Abstract
We investigated whether the brown seaweed Alariaceae Ecklonia cava (E. cava) has immunological effects on splenocytes in vitro. For that purpose, we prepared an enzymatic extract from E. cava (ECK) by using the protease, Kojizyme. Here, ECK administered to ICR mice dramatically enhanced the proliferation of their splenocytes and increased the number of their lymphocytes, monocytes and granulocytes. In flow cytometry assays performed to identify in detail the specific phenotypes of these proliferating cells after ECK treatment, the numbers of CD4+ T cells, CD8+ T cells and CD45R/B220+ B cells increased significantly compared to those in untreated controls. In addition, the mRNA expression and production level of Th1-type cytokines, i.e., TNF-α and IFN-γ, were down-regulated, whereas those of Th2-type cytokines, i.e., IL-4 and IL-10, were up-regulated by ECK. Overall, this dramatic increase in numbers of splenocytes indicated that ECK could induce these cells to proliferate and could regulate the production of Th1- as well as Th2-type cytokines in immune cells. These results suggest that ECK has the immunomodulatory ability to activate the anti-inflammatory response and/or suppress the proinflammatory response, thereby endorsing its usefulness as therapy for diseases of the immune system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seaweeds are a widely available source of biomass, considering that more than 2 million tons are either harvested from the oceans or cultured annually for food or phycocolloid production. The three basic types of seaweeds are brown (Phaeophyta), red (Rhodophyta), and green (Chlorophyta), divided according to the dominant pigment, respectively: xanthophylls, fucoxanthin, phycoerythrin, and chlorophyll a and b. The brown seaweeds abound in fucoxanthin pigment and polysaccharides that possess many bioactive properties (Ruperez and Saura-Calixto, 2001; Siriwardhana et al., 2004). For example, the brown seaweed Alariaceae Ecklonia cava (E. cava) is rich in xanthopophyll; fucoxanthin; vitamins; vitamin precursors such as α-tocopherol, β-carotene, niacin, thiamin, and ascorbic acid; and polysaccharides such as fucoidan, alginates, fucans, and laminarans, which are water-soluble dietary fibers and phycocolloids. E. cava grows plentifully near Jeju Island of Korea (Guiry and Bulunden, 1991) and, reportedly, contains a richer supply of total polyphenolic compounds than do other brown seaweeds (Heo et al., 2005a). These polyphenolic compounds of brown seaweeds have been called phlorotannins, and those of E. cava are the phenolic secondary metabolites eckol (a closed-chain trimer of phloroglucinol), 6,6′-bieckol (a hexamer), dieckol (a hexamer), phlorofucofuroeckol (a pentamer), and triphlorethol-A, all with biological activities (Kang et al., 2005a, b). Contained in the Ecklonia species alone are oxygen-radical scavengers (Kang et al., 2004a), antiplasmin inhibitors (Fukuyama et al., 1989), antimutagens (Lee et al., 1998), bactericidal agents (Nagayama et al., 2002), HIV-1 reverse transcriptase and protease inhibitors (Ahn et al., 2004b), tyrosinase inhibitors (Kang et al., 2004b), and cell damage inhibitors (Kang et al., 2005a, b). Our previous studies have demonstrated that enzymatic extracts of E. cava also act as antioxidants in vitro, anticancer agents, anticoagulants, and matrix metalloproteinase inhibitors (Heo et al., 2005a, b; Athukorala et al., 2006; Kim et al., 2006a, b). Other investigators have described brown seaweed extracts that provide immune resistance, immune function, and antitumor activity (Maruyama et al., 2003; Saker et al., 2004; Yeh et al., 2006). However, no immune activities of extracts from E. cava have been reported nor have their biological mechanisms been elucidated. In the present study, we used an enzymatic extraction technique that releases bioactive compounds by breaking down the cell walls of E. cava, which consist of carbohydrates, proteins, glycoproteins, and lipoproteins. Therefore, we investigated the immune effects of an E. cava enzymatic extract (ECK) on splenocytes in vitro.
Materials and Methods
Mice
All experiments were performed with ICR mice, ages 8 to 9 weeks, purchased from SLC (Yokohama, Japan). The animals were housed, five to a cage, in conventional animal facilities with NIH-07 diet and water ad libitum under constant temperature (23 ± 1°C) according to the internationally accepted guideline.
Preparation of ECK
E. cava was collected from the coast of Jeju Island, South Korea, washed with fresh water, freeze-dried, and pulverized into powder with a grinder. The enzymatic extract was prepared in accordance with the method developed by Heo et al. (2003). One gram of the dried E. cava was homogenized with 100 ml of distilled water (pH 6.0) and mixed with 100 μg of Kojizyme (Novo Nordisk, Bagsvaerd, Denmark). The reaction with this enzyme was conducted at 40°C for 12 h. Afterward, the digest was boiled for 10 min at 100°C to inactivate the Kojizyme. The product was clarified by 20 min of centrifugation at 3000 g to remove any unhydrolyzed residue. Finally, the enzymatic extract, ECK, obtained after filtration of the supernatant, was adjusted to pH 7.0 and stored for use in experiments.
Chemical Analysis of ECK
The approximate chemical composition of ECK was established by measuring its content of ash at 550°C in a dry furnace and content of moisture from the weight difference after drying of samples according to the AOAC method. In addition, ECK was divided into two phases by the addition of EtOH to yield polyphenols from the supernatant and crude sulfated polysaccharides from the precipitate. Then, to analyze the monosaccharide content of ECK, the crude sulfated polysaccharide was hydrolyzed in a sealed glass tube with 2 M trifluoroacetic acid for 4 h at 100°C. After digestion with 6 N of HCl for 4 h, 0.055 and 2.75 μg of the sample were applied separately to CarboPacc PA1 (4.5 × 250 mm, Dionex, Sunnyvale, CA) with a CarboPac PA1 cartridge (4.5 × 50 mm) column to analyze neutral and amino sugars, respectively. The column was eluted with 16 mM of NaOH at a 1.0 ml/min flow rate. Each sugar of the sample was detected via a ED50 Dionex electrochemical detector, and data were analyzed via Peack Net software. In addition, the sulfate content of crude sulfated polysaccharides was analyzed via the BaCl2/galation method (Saito et al., 1968). Subsequently, the content of polyphenols was determined according to a modified version of the Folin-Ciocalteu method (Waterman and Mole, 1994). The components of the preparation are expressed as wt/wt (g/100 g) or %, respectively.
T-Cell Culture
T cells were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 1% (vol/vol) minimum essential medium (Gibco BRL, Life Technologies, Paisley, UK), 2 mM glutamine (Flow Laboratories, Irvine, UK), 50 IU/ml of penicillin, 50 mg/ml of streptomycin, and 10% (vol/vol) fetal bovine serum (FBS) (all from Gibco).
Preparation of Spleen Cells
Spleens were removed aseptically from ICR mice, and single splenocytes were suspended in T-cell medium containing 10% FBS and 1% antibiotic (100 U/ml of penicillin and 100 mg/ml of streptomycin) and then separated. Red blood cells were lysed by immersion in lysis buffer (BD Biosciences Pharmingen, San Jose, CA) at room temperature for 10 min. After washing with Dulbecco’s phosphate buffered saline (DPBS, Gibco BRL, Life Technologies, New York, USA), the purified cells were obtained and used directly for experiments in which they were treated with ECK or a vehicle, hereafter called “untreated cells”
Trypan Blue Dye Exclusion Test
To determine the extent to which ECK affected the viability of splenocytes, we cultured 4 × 105 mouse cells with or without ECK. After 48 h, trypan blue dye was added to the splenocyte-containing culture plates at the proper dilution factor recommended by the supplier (Sigma-Aldrich, St Louis, MO). The number of the viable cells in both cultures, i.e., those stained by trypan blue dye, was counted via a hematometer.
[3H]Thymidine Incorporation Assay
The proliferative ability of T cells was tested by the standard assay based on the principle that the thymidine base of DNA sequences in these cells is replaced with [3H]thymidine (Amersham, Arlington Heights, IL). For this assay, 4 × 105 T cells were treated with ECK or concanavalin A (Con A; Sigma) and cultured in 96-well round-bottom microtiter plates (Nunc, Copenhagen, Denmark). Ten microliters of ECK or Con A were added into wells at final concentrations of 50, 100, or 500 μg/ml of ECK or 5 μg/ml of Con A. After incubation for 3 days at 37°C, 95% humidity, and 5% CO2, 1 μCi of [3H]thymidine (specific activity 42 Ci/mmol) was added to the cells, and the plates were incubated for an additional 18 h. The cells were then harvested onto glass fiber filters via an automatic cell harvester. The amount of radioactivity incorporated into DNA was determined in a liquid scintillation spectrometer.
Flow Cytometry Assay
The flow cytometry assay, which simultaneously measures multiple characteristics of single cells at a rapid rate, was used in our experiment to detect accessory molecule expression on spleen cells after fluorescence-activated cell sorting (FACS). In brief, splenocytes were harvested and washed with DPBS (Gibco BRL). The cells were blocked with an antimouse IgG solution in PBS for 15 min at 4°C to inhibit nonspecific staining, and then stained with fluorescently labeled monoclonal antibodies (mAbs) to the mouse antigens of interest at optimal concentrations for an additional 15 min at 4°C. Abs (BD Biosciences, San Jose, CA) were directly labeled with the following fluorescent tags: fluorescein isothiocyanate (FITC) or phycoerythrin (PE) for CD4 [H129.19 and rat (Lou/WS1)IgG2a,κ], CD8 [53–6.7 and rat IgG2a,κ], CD11b [M1/70 and rat(DA) IgG2b,κ], CD11c [HL3 and American Hamster IgG1*,λ2], and CD45R/B220 [RA3-6B2 and rat IgG2a,κ]. Those used in these experiments were CD4-FITC as a specific marker for T-helper (Th) lymphocytes and NK-T lymphocytes, CD8-PE as a specific marker for cytolytic T lymphocytes and dendritic cells, CD11b as a specific marker for granulocytes and macrophages, CD11c as a specific marker for NK cells and dendritic cells, and CD45R/B220-FITC as a specific marker for pan B lymphocytes and abnormal T cells. Appropriate isotype controls were always included. After centrifugation, the cells were fixed with 1% formalin, and 20,000 viable cells per treatment (as determined by light scatter profiles) were analyzed via a BD FACSCalibur™ flow cytometer and CellQuest software (BD Biosciences).
CFSE-Labeled Cell Proliferation Assay
To ascertain the proliferation and differentiation of ECK-treated splenocytes, we performed a carboxy fluorescein diacetate succinimidyl ester (CFSE) assay. Normally, after cell division, CFSE labeling is distributed equally between daughter cells, each of which is, therefore, half as fluorescent as the parent. For this assay, 6 × 107 splenocytes were suspended in warm RPMI 1640 medium without FBS and mixed with a working solution of 50 μM CFSE-FITC (final concentration 0.5 μM). After incubation in the dark at 37°C for 10 min, 50 ml of cold RPMI 1640 medium containing 1% FBS was added to stop the uptake of CFSE dye. The CFSE-labeled cells were washed in cold RPMI and resuspended in RPMI 1640 medium containing 10% FBS and 1% antibiotic. Then, 3 × 106 CFSE-labeled cells were incubated with 100 μg/ml of ECK or vehicle at 37°C for 3 or 5 days, and the medium was replaced with fresh medium containing 10% FBS and 1% antibiotic at 3 days. After 3 or 5 days, the CFSE-labeled cells were harvested and stained with mAbs such as R-PE-anti-CD4 [H129.19 and rat(Lou/WS1)IgG2a,κ], which is a specific marker for Th lymphocytes and NK-T lymphocytes; R-PE anti-CD8 [53-6.7 and rat IgG2a,κ], which is a specific marker for cytolytic T lymphocytes and dendritic cells; CD45R/B220 [RA3-6B2 and rat IgG2a,κ], which is a specific marker for pan B lymphocytes and abnormal T cells, granulocytes, and macrophages; and CD11c as a specific marker for NK cells and dendritic cells. Data were collected for 50,000 cells and analyzed via a BD FACSCalibur™ flow cytometer and CellQuest software (BD Biosciences). Proliferating lymphocyte subsets (CD4, CD8, and CD45R/B20) were distinguished via multiparameter flow cytometry.
Intracellular Cytokine Staining Assay
Because the production of interleukin (IL)-2 is a key event in Th1 and Th2 cell activation, we applied intracellular cytokine staining to check whether lymphocytes induced to proliferate by ECK exposure make IL-2. In brief, splenocytes incubated for 3 days with ECK were reincubated with a cocktail containing brefeldin-A, ionomycin, and Phorbol 12-myristate 13-acetate (PMA) for intracellular IL-2 staining. After 4 h of stimulation, the cells were harvested and washed with DPBS (Gibco BRL). The cells were blocked with anti-mouse IgG solution in PBS for 15 min at 4°C to inhibit nonspecific staining, and then stained with fluorescently labeled CD3 [145-2C11 and American Hamster IgG1*,κ]-FITC (BD Biosciences), a specific marker for mature T cells, at optimal concentration for an additional 15 min at 4°C. Then, the cells were reacted with the IL-2 mAb [JES6-5H4 and rat IgG2b]-PE (BD Biosciences). After the reaction, the cells were washed and fixed with 1% formalin. Twenty thousand viable cells per treatment (as determined by light scatter profiles) were analyzed via a BD FACSCalibur™ flow cytometer and CellQuest software (BD Biosciences).
RNA Preparation
Splenocytes were incubated with ECK for 48 h before RNA extraction. After incubation, the cells were harvested and lysed in the Trizol reagent (Molecular Research Center, Cincinnati, OH). The addition of chloroform (Sigma-Aldrich) and incubation for 5 min at 4°C followed. Supernatants obtained after centrifugation were mixed with isopropanol (Sigma), and the resulting RNA pellets were washed with EtOH and stored at −20°C until use.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
The cDNA was synthesized with RNA purified from splenocytes via a Promega A3500 kit, according to manufacturer’s instructions (Promega, San Luis Obispo, CA). PCR of this cDNA and the primer (Bioneer, Daejeon, South Korea), shown in Table 1, was performed for 40 cycles with a 5-min denaturing step at 94°C, a 1-min annealing step at 55 to 60°C, and a 20-min extension phase at 72°C using the TaKaRa PCR machine (Takara Bio, Shiga, Japan). PCR products were run on a 1.5% EtBr/agarose gel and visualized by UV transillumination.
ELISA
To identify the effects of ECK on the production of interferon (IFN)-γ, tumor necrosis factor (TNF)-α, IL-4, and IL-10, splenocytes were incubated with ECK, and the supernatants were collected from the cell suspensions. In addition, Con A (5 μg/ml) was used to promote T-cell proliferation and production of cytokines. Then, these supernatants were analyzed according to the manufacturer’s recommendations with mouse cytokine-specific ELISA kits (Biosource International, Camarillo, CA). Cytokine levels were calculated via standard curves using recombinant murine cytokines.
Statistical Analysis
Data were analyzed via the SPSS package for Windows (Version 10). Values are expressed as means ± standard error (SE). P < 0.05 is considered significant.
Results
ECK has an Abundance of Polyphenols and Crude Sulfated Polysaccharides
The composition of bioactive materials of interest in ECK was sought by measuring the extract’s moist and dry (ash) contents. The yield of ECK was about 52.15%, and contained significantly rich portions of polyphenols and crude sulfated polysaccharides (about 59.73%), as expressed in Table 2. In addition, the fucose and sulfate groups constituted about 72.25% and 6.17% respectively, of the crude sulfated polysaccharides. Because fucoidan usually contains large amounts of fucose and sulfates, this crude sulfated polysaccharide in our ECK can be regarded as fucoidan with that agent’s innate ability to activate or suppress immune cell functions.
ECK Enhances the Viability and Proliferation of Splenocytes
The viability of splenocytes incubated for 48 h with ECK was quantified by assessing the exclusion of trypan blue dye. As shown in Figure 1, the viability of ECK-treated splenocytes increased significantly above that of untreated, control cells (P < 0.01). In addition, the number of the viable cells increased incrementally as the concentrations of ECK rose from 50 μg/ml to 100 μg/ml, indicating the beneficial effect of ECK on the survival of immune cells.
Effect of ECK on the viability of splenocytes. Four hundred thousand cells were or were not treated with the ECK and placed into culture plates. After 2 days, trypan blue dye was added to the cells at the proper dilution factor recommended by the supplier. The number of the viable cells in both cultures, i.e., those stained by trypan blue, was counted via a hematometer. Experiments were performed in triplicate. Statistical evaluation was performed to compare the experimental groups and corresponding control groups. *P < 0.01.
Next, to confirm that ECK similarly benefits splenocytes’ proliferative ability, we used a [3H]thymidine incorporation assay. Accordingly, the proliferation of these ECK-treated cells was significantly higher than that of untreated cells at a comparative magnitude and increased with rising concentrations of ECK from 50 μg/ml to 500 μg/ml in a dose-dependent manner (P < 0.01; Figure 2). Presumably, ECK may promote an enhanced viability and proliferation of immune cells.
Effect of ECK on the proliferation of splenocytes. Four hundred thousand viable cells from each culture were transferred to wells of a 96-well microtiter tissue culture plate in triplicate at the concentrations indicated (50 to 500 μg/ml). After incubation with ECK for 3 days, proliferation of splenocytes was measured via the incorporation of [3H] thymidine. Experiments were performed in triplicate, and data are expressed as average percentage change from untreated controls ± SD. Background proliferation was 5009 ± 466 cpm; Con A-induced proliferation was 89,646 ± 8103 cpm. Statistical evaluation was performed to compare the experimental groups and corresponding control groups. *P < 0.01.
ECK Increases the Populations of T and B Cells
To define further the specific cell population stimulated by ECK, the splenocytes were analyzed via the correlated measurements of forward-angle light scatter plotted against right-angle light scatter. In a cytogram, three main clusters could be seen that related to the three major cell types: lymphocytes, monocytes, and polymorphonuclear granulocytes. Subsequently, the ability of splenocytes to interact with antibody markers was tested and subjected to FACS analysis. We found that the numbers of lymphocytes, monocytes, and granulocytes increased compared to those of untreated cells on 3 days or 5 days after ECK treatment. In particular, the number of lymphocytes (P < 0.005, on 3 days after ECK treatment) and granulocytes (P < 0.05, on 5 days after ECK treatment) was significantly increased by ECK as incubation progressed (Figure 3). Subsequently, the splenocytes were incubated with ECK for 48 h and stained with fluorescein-conjugated anti-CD4, CD8, CD11b, CD11c, and CD45R/B220 mAbs. Again, the numbers of CD4+ T cells, CD8+ T cells, and CD45R/B220+ pan B cells were markedly upregulated by ECK compared with untreated cells (Figure 4), although the elevation of CD11b+ macrophages, dendritic cells, and CD11c+ NK cells did not reach a level of statistical significance (data not shown). However, the number of CD8+ cytolytic T cells was elevated twofold above that of control cells. Moreover, the specific populations of CD4+ Th cells and CD45R/B220+ pan B cells were larger by about 14.5% and 12.1%, respectively. These results indicate that ECK not only stimulates lymphocytes in general but also increases the quantities of specific cell phenotypes.
Gated lymphocytes, monocytes and granulocytes population analysis of proliferating splenocytes incubated with ECK for 3 (A) or 5 days (B). The cells were cultured in the absence or presence of ECK (100 μg/ml) for 3 days or 5 days. After incubation, the cell population was measured via FACS analysis. The cultured splenocytes were analyzed and the correlated measurements of forward-angle light scatter plotted against right-angle light scatter. In a cytogram, three main clusters could be seen which related to the three major cell types: lymphocytes, monocytes, and polymorphonuclear granulocytes. Data are means ± SD of three experiments. Statistical evaluation was performed to compare the ECK-treated cells with corresponding untreated cells, respectively. *P < 0.05, **P < 0.005. Three independent experiments were performed.
Phenotypic analysis of proliferating splenocytes after ECK treatment. After incubation for 2 days, the expression level of each cell type was measured via FACS analysis using anti-CD4, CD8, and CD45R/B220 mAbs. The cells were cultured in the absence or presence of ECK (100 μg/ml) for 2 days. Then, the cells were blocked by anti-mouse IgG and stained by the mAbs as indicated. The results are representative of three separate experiments (n = 3).
ECK Enhances the Proliferation and Differentiation of CFSE-Labeled T and B Cells
To confirm whether ECK enhances the differentiation and proliferation of T and B cells, we measured the progressive decrease in intensity of the CFSE dye in each succeeding T- and B-cell generation. Normally, CFSE-FITC is transferred from parent cells to daughter cells. As shown in Figure 5, the proliferation of CFSE-labeled CD4+ Th cells, CD8+ cytolytic T cells, and CD45R/B220+ pan B cells was increased by ECK in comparison with untreated CFSE-labeled cells. Notably, the number of CFSE-labeled CD45R/B220+ pan B cells was about 20% larger than that of untreated cells. These results indicated that ECK not only increases the population of specific cell phenotypes but also induces succeeding generations of T and B cells.
Proliferation analysis in CFSE-labeled cells after ECK treatment. After incubation for 5 days, the expression level was measured via FACS analysis using anti-CD4, CD8, and CD45R/B220 mAbs. The CFSE-labeled cells were cultured in the absence or presence of ECK (100 μg/ml) for 5 days. Then, the cells were blocked by antimouse IgG and stained by the mAbs as indicated. The results are representative of three separate experiments (n = 3).
ECK Augments the Production Level of IL-2 in T Lymphocytes
To investigate the production of IL-2 known as a cytokine that stimulates Th1- and Th2-type lymphocytes, we performed an intracellular staining assay of lymphocytes combined with ECK. As shown in Figure 6, ECK markedly upregulated the production of IL-2 in CD3+ mature T cells compared with untreated cells. Moreover, ECK showed an about twofold improvement in the number of CD3+ mature T cells secreting IL-2. These results suggest that IL-2 production augmented by ECK might induce the proliferation and differentiation of T lymphocytes.
Intracellular IL-2 analysis in CD3+ lymphocytes after ECK treatment. After incubation for 72 h, the expression level was measured via FACS analysis using anti-CD3-FITC mAb and IL-2-PE mAb. Then, the cells were blocked by antimouse IgG and stained by the anti-CD3-FITC mAb and IL-2-PE mAb as indicated. The results are representative of three separate experiments (n = 3).
ECK Modulates the Expression Level of mRNA Between Th1- and Th2-Type Cytokines
The specific cytokines produced by polarized Th1 (helper) and Th2 (cytolytic) cells are the primary effectors that promote the differentiation of precursor Th cells, but these cytokines also cross-regulate the other subset’s functional activity. Presumably, if ECK contributes to the proliferation and changes the phenotype of immune cells, ECK can then alter and even enhance their immunological functions. Therefore, we determined the effects of ECK on cytokine mRNA in these numerically upregulated splenocytes. As shown in Figure 7, in fact, the mRNA expression levels of certain Th1-type cytokines, i.e., IL-1β, IFN-γ, and TNF-α, as well as the Th2-type cytokines, i.e., IL-4 and IL-10, were changed by ECK compared to that expressed by the untreated cells. Further, IFN-γ and TNF-α mRNA expression was significantly decreased by ECK (P < 0.01), although IL-1β mRNA expression did not reach statistical significance in ECK-treated cells. Conversely, the levels of IL-4 and IL-10 mRNA expression increased significantly after ECK exposure compared to that of untreated cells (P < 0.01). The expression levels of GAPDH mRNA used as an internal control on ECK-treated splenocytes were identical with those of untreated cells. The expression levels of the cytokines/GAPDH mRNA were then calculated to normalize the level of target cytokines to GAPDH mRNA (Figure 7B). Together, these results suggest that ECK modulates the release of Th2- and also the suppression of Th1-type cytokines, thereby regulating immunologic capacity.
Effect of ECK on the mRNA expression of cytokines by proliferating splenocytes (A); relative intensity of GAPDH expression indicating cytokine levels (B.) After incubation for 2 days, RNA from the cultured cells treated with ECK (100 μg/ml) or not treated was used for cDNA synthesis. The cDNA (1 μg) was amplified via PCR using gene-specific primers. PCR products were separated via 1% agarose gel electrophoresis and visualized with ethidium bromide staining. The expression levels of various target cytokines/GAPDH mRNA were calculated to normalize the level of target cytokines to GAPDH mRNA. Similar results were obtained in three separate experiments. *P < 0.01.
ECK Modulates the Production of Th1- and Th2-Type Cytokines
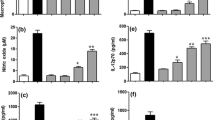
To assess whether ECK affected production of the cytokines IFN-γ, TNF-α, IL-4, and IL-10 in blastogenic cells stimulated by Con A, we quantified these cytokines via ELISA. Because supernatants from the splenocytes tested produced very low levels of cytokines without specific mitogen stimulation, Con A was used to promote T-cell proliferation and production of cytokines. As Figure 8 depicts, IFN-γ and TNF-α production was markedly increased by Con A, whereas ECK treatment significantly reduced the production of these two cytokines in Con A-stimulated blastogenic cells (P < 0.05). In particular, IFN-γ production decreased threefold in ECK-treated blastogenic cells. In contrast, the levels of IL-4 and IL-10 produced in ECK-treated blastogenic cells were significantly higher than that of untreated cells (P < 0.005 and P < 0.005, respectively). Collectively, ECK upregulated the production of Th2-type cytokines yet downregulated the production of Th1-type cytokines in Con A-stimulated blastogenic cells. This immunomodulatory effect of ECK in Con A-stimulated blastogenic cells may be associated with the reduced production of IFN-γ and TNF-α and enhanced production of IL-4 and IL-10.
Effect of ECK on Th1 (IFN-γ and TNF-α) and Th2 (IL-4 and IL-10) cytokine production by Con A-stimulated blastogenic cells. (A) IFN-γ, (B) TNF-α, (C) IL-4, (D) IL-10. Splenocytes were stimulated with Con A (5 μg/ml), and these stimulated blastogenic cells were treated with ECK (100 μg/ml) for 3 days or not treated. The culture supernatants were collected, and concentrations of IL-1β, TNF-α, IL-4, and IL-10 were measured using commercially available ELISA kits. Spontaneous cytokine release: IFN-γ, 66 ± 21 pg/ml; TNF-α, 38 ± 21 pg/ml: IL-4, undetectable; IL-10, undetectable. Data are means ± SD of three experiments. Statistical evaluation was performed to compare the ECK-treated blastogenic cells with corresponding Con A alone-stimulated cells, respectively. *P < 0.05 **P < 0.005.
Discussion
In the present study, we demonstrated that ECK, a protease extract from brown seaweed, dramatically strengthened the survival and proliferation of splenocytes. In addition, functional subsets of both T and B cells were markedly increased by ECK treatment. This outcome supports the stimulatory effect of ECK on lymphocytes, particularly on the subsets of T and B cells involved in cytokine and antibody synthesis, e.g., the number of CD4+ Th cells was increased by ECK.
Still, distinctive cytokines released by Th1- and Th2-type cells clearly regulate specific immune responses. Among them, IL-2 is a key event in T cell activation. IL-2 was originally called a growth factor for antigen-stimulated T lymphocytes that allows these cells to enter into the S phase of the cell cycle and divide. After the activation phase takes place, T lymphocytes proliferate and differentiate to generate effector T cells. Thereby, Th precursor cells, which are functionally immature, may become Th1 or Th2 effector cells. These subsets are responsible for cell-mediated immunity and humoral responses, respectively (Liberman et al., 2003). In addition, IL-2 induces the secretion of Th1- and Th2-type cytokines (O’Garra and Arai, 2000). Th1-type cells have inflammatory functions and the ability to activate cytotoxicity by producing such proinflammatory cytokines as, IL-12, IFN-γ, and TNF-α. Th2-type cells can function to encourage antibody production, particularly IgE, eosinophil proliferation/function and also production of such anti-inflammatory cytokines as IL-4, IL-5, IL-6, IL-9, IL-10, and IL-13 (Mosmann and Sad, 1996). The distinctive and opposing effector functions of these cellular subsets are well known, as is the fact that extremes of either type can lead to disease. CD4+ T cells can be divided into Th1 and Th2 effector subsets and are defined based on the cytokines they secrete (Feili-Hariri et al. 2005). Moreover, any factor that affects the balance of either Th1/Th2 cell cytokines or antibody isotypes may play a role in the development or resolution of autoimmune disease (Schifitto et al. 2000; Chung 2001). The environmental milieu regulates the ensuing CD4+ T cells differentiation into a Th1 or a Th2 phenotype (Rao and Avni 2000).
Further, Th1 and Th2 cells have been found to reciprocally inhibit each other’s differentiation pathways. IFN-γ can inhibit Th2 development (Chung, 2001), and IL-4 has been found to inhibit Th1 development (Skapenko et al., 2004). Now, our results have shown that, after exposure to ECK, mRNA expression levels of the Th1-type cytokines IL-1β, IFN-γ, and TNF-α decreased markedly, whereas mRNA levels of the Th2-type cytokines IL-4 and IL-10 significantly increased as compared to those of untreated controls. ECK also increased the production of IL-4 and IL-10 but suppressed the production of IFN-γ and TNF-α in Con A-stimulated blastogenic cells. These results suggest that the activation of CD4+ T cells by ECK specifically regulates the expression of Th1- and Th2-type cytokine mRNAs, thereby inhibiting the development of Th1 cells via IL-10 and stimulating the differentiation of Th2 cells via IL-4. The apparent ability of ECK to modulate these two cytokines, as shown here, would be an important factor in regulating immune responses.
Although the literature contains a multitude of research results on lymphocyte subpopulations and cytokines modulated by ECK, the basic mechanisms underlying their interactions during immune reactions remain obscure. Recently, a transcription molecular mechanistic study revealed that eckol, a component of ECK, increased phosphorylation of extracellular signal-regulated kinase and the activity of NFκB (Kang et al., 2005b). Indeed, NFκB plays a critical role in the transcription of several genes involved in immune and inflammatory responses, cell proliferation/differentiation, and cell transformation. The possible involvement of the NFκB signal pathway and/or other pathways related to this molecule offers opportunities for future exploration. Also, the mechanism of the influence of ECK on signals related to modulation of immune responses needs clarification.
The ECK used in this study has numerous advantages such as its properties as a natural product, water solubility, great variation of constituents, multiple biological activities, high extraction efficiency, low cost, and minimal environmental pollution and toxicity compared to solvent extracts (Heo et al., 2003). We previously reported that many components from seaweeds isolated with the enzymatic extraction technique used here act as antioxidants (Heo et al., 2003, 2005a, b; Ahn et al., 2004a; Park et al., 2005), anticoagulants (Athukorala et al., 2006), and anticancer agents (Kim et al., 2006a). In contrast, solvent extracts sometimes provide extremely low recovery rates, do not meet strict regulations for use in the food industry, and yield only a limited recovery of water-soluble components in water extractions. Therefore, solvent-free ECK is a superior product for nutritional and therapeutic applications.
In addition, previous studies have reported that such physiologic properties as immune modulation, immune activation, and immune suppression are related to polyphenols containing phloroglucinol, eckol, and dieckol or fucoidan with its content of fucose, glucose, mannose, galactose, rhamnose, and xylose (Garcia et al., 2003; Choi et al. ,2005; Schepetkin et al., 2005; Meeran et al., 2006). As our present results show, ECK has high concentrations of polyphenols and especially crude sulfated polysaccharide with its abundance of fucose, a prospectively beneficial immunomodulator.
In the latter regard, researchers have indicated that optimal immunotherapy should restore or maintain a well-balanced Th1 and Th2 response (Patwardhan and Gautam 2005; Mosmann and Sad, 1996). Our results demonstrated that ECK has ideal immunostimulatory characteristics, in that it activates T cells and B cells, and also modulatory characteristics evident here in production of Th1- as well as Th2-type cytokines. ECK also seems to be a suitable agent for selectively modulating either Th1 or Th2 responses and, thereby, may provide a means of achieving T cell homeostasis. Therefore, ECK has the advantage of being a natural product that may be useful as therapy for immune-related diseases.
References
Ahn CB, Jeon YJ, Kang DS, Shin TS, Jung BM (2004a) Free radical scavenging activity of enzymatic extracts from a brown seaweed Scytosiphon lomentaria by electron spin resonance spectrometry. Food Res Intern 37, 253–258
Ahn MJ, Yoon KD, Min SY, Lee JS, Kim JH, Kim TG, Kim SH, Kim NG, Huh H, Kim J (2004b) Inhibition of HIV-1 reverse transcriptase and protease by phlorotannins from the brown alga Ecklonia cava. Biol Pharm Bull 27, 544–547
Athukorala Y, Jung WK, Vasanthan T, Jeon YJ (2006) An anticoagulative polysaccharide from an enzymatic hydrolysate of Ecklonia cava. Carbohydr Polym 66, 184–191
Choi EM, Kim AJ, Kim YO, Hwang JK (2005) Immunomodulating activity of arabinogalactan and fucoidan in vitro. J Med Food 8, 446–453
Chung F (2001) Anti-inflammatory cytokines in asthma and allergy: interleukin-10, interleukin-12, interferon-gamma. Mediators Inflamm 10, 51–59
Feili-Hariri M, Falkner DH, Morel PA (2005) Polarization of naive T cells into Th1 or Th2 by distinct cytokine-driven murine dendritic cell populations: implications for immunotherapy. J Leukoc Biol 78, 656–664
Fukuyama Y, Kodama M, Miura I, Kinzyo Z, Kido M, Nakayama Y, Takahashi H (1989) Structure of an anti-plasmin inhibitor, eckol, isolated from the brown alga Ecklonia kurome Okamura and inhibitory activities of its derivatives on plasma plasmin inhibitors. Chem Pharm Bull 37, 349–353
Garcia D, Leiro J, Delgado R, Sanmartin ML, Ubeira FM (2003) Mangifera indica L. extract (Vimang) and mangiferin modulate mouse humoral immune responses. Phytother Res 17, 1182–1187
Guiry MD, Bulunden G (1991) Seaweed Resource in Europe: Uses and Potential. (Chichester, UK: John Wiley & Sons)
Heo SJ, Jeon YJ, Lee J, Kim HT, Lee KW (2003) Antioxidant effect of enzymatic hydrolyzate from a kelp, Ecklonia cava. Algae 18, 341–347
Heo SJ, Park EJ, Lee KW, Jeon YJ (2005a) Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresour Technol 96, 1613–1623
Heo SJ, Park EJ, Lee KW, Jeon YJ (2005b) Antioxidative effect of proteolytic hydrolysates from Ecklonia cava on radical scavenging using ESR and H2O2-induced DNA damage. Food Sci 14, 614–620
Kang HS, Chung HY, Kim JY, Son BW, Jung HA, Choi JS (2004a) Inhibitory phlorotannins from the edible brown alga Ecklonia stolonifera on total reactive oxygen species (ROS) generation. Arch Pharm Res 27, 194–198
Kang HS, Kim HR, Byun DS, Son BW, Nam TJ, Choi JS (2004b) Tyrosinase inhibitors isolated from the edible brown alga Ecklonia stolonifera. Arch Pharm Res 27, 1226–1232
Kang KA, Lee KH, Chae SW, Koh YS, Yoo BS, Kim JH, Ham YM, Baik JS, Lee NH, Hyun JW (2005a) Triphlorethol-A from Ecklonia cava protects V79-4 lung fibroblasts against hydrogen peroxide induced cell damage. Free Radic Res 39, 883–892
Kang KA, Lee KH, Chae SW, Zhang R, Jung MS, Lee YG, Kim SY, Kim HS, Joo HG, Park JW, Ham YM, Lee NH, Hyun JW (2005b) Eckol isolated from Eclonia cava attenuates oxidative stress induced cell damage in lung fibroblast cells. FEBS Lett 579, 6295–6304
Kim KN, Lee KW, Song CB, Jeon YJ (2006a) Cytotoxic activity of green and brown seaweeds collected from Jeju Island against four tumor cell lines. J Food Sci Nutr 11, 17–24
Kim MM, Ta QV, Mendis E, Rajapakse N, Jung WK, Gyun HG, Jeon YJ, Kim SK (2006b) Phlorotannins in Ecklonia cava extract inhibit matrix metalloproteinase activity. Life Sci 79, 1436–1443
Lee JH, Kim ND, Choi JS, Kim YJ, Moon YH, Lim SY, Park KY (1998) Inhibitory effects of the methanolic extract of an edible brown alga, Ecklonia stolonifera and its component, phlorglucinol on aflatoxin B1 mutagenicity in vitro (Ames test) and on benzo(a)pyrene or N-methyl N-nitrosourea clastogenicity in vivo (mouse micronucleus test). Nat Prod Sci 4, 105–114
Liberman AC, Refojo D, Arzt E (2003) Cytokine signaling/transcription factor cross-talk in T cell activation and Th1-Th2 differentiation. Arch Immunol Ther Exp (Warsz) 51, 351–365
Maruyama M, Tamauchi H, Hasimoto M, Nakano T (2003) Antitumor activity and immune response of Mekabu fucoidan extracted from sporophyll of Undaria pinnatifida. In vivo (Athens, Greece) 17, 245–249
Meeran SM, Mantena SK, Katiyar SK (2006) Prevention of ultraviolet radiation-induced immunosuppression by (–)-epigallocatechin-3-gallate in mice is mediated through interleukin 12-dependent DNA repair. Clin Cancer Res 1, 2272–2280
Mosmann TR, Sad S (1996) The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today 17, 142–146
Nagayama K, Iwamura Y, Shibata T, Hirayama I, Nakamura T (2002) Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome. J Antimicrob Chemother 50, 889–893
O’Garra A, Arai N (2000) The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends Cell Biol 10, 542–550
Park PJ, Heo SJ, Park EJ, Kim SK, Byun HG, Heon BT, Jeon YJ (2005) Reactive oxygen scavenging effect of enzymatic extracts from Sargassum thunbergii. J Agric Food Chem 53, 6666–6672
Patwardhan B, Gautam M (2005) Botanical immunodrugs: scope and opportunities. Drug Discov Today 10, 495–502
Rao A, Avni O (2000) Molecular aspects of T-cell differentiation. Br Med Bull 56, 969–984
Ruperez P, Saura-Calixto F (2001) Dietary fibers and physiochemical properties of edible seaweeds. Eur Food Res Technol 212, 349–354
Saito H, Yamagata T, Suzuki S (1968) Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem 243, 1536–1542
Saker KE, Fike JH, Veit H, Ward DL (2004) Brown seaweed-(Tasco) treated conserved forage enhances antioxidant status and immune function in heat-stressed wether lambs. J Anim Physiol Anim Nutr 88, 122–130
Schepetkin IA, Faulkner CL, Nelson-Overton LK, Wiley JA, Quinn MT (2005) Macrophage immunomodulatory activity of polysaccharides isolated from Juniperus scopolorum. Int Immunopharmacol 5, 1783–1799
Schifitto G, McDermott MP, Evans T, Fitzgerald T, Schwimmer J, Demeter L, Kieburtz K (2000) Autonomic performance and dehydroepiandrosterone sulfate levels in HIV-1-infected individuals: relationship to TH1 and TH2 cytokine profile. Arch Neurol 57, 1027–1032
Siriwardhana N, Jeon YJ, Kim SH, Ha JH, Heo SJ, Lee KW (2004) Enzymatic hydrolysis for effective extraction of antioxidative compounds from Hizikia fusiformis. Algae 19, 59–68
Skapenko A, Niedobitek GU, Kalden JR, Lipsky PE, Schulze-Koops H (2004) IL-4 exerts a much more profound suppression of Th1 immunity in humans than in mice. J Immunol 172, 6427–6434
Waterman PG, Mole S (1994) Analysis of Phenolic Plant Metabolites. (Oxford: Blackwell)
Yeh ST, Lee CS, Chen JC (2006) Administration of hot-water extract of brown seaweed Sargassum duplicatum via immersion and injection enhances the immune resistance of white shrimp Litopenaeus vannamei. Fish Shellfish Immunol 20, 332–345
Acknowledgment
This research was performed under a program of the Basic Atomic Energy Research Institute (BAERI), which is a part of the Nuclear R&D Programs funded by the Ministry of Science & Technology (MOST) of Korea. The authors thank P. Minick for editorial assistance.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Y-J. Jeon and Y. Jee contributed equally to this study.
Rights and permissions
About this article
Cite this article
Ahn, G., Hwang, I., Park, E. et al. Immunomodulatory Effects of an Enzymatic Extract from Ecklonia cava on Murine Splenocytes. Mar Biotechnol 10, 278–289 (2008). https://doi.org/10.1007/s10126-007-9062-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-007-9062-9