisera, each diluted to the indicated concentration (Fig. 1) in 5% non-fat dry milk/TBST. The filters were then washed four times 10 min in TBST and incubated for 1 h at room temperature with goat anti-rabbit antibodies conjugated with alkaline phosphatase (DIANOVA), diluted 1:5,000 in 5% non-fat dry milk/TBS. The membranes were washed as described above, and antibody reactions were detected with nitro-blue-tetrazolium and bromo-chloro-indolyl-phosphate.
SDS-PAGE of Gammarus roeseli hemolymph. Total hemolymph proteins from G. roeseli were separated on SDS-PAGE and stained with Coomassie Brilliant Blue R-250. (1–3) Hemolymph samples from three specimens. (4) Hemocyanin subunits at higher resolution. The positions of the marker proteins are shown on the left and right hand sides
Cloning and sequencing of hemocyanin cDNA
Gammarus roeseli specimens were collected as described, washed and shock-frozen in liquid nitrogen and ground to a fine powder under continuous addition of liquid nitrogen. Total RNA was extracted using the guanidinium thiocyanate method (Chirgwin et al. 1979). Poly(A)+RNA was purified by the aid of the PolyATract kit (PROMEGA). To minimise degradation by nucleases, RNA samples were stored at −80°C in 80% ethanol. A directionally cloned cDNA expression library was established using the Lambda ZAP-cDNA synthesis kit from STRATAGENE. The library was amplified once as described in the instructions to authors. Screening for hemocyanin-containing clones was carried out either with antisera prepared against G. roeseli hemocyanin or with antibodies against the hemocyanins of Astacus leptodactylus or Homarus americanus. Positive phagemid clones were converted to the pBK-CMV plasmid using the material provided by STRATAGENEwith its cDNA synthesis kit. The hemocyanin cDNA clones were sequenced on both strands by the commercial GENTERPRISE sequencing service (Mainz, Germany). The complete sequences of the clones were obtained by “primer” walking using specific oligonucleotides. Missing 5′-terminal regions of the cDNAs were obtained by 5′ RACE techniques, either employing the “tailing” strategy and three nested oligonucleotide primers (INVITROGEN), or the GeneRacer technique (INVITROGEN). In each case 0.5–1 μg of G. roeseli total RNA was applied. Sequences were obtained as described after cloning the PCR fragments into the pCR4-TA plasmid vector (INVITROGEN).
Sequence data analyses and phylogenetic studies
The tools provided with the ExPASy Molecular Biology Server of the Swiss Institute of Bioinformatics (http://www.expasy.org) were used for the analyses of DNA and amino acid sequences. Signal peptides were predicted using the online version of SignalP V1.1 (Nielsen et al. 1997). Sequences were assembled by hand using GeneDoc 2.6 (Nicholas and Nicholas 1997).
A final sequence of G. roeseli hemocyanin was aligned with all 22 available crustacean hemocyanin subunits. We also added the three crustacean pseudohemocyanins (cryptocyanins) and either the insect or chelicerate hemocyanin sequences (Hagner-Holler et al. 2004; Averdam et al. 2003). The previously published alignments were used as guidelines (Burmester 2001; Hagner-Holler et al. 2004). The complete alignment is available from the authors upon request. The sequences used in this study and their abbreviations in Figs 4 and 5 are: HamHcA, Homarus americanus hemocyanin subunit A (EMBL/GenBank accession number: AJ272095); PinHcA-C, Panulirus interruptus hemocyanin subunits a (P04254*; asterisks indicate protein database accession numbers), b (P10787*) and c (S21221*); PelHc1–4, Palinurus elephas hemocyanin subunit 1 (AJ344361), 2 (AJ344362), 3 (AJ344363) and 4 (AJ516004); CsaHc, Callinectes sapidus hemocyanin (AF249297); PvaHc, Penaeus vannamei hemocyanin (X82502); PvaHc1, P. vannamei hemocyanin subunit 1 (AJ250830); PleHc, Pacifastacus leniusculus hemocyanin (AF522504), CmaHc1–6; Cancer magister hemocyanin subunits 1 through 6 (AY861676 to AY861690; U48881); CmaCC1, C. magister cryptocyanin (AF091261); HamPHc1 and HamPHc2, H. americanus pseudo-hemocyanin 1 (AJ132141) and 2 (AJ132142).
Neighbour-joining trees were constructed by the program NEIGHBOR from the PHYLIP 3.6b2 package (Felsenstein 2004) on the basis of the protein distances obtained with PROTDIST, assuming Dayhoff’s PAM substitution matrix (Dayhoff et al. 1978). The robustness of each tree was tested by the bootstrap procedure (Felsenstein 1985) with 100 replications (program SEQBOOT). Bayesian phylogenetic analyses were performed by MrBayes 3.0beta4 (Huelsenbeck and Ronquist 2001) with the WAG matrix (Whelan and Goldman 2001), assuming a gamma distribution of substitution rates. Prior probabilities for all trees and for amino acid replacement models were equal, starting trees were random. Metropolis-coupled Markov chain Monte Carlo sampling was performed with one cold and three heated chains that were run for 100,000 generations. Trees were sampled every tenth generation. Posterior probabilities were estimated on the final 2,000 trees (burnin=8,000).
Linearised trees of crustacean hemocyanins were constructed as described (Kusche and Burmester 2001; Kusche et al. 2003). Briefly, a corrected matrix of protein distances was estimated assuming the WAG evolutions model with gamma distribution of rates. The matrix was imported into the Microsoft EXCEL XP spreadsheet program. Then the divergence times of proteins and groups of proteins were estimated on the basis of known fossil data of Crustacea. We assume that the branches leading to the Astacura on the one hand and to the Palinura plus Brachyura on the other, diverged 180 million years ago (MYA), and that of the Palinura and Brachyura split 150 MYA (Schram 1982; Briggs et al. 1993). These dates were used to infer amino acid replacement rates, which were then applied to estimate the lengths of branches. The confidence limits were calculated using the observed standard deviations of replacement rates.
Results
Biochemical characterisation of G. roeseli hemocyanin
Hemolymph samples from G. roeseli were withdrawn and investigated by SDS-PAGE (Fig. 1). In the Coomassie-stained gels, prominent protein bands were observed in the range of ∼80 kDa, which is the typical mass of crustacean hemocyanin subunit. No within-species variation of the electrophoretic pattern of hemocyanin subunits was observed when individuals were compared. Although the putative hemocyanin subunits made up the largest fraction by far, additional prominent protein bands were observed with apparent masses of about 105 and 200 kDa. Closer investigations of the putative hemocyanin bands on longer gels show the presence of at least five subunits, with apparent molecular masses of 90, 86, 82, 79 and 76 kDa. In electron microscopic studies, the hemolymph samples show the typical hexameric (1×6) hemocyanin structures with diameters of ∼12 nm, but no aggregation forms (Fig. 2). The hexameric structure of the G. roeseli hemocyanin was further confirmed by size-exclusion studies (not shown).
Molecular cloning and sequencing of G. roeseli hemocyanin-cDNA
A cDNA library was constructed from G. roeseli mRNA that has been extracted from total animals. This library was screened with specific polyclonal antibodies, which had been raised against G. roeseli hemocyanin purified by SDS-PAGE. In several independent experiments, a total of about 20 positive clones were identified. The sizes of the cDNA-inserts were small and mostly in the range of 500–700 bp. The longest cDNA clone measured about 1.2 kb. We sequenced a total of eight of these clones, which correspond to four distinct clone types. They turned out to resemble the C-terminal ends of crustacean hemocyanins in a BLASTX search. However, none of the clones were complete at its 5′ end, with large section of the putative coding sequence missing. Thus we carried out several 5′ RACE experiments, which eventually gave rise to two distinct complete 5′ sequences. To obtain the full cDNA sequence, we constructed several sets of specific primers. These were applied to the RNA in RT-PCR, or to the cDNA library in PCR experiments. However, although we tested a large number of primers with various combinations, no specific amplificate could be obtained. Therefore, the complete G. roeseli hemocyanin cDNA sequence was obtained from three overlapping fragments and named GroHc1 (EMBL/GenBank accession number: AJ937836). While 1241 bp of its 3′ end derive from the cDNA library, the remaining 5′ sequences were obtained by two successive RACE experiments. A second tentative sequence (GroHc2) was derived from the fragments, which, however, misses 337 bp of the middle region. It is therefore possible that the fragments do not represent a single hemocyanin subunit cDNA.
The deduced GroHc1 cDNA sequence covers a total of 2150 bp, plus a poly-A tail of 20 bp. The 5′ UTR comprises 69 bp, the 3′ UTR 62 bp. A putative non-consensus polyadenylation signal (AATATA) is present 17 bp upstream of the poly-A tail. The open reading frame of GroHc1 (2019 bp) translates into a nascent protein of 672 amino acids with a calculated mass of 76,352 Da (Fig. 4). A putative N-terminal signal peptide for transmembrane transport of 15 amino acids was predicted by computer analysis. Thus the native protein has a mass of 74.7 kDa (Fig. 1). No N-glycosylation sites were detected in the G. roeseli hemocyanin amino acid sequence by computer analyses.
Hemocyanin sequence comparisons within G. roeseli and among Crustacea
BLASTP searches of the non-redundant protein database at GenBank show the highest degree of sequence similarity of the G. roeseli hemocyanin sequence with H. americanus hemocyanin A (EMBL/GenBank acc. no. AJ272095). The proteins share 65.4% of the amino acids, and are 84.1% similar when isofunctional amino acids were considered (based on a PAM250 matrix). Other crustacean hemocyanin subunits display somewhat lower identity scores, which are in the range of 57–64%. While SDS-PAGE analyses imply the presence of distinct hemocyanin subunits (Fig. 1), four of them are represented in the cDNA library. Additional sequences that do not match GroHc1 were obtained in the RACE experiments. Taken together, the data show that at least four subunits can be assigned to two distinct hemocyanin types, which differ within the coding region in about 15–25% on the nucleotide level, and 8–16% on the amino acid level. The variations within each hemocyanin types are smaller, with differences of 2–5% on the nucleotide level. No indel was observed within the coding region of the G. roeseli hemocyanin sequence fragments. GroHc1 and the tentative partial sequence GroHc2 are the two representatives of the hemocyanin types from G. roeseli. The two hemocyanin sequences differ in 15.3% of the 1,682 bp overlapping nucleotides in the coding region, which translates into a 10.0% difference on the level of amino acids.
Molecular phylogenetic studies
The GroHc1 sequence was aligned with the other crustacean hemocyanins (Fig. 3). Phylogenetic analyses were carried out using the distance matrix based neighbour-joining method or by Bayesian inference. We employed either the chelicerate or the insect hemocyanins as outgroups. In neighbour-joining trees, the position of the G. roeseli was found to be unstable. In some trees, it associates with the β-type subunits of decapod hemocyanins (Markl 1986). In other trees, it was found at the basis of the branch leading to α and γ-hemocyanin subunits. Given the uncertain position of the G. roeseli hemocyanin and the long branches of the pseudohemocyanins, all bootstrap values except those of most terminal branches were low.
Amino acid sequence alignment of selected crustacean hemocyanin subunits. The conserved residues are shaded in grey, the copper-binding histidines in black. The signal peptides are underlined. The abbreviations are: GroHc1, G. roeseli hemocyanin subunit 1 (GroHc1, accession number AJ937836); HamHcA, H. americanus hemocyanin A (AJ272095); PleHcB, P. leniusculus hemocyanin subunit β (AF522504); PinHcC, P. interruptus hemocyanin subunit c (S21221)
In Bayesian analyses, we obtained a strong support (1.00 posterior probability) for all branches of the tree, except of the relationship among the four P. elephas hemocyanin subunits. In each analysis, we carried out three independent runs with one cold and three hot chains, which, however, resulted in identical topologies (Fig. 4). We also observed no effect of outgroup selection (chelicerate or insect hemocyanins). We found the WAG model to give the highest likelihood scores, but other evolution models (JTT, BLOSUM, PAM) still resulted in the same tree. The Bayesian trees clearly show that within crustacean hemocyanins the branch leading to the β-type hemocyanin subunits diverged first. The pseudohemocyanins combine with the γ-type hemocyanin subunits, and this clade forms a common branch with the α-type subunits. The G. roeseli hemocyanin (GroHc1) is sister to this group, meaning that the branch leading to the amphipod hemocyanins diverged after the β- and α/γ-type hemocyanins separated, but before the α- and γ-types split. When included in the analysis, the tentative second G. roeseli sequence (GroHc2) groups with GroHc1.
Evolution of the crustacean hemocyanin subunits. An alignment of selected arthropod hemocyanins and phenoloxidases were analysed by MrBayes, assuming a WAG model of evolution with gamma-distribution of rates. All nodes except of that indicated are supported with an estimated Bayesian posterior probability of 1.0. The bar represents 0.1 PAM distance
Although it is obvious that replacement rates are variable among crustacean hemocyanins, evolutionary rates appear to be fairly constant within the clades of the subunit types (cf. Fig. 4). We can therefore approximate divergence times by assuming local molecular clocks and by employing known fossil data, as explained in the “Materials and methods” section. On the basis of a WAG model of protein evolution with gamma-distribution of rates, we calculated that the malacostracan hemocyanin subunits start to diversify about 315±19 MYA (Fig. 5). The separation of α- and γ-types occurred about 258±10 MYA, and the pseudohemocyanins emerged 210±32 MYA. The divergence of amphipod and the other hemocyanins was calculated to have occurred about 285±24 MYA. On the basis of the partial sequences, GroHc1 and GroHc2 were estimated to have split about 40–50 MYA.
A molecular timescale of crustacean hemocyanin evolution. The branch lengths were calculated with the WAG matrix, error bars (standard deviation of the mean) are given in grey. The molecular clock was calibrated assuming that the Astacura and Palinura diverged 180 MYA, and the Brachyura and Palinura 150 MYA (Briggs et al. 1993)
Discussion
Although there is no doubt that hemocyanins must have been present in the last common ancestors of all present-day Crustacea, today they are apparently restricted to the Malacostraca. No data on hemocyanins are currently available from the Leptostraca, which are considered as the most “primitive” malacostracan taxon. The Eumalacostraca comprise four taxa: Syncarida, Eucarida, Hoplocarida and Peracarida. So far, hemocyanins have been studied mainly in the Decapoda, which is the most prominent eucarid taxon (Markl 1986; Markl et al. 1986; Stöcker et al. 1988; Burmester 2002). The hoplocarid mantis shrimp Squilla mantis harbours an unusual 2×6-mer (Bijlholt and van Bruggen 1986). Data on hemocyanins from the Peracarida (Isopoda, Amphipoda, Mysidacea and Euphausiacea) are actually sparse (Terwilliger et al. 1979; van Holde and Brenowitz 1981; Chan and Weeks 1992; Hodgson and Spicer 2001; Spicer and Hodgson 2003; Pless et al. 2003), and complete hemocyanin sequences had been unknown from outside the Decopoda. Therefore, here we provide the first sequence from a non-decapod crustacean taxon.
Structure and subunit composition of G. roeseli hemocyanin
Biochemical characterisation identifies G. roeseli hemocyanin as a simple hexamer (1×6) consisting of at least five distinct subunits that can be separated by SDS-PAGE. This agrees with previous studies on gammarid and other amphipod species, which have shown between two and eight distinct hemocyanins subunits (Hodgson and Spicer 2001). The molecular masses of G. roeseli hemocyanin subunits (77 to 91 kDa) are within the same range found for the other Amphipoda. A large number of subunits have generally been associated with the ability of a hemocyanin to display highly cooperative oxygen binding (Markl and Decker 1992). In fact, it has been demonstrated that hemocyanins from other aquatic amphipods display a high cooperativity and a pronounced Bohr effect (Spicer and Taylor 1994).
Hemocyanin subunit types in Malacostraca
Given the close evolutionary relationship, it is not surprising that the G. roeseli hemocyanin subunit sequence resembles those of the other Malacostraca (Fig. 3). Immunological and molecular phylogenetic studies had implied the existence of three distinct types of hemocyanin subunits in the Decapoda, which have been termed α, β and γ (Markl 1986; Markl et al. 1986; Burmester 2002; Kusche et al. 2003). Based on comparison of cross-reactions with various polyclonal antibodies, Markl and co-workers suggested that β and γ-type hemocyanin subunits are in fact restricted to the Decapoda, whereas α-type subunits should occur in all Malacostraca, including the Amphipoda (Markl 1986; Markl et al. 1986). Thus, α-type subunits were considered as the basic building block of a malacostracan hemocyanin. However, here we show that the hemocyanin from the amphipod G. roeseli cannot be assigned to any particular subunit-type, but forms a separated branch allied to both, α-and γ-type subunits. According to the phylogenetic analyses, true α-subunits only emerged within the Decapoda, and cannot be found in non-decapod species, as had been previously assumed.
The previous misinterpretations of the immunological data are not surprising, because α-subunits have the slowest rate of evolution among crustacean hemocyanins (cf. Fig. 4). In fact, although phylogenetically not particularly related, GroHc1 displays the highest degree of resemblance with H. americanus hemocyanin A, which is an α-type subunit. Thus antibodies that had been raised against α-subunits should give the strongest cross-reaction with the non-decapod hemocyanins because here conservation of epitopes is most likely. Earlier studies have provided valuable information to some extent, e.g., by correctly identifying the relationship among α-, β- and γ-type subunits (Markl 1986; Markl et al. 1986). However, the present analysis clearly shows the power of phylogenetic inference using molecular sequences.
Complex evolution of crustacean hemocyanins
It may be assumed that the G. roeseli hemocyanin separated from the α- and γ-type subunits at the time the Decapoda and Amphipoda, diverged. While the earliest fossil record of the Decapoda stems from about 250 MYA, the earliest Amphipoda were obtained only from the upper Eocene (Schram 1982; Briggs et al. 1983). However, other Peracarida (Isopoda, Mysidacea) were found in Carboniferous strata about 300 MYA old. We have calculated that amphipod and decapod hemocyanins separated 285±24 MYA (Fig. 5), which is in fact close to fossil date of eucarid and peracarid divergence. Phylogenetic analyses further show that β-type hemocyanin subunits already emerged before the divergence of eucarid and peracarid Crustacea, probably in the stemline of the Malacostraca at an estimated time of about 315 MYA (Figs. 4 and 5). β-type hemocyanins were thus present in the last common ancestor of Eucarida and Peracarida, but have not been detected so far in species other than the Decapoda. This may be interpreted as multiple independent gene loss events, or may be due to insufficient studies of the non-decapod Crustacea. Nevertheless, up to eight-hemocyanin subunits may occur in Amphipoda (Hodgson and Spicer 2001), demonstrating that after the emergence of this taxon, gene duplication events independently lead to the formation of complex hemocyanins.
The present study shows that crustacean hemocyanin evolution appears to be more complex than previously thought. It also provides further evidence that the hemocyanin subunit evolution in the malacostracan Crustacea strikingly differs from that in Chelicerata (Markl 1986; Markl et al. 1986; Burmester 2001). Some chelicerate hemocyanins display subunit structures that have been retained for more than half a billion years (Markl 1986; Voit et al. 2000; Averdam et al. 2003). By contrast, there is much more variability in terms of subunit organisation in the Crustacea. Gene duplication and gene loss were apparently common during Crustacean evolution, and new hemocyanin subunits frequently emerged to accommodate to specific ecological and physiological requirements. Even today, hemocyanin subunit composition and quaternary structures may differ between closely related species, and may vary even within populations (Markl 1986; Markl et al. 1986; Stöcker et al. 1988; Mangum and Joy 1997). In addition, some subunits may be expressed only in certain developmental stages or under specific physiological conditions (Markl and Decker 1992; Durstewitz and Terwilliger 1997; Terwilliger 1998). This has never been observed so far in Chelicerata or Myriapoda (Burmester 2002).
Abbreviations
- Hc:
-
Hemocyanin
- MYA:
-
Million years ago
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
References
Averdam A, Markl J, Burmester T (2003) Subunit sequences of the 4×6-mer hemocyanin from the golden orb-web spider Nephila inaurata: intramolecular evolution of the chelicerate hemocyanin subunits. Eur J Biochem 270:3432–3439
Bijlholt M, van Bruggen EF (1986) A model for the architecture of the hemocyanin from the arthropod Squilla mantis (Crustacea, Stomatopoda). Eur J Biochem 155:339–344
Bradford MM (1976) A rapid and sensitive method for quantification of microgram quantities of proteins using the principle of protein dye binding. Anal Biochem 72:248–254
Briggs DEG, Weedon MJ, White MA (1993) Arthropoda (Crustacea excluding Ostracoda). In: Benton MJ (ed) The fossil record 2. Chapman and Hall, London, pp 321–342
Burmester T (1999) Identification, molecular cloning and phylogenetic analysis of a non-respiratory pseudo-hemocyanin of Homarus americanus. J Biol Chem 274:13217–13222
Burmester T (2001) Molecular evolution of the arthropod hemocyanin superfamily. Mol Biol Evol 18:184–195
Burmester T (2002) Origin and evolution of arthropod hemocyanins and related proteins. J Comp Physiol B 172:95–117
Burmester T, Scheller K (1996) Common origin of arthropod tyrosinase, arthropod hemocyanin, insect hexamerin and dipteran arylphorin receptor. J Mol Evol 42:713–728
Chan HM, Weeks JM (1992) The subunit structural composition of amphipod hemocyanin Crustacea; Amphipoda; Talitridae. Comp Biochem Physiol 101B:567–572
Chirgwin JM, Przbyla AE, MacDonald RJ, Rutter WJ (1979) Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 18:5294–5299
Dayhoff MO, Schwartz RM, Orcutt BC (1978) A model of evolutionary change in proteins. In: Dayhoff MO (ed) Atlas of protein sequence structure, vol 5, suppl 3. National Biomedical Research Foundation, Washington DC, pp 345–352
Durstewitz G, Terwilliger NB (1997) Developmental changes in hemocyanin expression in the Dungeness crab, Cancer magister. J Biol Chem 272:4347–4350
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Felsenstein J (2004) PHYLIP (Phylogeny Inference Package) version 3.6b. Distributed by the author. Department of Genetics, University of Washington, Seattle
Hagner-Holler S, Schoen A, Erker W, Marden JH, Rupprecht R, Decker H, Burmester T (2004) A respiratory hemocyanin from an insect. Proc Natl Acad Sci USA 101:871–874
Harris JR, Agutter PS (1970) A negative staining study of human erythrocyte ghosts and rat liver nuclear membranes. J Ultrastruc Res 33:219–232
Hodgson E, Spicer JI (2001) Subunit compositions of crustacean hemocyanins are species-specific: evidence from non-decapod species. Comp Biochem Physiol A 128:873–888
van Holde KE, Brenowitz M (1981) Subunit structure and physical properties of the hemocyanin of the giant isopod Bathynomus giganteus. Biochemistry 20:5232–5239
van Holde KE, Miller KI (1995) Hemocyanins. Adv Protein Chem 47:1–81
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755
Immesberger I, Burmester T (2004) Phenoloxidase-like proteins of the tunicate Ciona intestinalis and the origin of the arthropod hemocyanin superfamily. J Comp Physiol B 174:169–180
Jaenicke E, Decker H, Gebauer W, Markl J, Burmester T (1999) Identification, structure and properties of hemocyanins from diplopod Myriapoda. J Biol Chem 274:29071–29074
Kusche K, Burmester T (2001) Sequence and evolution of lobster hemocyanins. Biochem Biophys Res Commun 282:887–892
Kusche K, Hembach A, Hagner-Holler S, Gebauer W, Burmester T (2003) Complete subunit sequences, structure and evolution of the 6×6-mer hemocyanin from the common house centipede, Scutigera coleoptrata. Eur J Biochem 270:2860–2868
Mangum CP (1983) Oxygen transport in the blood. In Bliss DE, Mantel LH (eds) The biology of Crustacea, vol 5. Academic Press, New York, pp 373–429
Mangum CP, Joy PJ (1997) Hemocyanin subunit composition in the American lobster Homarus americanus. J Crust Biol 17:1–5
Mangum CP, Scott JL, Black REL, Miller KI, van Holde KE (1985) Centipedal hemocyanin: its structure and implication for arthropod phylogeny. Proc Natl Acad Sci USA 82:3721–3725
Markl J (1986) Evolution and function of structurally diverse subunits in the respiratory protein hemocyanin from arthropods. Biol Bull (Woods Hole, MA) 171:90–115
Markl J, Decker H (1992) Molecular structure of the arthropod hemocyanins. Adv Comp Environ Physiol 13:325–376
Markl J, Hofer A, Bauer G, Markl A, Kemptner B, Brenzinger M, Linzen B (1979) Subunit heterogeneity in arthropod hemocyanins: II. Crustacea. J Comp Physiol 133:67–175
Markl J, Stöcker W, Runzler R, Precht E (1986) Immunological correspondences between the hemocyanin subunits of 86 arthropods: evolution of a multigene protein family. In: Linzen B (ed) Invertebrate oxygen carriers. pp 281–292
Miller KI, Eldred NW, Arisaka F, van Holde KE (1977) Structure and function of hemocyanin from thalassinid shrimp. J Comp Physiol 115B:171–184
Nicholas KB, Nicholas HB Jr (1997) GeneDoc: analysis and visualization of genetic variation. http://www.psc.edu/biomed/genedoc/
Nielsen H, Engelbrecht J, Brunak S, von Heijne G (1997) Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng 10:1–6
Pless DD, Aguilar MB, Falcon A, Lozano-Alvarez E, Heimer de la Cotera EP (2003) Latent phenoloxidase activity and N-terminal amino acid sequence of hemocyanin from Bathynomus giganteus, a primitive crustacean. Arch Biochem Biophys 409:402–410
Sambrook J, Russell D (2000) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press
Schram FR (1982) The fossil record and evolution of Crustacea. In: Abele LG (ed) The biology of Crustacea, vol 1. Academic, New York, pp 93–147
Spicer JI, Hodgson E (2003) Structural basis for salinity-induced alteration in oxygen binding by hemocyanin from the estuarine amphipod Chaetogammarus marinus (L.). Physiol Biochem Zool 76:843–849
Spicer JI, Taylor AC (1994) Oxygen-binding by hemocyanins from an ecological series of amphipod crustaceans. Mar Biol 120:231–237
Stöcker W, Raeder U, Bijlholt MMC, Wichertjes T, van Bruggen EFJ, Markl J (1988) The quaternary structure of four crustacean two-hexameric hemocyanins: immunocorrelation, stoichiometry, reassembly and topology of individual subunits. J Comp Physiol 158B:271–289
Terwilliger NB (1998) Functional adaptations of oxygen-transport proteins. J Exp Biol 201:1085–1098
Terwilliger NB, Terwilliger RC, Applestein M, Bonaventura C, Bonaventura J (1979) Subunit structure and oxygen binding by hemocyanin of the isopod Ligia exotica. Biochemistry 18:102–108
Terwilliger NB, Dangott LJ, Ryan MC (1999) Cryptocyanin, a crustacean molting protein: evolutionary links to arthropod hemocyanin and insect hexamerins. Proc Natl Acad Sci USA 96:2013–2018
Voit R, Feldmaier-Fuchs G, Schweikardt T, Decker H, Burmester T (2000) Complete sequence of the 24 mer hemocyanin of the tarantula Eurypelma californicum: structure and intramolecular evolution of the subunits. J Biol Chem 275:39339–39344
Weber R, Vinogradov SN (2001) Nonvertebrate hemoglobins: functions and molecular adaptations. Physiol Rev 81:569–628
Whelan S, Goldman N (2001) A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol Biol Evol 18:691–699
Acknowledgements
We thank J. Markl for the excellent working facilities and support, C. Stürzbecher for the specimens of our initial studies, and W. Gebauer for his help with electron microscopy. This work is supported by a grant of the Deutsche Forschungsgemeinschaft (Bu956/3 and 5). The nucleotide sequence reported in this paper (GroHc1) has been submitted to the EMBL/GenBankTM Nucleotide Sequence Databases under the accession number AJ937836.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier
Rights and permissions
About this article
Cite this article
Hagner-Holler, S., Kusche, K., Hembach, A. et al. Biochemical and molecular characterisation of hemocyanin from the amphipod Gammarus roeseli: complex pattern of hemocyanin subunit evolution in Crustacea. J Comp Physiol B 175, 445–452 (2005). https://doi.org/10.1007/s00360-005-0012-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-005-0012-4