Abstract
An actinomycete strain 7326 producing cold-adapted α-amylase was isolated from the deep sea sediment of Prydz Bay, Antarctic. It was identified as Nocardiopsis based on morphology, 16S rRNA gene sequence analysis, and physiological and biochemical characteristics. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and zymogram activity staining of purified amylase showed a single band equal to a molecular mass of about 55 kDa. The optimal activity temperature of Nocardiopsis sp. 7326 amylase was 35°C, and the activity decreased dramatically at temperatures above 45°C. The enzyme was stable between pH 5 and 10, and exhibited a maximal activity at pH 8.0. Ca2+, Mn2+, Mg2+, Cu2+, and Co2+ stimulated the activity of the enzyme significantly, and Rb2+, Hg2+, and EDTA inhibited the activity. The hydrolysates of soluble starch by the enzyme were mainly glucose, maltose, and maltotriose. This is the first report on the isolation and characterization of cold-adapted amylase from Nocardiopsis sp.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microorganisms living in extreme environments, such as deep sea and Polar Regions, presumably have developed particular characteristics that allow them to thrive in such environments (Takami et al. 1997). For example, their cold-active or cold-adaptive enzymes have received a great deal of attention because they are essential in some fundamental scientific study areas and have the ability to withstand certain industrial reaction conditions (Deming 1998). Recent microbial studies of the deep sea have led to significant new discoveries of unusual microbial diversity, new species, and cold-adaptive enzymes (Feller 2003; Feller and Gerday 2003; Zeng et al. 2003, 2006), which were regarded as critical elements for the survival of the microorganisms in extreme environments (Chessa et al. 1999). Likewise, the high activity of cold-adaptive enzymes at low and moderate temperatures offers potential economic benefits (Russell 1998; Gerday et al. 2000; Cavicchioli et al. 2002).
Actinomycetes are one of the most investigated groups because they constitute a potential source of biotechnologically interesting substances (Lealem and Gashe 1994). Nocardiopsis strains are distributed ubiquitously in the environment (Kroppenstedt and Evtushenko 2002). They are frequently isolated from habitats with moderate to high salt concentrations such as saline soil or marine sediments (Evtushenko et al. 2000; Al-Zarban et al. 2002) and salterns (Chun et al. 2000). Here we first report the purification and characterization of a cold-adaptive α-amylase secreted by a Nocardiopsis strain that was isolated from deep sea sediment of Prydz Bay, Antarctic. The culture has been deposited in the Marine Culture Collection of China (http://www.mccc.org.cn) as Nocardiopsis sp. 7326.
Materials and Methods
Sample Collection
The deep sea sediment was collected via multicore sampler at a depth of 900 m of site PN5-6 (740°25′E, 66°55′S) during the 21st cruise of Chinese Antarctic Research (Nov. 2004–Mar. 2005). The sediment was transferred to sterile Falcon tubes in clean bench and kept aseptically at 4°C until usage.
Strain Isolation and Culture Conditions
The sediment was diluted with artificial seawater (ASW) containing 0.3% NaCl, 0.07% KCl, 0.53% MgSO4·7H2O, 1.08% MgCl2·6H2O, and 0.1% CaSO4·7H2O and the supernatants were grown on glycerol/asparagines (ISP-5) medium containing 0.5% starch under 10°C for 5 days. The single colony was isolated after being picked and cultured on ISP-5 agar medium repetitively and verified by microscope. The strains were preserved on ISP-2 medium (Pridham et al. 1956), supplemented with 1% (wt/vol) agar starch slants at 4°C.
The seed cultural medium (A) was ISP-5 medium supplemented with 1% (wt/vol) starch and ASW (pH 7.5). The optimal amylase-producing medium (B) contained 2.5% (wt/vol) starch, 1% glucose, 0.05% K2HPO4, and ASW (pH 8.0). The seed culture was prepared in Erlenmeyer flasks by inoculating a 2.0-ml spore suspension containing 3.6 to 4.0 × 108 UFC ml−1 into 50 ml of presterilized medium A and cultivating under agitation at 180 rpm for 48 h. Then 50 ml of the seed culture was added to 500 ml of medium B and was incubated at 20°C for 120 h. Samples (5 ml) were taken from each of three replicate flasks at 12-h intervals. The cell-free supernatant containing α-amylase was harvested by centrifugation at 4000 rpm for 15 min at 4°C and subjected to purification and characterization.
Identification of Strain 7326
The genome DNA was extracted from the strain and used as template for polymerase chain reaction (PCR) amplification of the 16S rRNA fragment according to the method described previously (Rainey et al. 1996). The primers used were Eubac27F: 5′-AGAGTTTGATCCTGGCTCAG-3′ and Eubac1492R: 5′-GGTTACCTTGTTACGACTT-3′ (DeLong 1992). The PCR product was cloned into pMD18-T (Takara) and sequenced by Shanghai Sangon Biological Engineering Technology & Services Company. The 16S rRNA gene sequence was checked for similarities to 16S rRNA sequences in the EMBL and RDPII (http://rdp.cme.msu.edu) database. The alignment and phylogenetic analysis of sequences were achieved via DNAMAN (Lynnon Biosoft). Tests of physiological and biochemical characteristics were carried out according to the methods described previously (Schipper et al. 2002).
Purification of Amylase
Amylase was purified at 4°C as follows. The cell-free supernatant was subjected to ammonium sulfate precipitation, and the protein precipitating at 60% saturation was resuspended in 20 mM Tris-HCl buffer (pH 8.0) and dialyzed against the same buffer for 18 h. The prepurified protein was analyzed via anion-exchange chromatography on a DEAE Sepharose CL-6B column (5 × 30 cm) that had been preequilibrated with 20 mM Tris-HCl (pH 8.0) containing 5% glycerol. The protein was eluted with 20 mM Tris-HCl buffer (pH 8.0) containing 10 mM CaCl2 in a linear gradient between 0 and 0.8 M NaCl. The active fractions were applied to a Sephadex G-75 column (1 × 45 cm) and eluted with 20 mM Tris-HCl buffer (pH 8.0). Finally, the resulting enzyme preparation was desalted and concentrated by dialysis and lyophilization.
The purified amylases were identified via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and zymogram activity staining. After electrophoresis, the gel was stained with Coomassie Brilliant Blue R-250. For activity staining, the gel was suspended in 20% (vol/vol) isopropanol and incubated for 30 min, then transferred to 20 mM Tris-HCl buffer (pH 8.0) and incubated for 30 min. Amylolytic activity was detected by placing the renatured gel onto an agarose gel containing 0.8% soluble starch, and incubating at 35°C for 3 h. The transparent band on the amylase-containing agarose gel was examined after it was flooded with I2 –KI solution.
Assay of Enzyme Activity and Protein Concentration
Amylase activity was determined by detecting the amount of reducing sugars liberated. The reaction mixture (1 ml) contained 0.25 ml of 2% soluble starch, 0.25 ml of 0.4 M Tris-HCl buffer (pH 8.0), and 0.5 ml of enzyme. The reaction was terminated by addition of 2 ml of 3,5-dinitrosalicylic acid reagent after incubation at 35°C for 30 min (Sengupta et al. 2000). One unit of enzyme activity was defined as the amount of enzyme that released 1 μmol of reducing sugar as glucose per minute under the assay conditions. The protein concentration was measured with bovine serum albumin as a standard (Lowry et al. 1951). All measurements in this experiment were made in triplicate.
Thin-Layer Chromatography (TLC) Analysis
The hydrolysis products of starch were submitted to TLC on silica gel plates. Enzymes were incubated with 1% of soluble starch in 10 mM Tris-HCl buffer (pH 8.0) at 35°C for 5 h. Aliquots (5 μl) of the reaction mixtures were chromatographed on a silica gel (Merck) with chloroform–acetic acid–ddw (18:21:3, by vol), and the products were detected by spraying the gel with aniline–diphenylamine–phosphate followed by baking at 120°C for 30 min. Glucose (G1), maltose (G2), maltotriose (G3), and maltotetraose (G4) were the standards.
Effects of pH, Metal Ions, EDTA, and Temperature on Activity
The optimal pH for amylase activity was determined at 35°C in 50 mM acetate–NaOH (pH 4.0 to 5.7), phosphate–NaOH buffer (pH 5.7 to 6.3), 3-(N-morpholino)propanesulfonic acid (MOPS)–NaOH (pH 6.5 to 7.8), Tris-HCl (pH 7.8 to 8.6), 2-(N-cyclohexylamino)ethane (CHES)–NaOH (pH 8.7 to 9.5), and glycine–NaOH (pH 9.5 to 11.0 ) . All pH values were adjusted at room temperature, and the pH stability of the amylase was evaluated by determining the residue activities after a 24-h incubation in the aforementioned buffers at 4°C. The effects of metal ions and EDTA on amylase were examined by determining the activities after 30-min incubation at 35°C in 50 mM Tris-HCl (pH 8.0) buffers containing various metal ions at 5 mM.
The effects of temperature on amylolytic activity were determined by measuring the activity at different temperatures. Before thermostability studies, the enzyme was dialyzed extensively against 50 mM Tris-HCl (pH 8.0) containing 2 mM EDTA and then dialyzed twice against the same buffer without EDTA. Thermostability of the amylase was examined by measuring the residual activities of EDTA-treated enzyme fractions that were preincubated at 15°C, 25°C, 35°C, 45°C, and 55°C in 50 mM Tris-HCl (pH 8.0) containing 5 mM Ca2+ for different periods of time.
Nucleotide Sequence Accession Numbers
The nucleotide sequence of the 16S rRNA gene has been deposited in EMBL under accession no. AM111064.
Results and Discussion
Identification of Strain 7326
Strain 7326 can grow at 0°C, and the optimum and highest temperatures for its growth were 20°C and 37°C, respectively. Its vegetative hyphae were long and slim (0.15 to 0.3 μm in width), well-developed, and fragmented under light microscopy (Figure 1). Short spore chains were borne on the aerial hyphae. Spores (dimensions 0.4 to 0.6°0.6 to 1.0 μm) were rod-shaped, smooth-surfaced, and nonmotile. Strain 7326 could not grow on most of the actinomycete-special agar mediums without additional NaCl. Nevertheless, the colonies were well growing, aerobic, Gram-positive, coarsely wrinkled to folded, and white in color on these mediums containing 20 to 100 g/L of NaCl. Aerial filaments were well developed and abundant. Strain 7326 could utilize the following substrates as the sole source of carbon and energy: d-arabinose, d-fructose, d-galactose, glycerol, mannitol, d-mannose, raffinose, d-ribose, sorbitol, sucrose, d-trehalose, and d-xylose. It could utilize the l-alanine, l-asparagine, l-glutamic acid, l-histidine, l-phenylalanine, l-serine, and l-threonine as sole source of nitrogen. The sources of carbon and nitrogen that could not be utilized by strain 7326 included cellobiose, d-glucose, inositol, lactose, d-maltose, L-rhamnose, d-xylitol, l-cystine, l-methionine, l-glycine, and l-valine. The 16S rRNA gene analysis showed that strain 7326 was most closely related to the genus Nocardiopsis (Figure 2), with the highest levels of similarity (99.454%) to Nocardiopsis sp. 20088 (AY336519). Thus we placed this strain in the genus Nocardiopsis, as Nocardiopsis sp. 7326. Data obtained from RDP II (Ribosomal Database Project) also suggested that strain 7326 was a member of the Nocardiopsis genus.
Phylogenetic analysis of Nocardiopsis sp. 7326. The tree was constructed by neighbor-joining method based on 16S rRNA sequences. The scale bar shows 0.01 substitutions per base position. Numbers refer to bootstrap values for each node out of a total of 100 replicate resamplings. The numbers in the bracket are the EMBL accession numbers of the 16S rRNA sequences of reference bacteria. Streptomyces megasporus, Bacillus subtilis, and Escherichia coli were used as the outgroup.
Purification of Nocardiopsis sp. 7326 Amylase
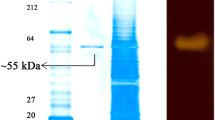
The purification procedure of the Nocardiopsis sp. 7326 extracellular amylase is summarized in Table 1. The amylase exhibited a specific activity of 548 U/mg, corresponding to a purification factor of 21-fold and a total yield of 10.1%. The purified protein showed a single band equal to a molecular mass of about 55 kDa on SDS-PAGE and activity staining gel, confirming high enzyme purity (Figure 3).
TLC analysis
TLC was used to analyze the hydrolysis patterns of soluble starch digested by the purified amylase (Figure 4). The main hydrolysis products of soluble starch were mainly G1 (glucose), G2 (maltose), and G3 (maltotriose) and included a little G4 (maltotetraose). The main hydrolysis product from G2 was G1, mainly G1 and a little G2 from G3, and mainly G1 and a little G2 and G3 from G4. The concentration of the resulting sugars increased with increase of the incubation time. These hydrolysis patterns revealed that amylase from strain 7326 functioned as a typical amylase to hydrolyze the α-(1,4)-glycosidic linkage only.
TLC analysis of hydrolysis products of soluble starch digested by Nocardiopsis sp. 7326 amylase. Lane 1, maltose (G2); lane 2, product generated from G2; lane 3, maltotriose (G3); lane 4, products generated from G3; lane 5, maltotetraose (G4); lane 6, products generated from G4; lane 7, products generated from soluble starch; Std, standard marker.
Effects of Temperature and pH on Amylase Activity
Nocardiopsis sp. 7326 showed maximal enzymatic activity at 35°C, and maintained 38% of its highest activity at 0°C (Figure 5), which was in agreement with the character of the cold-adaptive enzymes (Gerday et al. 2000; Cavicchioli et al. 2002; D’Amico et al. 2003; Feller 2003; Feller and Gerday 2003). The amylase retained more than 70% of the highest activity between 20°C and 45°C. Up to now, the optimum temperatures for enzymatic activity of α-amylases that were normally used in industry were ordinarily between 50°C and 55°C (Declerck et al. 2003). As shown in Figure 6, the amylase retained most of the activity after incubation at 15°C or 25°C for 60 min, while under its optimum activity temperature the amylase lost half of its activity after 40 min of incubation. The activity decreased to 18% after 30-min treatment at 55°C, reflecting the thermosensitivity of the enzyme. The special properties of higher catalytic efficiency at low temperature and more thermosensitivity than that of its mesophilic counterparts (Lee et al. 1994; Igarashi et al. 1998; Hagihara et al. 2001; Yang et al. 2004; Ballschmiter et al. 2006) are the typical characteristics of cold-adaptive amylase.
Figure 7 shows the pH stability and optimal pH of Nocardiopsis sp. 7326 amylase with soluble starch as a substrate. The enzyme was stable between pH 5 and 10 after 24 h of incubation at 4°C. The optimal pH for enzymatic activity was found to be around 8.0, and the amylase retained more than 60% of the highest activity at pH 7 to 9. As a comparison, the optimal pH of a thermostable α-amylase from Nocardiopsis sp. endophyte was pH 5.0 (Stamford et al. 2001).
Effect of pH on the stability (▴) and activity ( ) of Nocardiopsis sp. 7326 amylase. Reaction mixtures were buffered with 50 mM acetate–NaOH (pH 4.0, pH 4.5), phosphate–NaOH buffer (pH 5.7), MOPS–NaOH (pH 6.5, pH 7.0, pH 7.5), Tris–HCl (pH 7.8, pH 8.0, pH 8.6), CHES–NaOH (pH 9.0, pH 9.5), glycine–NaOH (pH 10.0, pH 11.0).
) of Nocardiopsis sp. 7326 amylase. Reaction mixtures were buffered with 50 mM acetate–NaOH (pH 4.0, pH 4.5), phosphate–NaOH buffer (pH 5.7), MOPS–NaOH (pH 6.5, pH 7.0, pH 7.5), Tris–HCl (pH 7.8, pH 8.0, pH 8.6), CHES–NaOH (pH 9.0, pH 9.5), glycine–NaOH (pH 10.0, pH 11.0).
Effects of Metal Ions and EDTA on Amylase Activity
The effects of metal ions on the activity of Nocardiopsis sp. 7326 amylase showed that the enzyme was activated by Ca2+, Mn2+, Mg2+, Cu2+, and Co2+. However, it was inhibited by Rb2+, Hg2+, and EDTA (Table 2). In previous reports, most amylases activity were inhibited in the presence of Ni2+, Cd2+, Cu2+, Ag+, Pb2+, Fe2+, and Zn2+. For example, the α-amylases from Bacillus sp. strain KSM-1378 (Cordeiro et al. 2002) and Bacillus firmus (Igarashi et al. 1998) were strongly inhibited by Ni2+, Cd2+, Zn2+, and Hg2+; the α-amylase from Thermus sp. was strongly inhibited by Cu2+ and Fe2+ (Shen et al. 1998); and the α-amylases from B. subtilis, B. amyloliquefaciens I, and B. amyloliquefaciens II were strongly inhibited by Zn2+, Ag+, Cu2+, and Fe2+ (Elif and Velittin 2000). However, the activity of Nocardiopsis sp. 7326 amylase was not affected by Zn2+, Ni2+, and Fe2+, and was even activated by Cd2+ and Cu2+. The unusual property might be related to the special structure of the amylase, and the mechanism requires further research.
Conclusion
Many cold-active or cold-adapted amylases have been reported from microbial origins (Feller et al. 1999; Groudieva et al. 2004; D’Amico et al. 2006; Siddiqui et al. 2006). To our knowledge, this is the first study on purification and characterization of a cold-adapted α-amylase from the genus Nocardiopsis. On the other hand, only one α-amylase from Nocardiopsis sp. had been studied (Stamford et al. 2001) up to now, which showed the highest activity at 70°C and pH 5.0. It exhibited thermostable properties as indicated by retention of 100% of residual activity at 70°C and 50% of residual activity at 90°C for 10 min. These features have potential for industrial applications. In this study, the most desirable properties of the Nocardiopsis sp. 7326 amylase were its high activity at low temperature and stability at alkaline pH, which also permitted its biotechnological application in various industries. For example, it could be applied as a detergent additive, as a desizing agent in textile processing, and in the food industry.
The Nocardiopsis sp. 7326 was a psychrotroph exhibiting sigmoidal growth even at 0°C. Based on the 16S rRNA sequence analysis, it was closely related to Nocardiopsis antarctica, which was not studied for enzymatic activity (Rainey et al. 1996). Further, the different characteristics toward metal ions, such as the sensitivity to Zn2+, Ni2+, and Fe2+ and the activation by Cd2+ and Cu2+, indicated a special structure of Nocardiopsis sp. 7326 amylase. These results would make intriguing further research on gene expression and regulation and the protein structure in psychrophilic Nocardiopsis.
References
Al-Zarban SS, Abbas I, Al-Musallam AA, Steiner U, Stackebrandt E, Kroppenstedt RM (2002) Nocardiopsis halotolerans sp. nov., isolated from salt marsh soil in Kuwait. Int J Syst Evol Microbiol 52, 525–529
Ballschmiter M, Futterer O, Liebl W (2006) Identification and characterization of a novel intracellular alkaline alpha-amylase from the hyperthermophilic bacterium Thermotoga maritima MSB8. Appl Environ Microbiol 72, 2206–2211
Cavicchioli R, Siddiqui KS, Andrews D, Sowers KR (2002) Low-temperature extremophiles and their applications. Curr Opin Biotechnol 13, 253–261
Chessa JP, Feller G, Gerday C (1999) Purification and characterization of the heat-labile alpha-amylase secreted by the psychrophilic bacterium TAC 240B. Can J Microbiol 45, 452–457
Chun J, Bae KS, Moon EY, Jung SO, Lee HK, Kim SJ (2000) Nocardiopsis kunsanensis sp. nov., a moderately halophilic actinomycete isolated from a saltern. Int J Syst Evol Microbiol 50(Pt 5), 1909–1913
Cordeiro CAM, Martins MLL, Luciano AB (2002) Production and properties of α-amylase from thermophilic Bacillus sp. Braz J Microbiol 33, 57–61
D’Amico S, Gerday C, Feller G (2003) Temperature adaptation of proteins, engineering mesophilic-like activity and stability in a cold-adapted alpha-amylase. J Mol Biol 332, 981–988
D’Amico S, Sohier JS, Feller G (2006) Kinetics and energetics of ligand binding determined by microcalorimetry: insights into active site mobility in a psychrophilic alpha-amylase. J Mol Biol 358, 1296–1304
Declerck N, Machius M, Joyet P, Wiegand G, Huber R, Gaillardin C (2003) Hyperthermostabilization of Bacillus licheniformis alpha-amylase and modulation of its stability over a 50 degrees C temperature range. Protein Eng 16, 287–293
DeLong EF (1992) Archaea in coastal marine environments. Proc Natl Acad Sci USA 89, 5685–5689
Deming JW (1998) Deep ocean environmental biotechnology. Curr Opin Biotechnol 9, 283–287
Elif S, Velittin G (2000) Increase of the α-amylase yield by some Bacillus strains. Turk J Biol 24, 299–308
Evtushenko LI, Taran VV, Akimov VN, Kroppenstedt RM, Tiedje JM, Stackebrandt E (2000) Nocardiopsis tropica sp. nov., Nocardiopsis trehalosi sp. nov., nom. rev. and Nocardiopsis dassonvillei subsp. albirubida subsp. nov., comb. Nov. Int J Syst Evol Microbiol 50(Pt 1), 73–81
Feller G (2003) Molecular adaptations to cold in psychrophilic enzymes. Cell Mol Life Sci 60, 648–662
Feller G, Gerday C (2003) Psychrophilic enzymes, hot topics in cold adaptation. Nat Rev Microbiol 1, 200–208
Feller G, D’Amico D, Gerday C (1999) Thermodynamic stability of a cold-active alpha-amylase from the Antarctic bacterium Alteromonas haloplanctis. Biochemistry 38, 4613–4619
Gerday C, Aittaleb M, Bentahir M, Chessa JP, Claverie P, Collins T, D’Amico S, Dumont J, Garsoux G, Georlette D, Hoyoux A, Lonhienne T, Meuwis MA, Feller G (2000) Cold-adapted enzymes, from fundamentals to biotechnology. Trends Biotechnol 18, 103–107
Groudieva T, Kambourova M, Yusef H, Royter M, Grote R, Trinks H, Antranikian G (2004) Diversity and cold-active hydrolytic enzymes of culturable bacteria associated with Arctic sea ice, Spitzbergen. Extremophiles 8, 475–488
Hagihara H, Igarashi K, Hayashi Y, Endo K, Ikawa-Kitayama K, Ozaki K, Kawai S, Ito S (2001) Novel alpha-amylase that is highly resistant to chelating reagents and chemical oxidants from the alkaliphilic Bacillus isolate KSM-K38. Appl Environ Microbiol 67, 1744–1750
Igarashi K, Hatada Y, Hagihara H, Saeki K, Takaiwa M, Uemura T, Ara K, Ozaki K, Kawai S, Kobayashi T, Ito S (1998) Enzymatic properties of a novel liquefying alpha-amylase from an alkaliphilic Bacillus isolate and entire nucleotide and amino acid sequences. Appl Environ Microbiol 64, 3282–3289
Kroppenstedt RM, Evtushenko LI (2002) The Family Nocardiopsaceae. In Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E (eds), The Prokaryotes. An Evolving Electronic Resource for the Microbiological Community. http://141.150.157.117,8080/prokPUB/index.htm
Lealem F, Gashe BA (1994) Amylase production by a Gram-positive bacterium isolated from fermenting tef (Eraglostis tef). J Appl Bacteriol 77, 348–352
Lee SP, Morikawa M, Takagi M, Imanaka T (1994) Cloning of the aapT gene and characterization of its product, alpha-amylase-pullulanase (AapT), from thermophilic and alkaliphilic Bacillus sp. strain XAL601. Appl Environ Microbiol 60, 3764–3773
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193, 265–275
Pridham TG, Anderson P, Foley C, Lindenfelser LA, Hesseltine CW, Benedict RG (1956) A selection of media for maintenance and taxonomic study of Streptomyces. Antibiot Annu 1, 947–953
Rainey FA, Ward-Rainey N, Kroppenstedt RM, Stackebrandt E (1996) The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage, proposal of Nocardiopsaceae fam. Nov. Int J Syst Bacteriol 46, 1088–1092
Russell NJ (1998) Molecular adaptations in psychrophilic bacteria, potential for biotechnological applications. Adv Biochem Eng Biotechnol 61, 1–21
Schipper A, Bosecker K, Willscher S, Sproer C, Schumann P, Kroppenstedt RM (2002) Nocardiopsis metallicus sp. nov., a metal-leaching actinomycete isolated from an alkaline slag dump. Int J Syst Evol Microbiol 52, 2291–2295
Sengupta S, Jana ML, Sengupta D, Naskar AK (2000) A note on the estimation of microbial glycosidase activities by dinitrosalicylic acid reagent. Appl Microbiol Biotechnol 53, 732–735
Shen G, Saha B, Lee Y, Bhatnagar L, Zeikus J (1998) Purification and properties of an extracellular α-amylase from Thermus sp. Bot Bull Acad Sci 36, 195–200
Siddiqui KS, Poljak A, Guilhaus M, De Francisci D, Curmi PM, Feller G, D’Amico S, Gerday C, Uversky VN, Cavicchioli R (2006) Role of lysine versus arginine in enzyme cold-adaptation: modifying lysine to homo-arginine stabilizes the cold-adapted alpha-amylase from Pseudoalteramonas haloplanktis. Proteins 64, 486–501
Stamford TL, Stamford NP, Coelho LC, Araujo JM (2001) Production and characterization of a thermostable alpha-amylase from Nocardiopsis sp. endophyte of yam bean. Bioresour Technol 76, 137–141
Takami H, Inoue A, Fuji F, Horikoshi K (1997) Microbial flora in the deepest sea mud of the Mariana Trench. FEMS Microbiol Lett 152, 279–285
Yang SJ, Lee HS, Park CS, Kim YR, Moon TW, Park KH (2004) Enzymatic analysis of an amylolytic enzyme from the hyperthermophilic archaeon Pyrococcus furiosus reveals its novel catalytic properties as both an alpha-amylase and a cyclodextrin-hydrolyzing enzyme. Appl Environ Microbiol 70, 5988–5995
Zeng R, Zhang R, Zhao J, Lin N (2003) Cold-active serine alkaline protease from the psychrophilic bacterium Pseudomonas strain DY-A: enzyme purification and characterization. Extremophiles, 7, 335–337
Zeng R, Xiong P, Wen J (2006) Characterization and gene cloning of a cold-active cellulase from a deep sea psychrotrophic bacterium Pseudoalteromonas sp. DY3. Extremophiles 10, 79–82
Acknowledgments
This work was supported by National Natural Science Funds of China (No. 40406029).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, JW., Zeng, RY. Purification and Characterization of a Cold-Adapted α-Amylase Produced by Nocardiopsis sp. 7326 Isolated from Prydz Bay, Antarctic. Mar Biotechnol 10, 75–82 (2008). https://doi.org/10.1007/s10126-007-9035-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-007-9035-z