Abstract
Background
A significant body weight loss (BWL) is observed during 1 month after gastrectomy for gastric cancer. However, it remains unclear which body component mainly accounts for the weight loss.
Methods
Two-hundred forty-four patients who underwent gastrectomy for gastric cancer between May 2010 and October 2013 were examined. Body weight and composition were evaluated by a bioelectrical impedance analyzer within 1 week before surgery (first measurement), at 1 week after surgery (second measurement), and at 1 month after surgery (third measurement). The changes in the early period were defined as the differences until the second measurement, and those in the late period were defined as the differences from the second to the third measurement.
Results
Total BWL within 1 month was −3.4 kg, and the rate of body weight at 1 month to the preoperative body weight was 94.1 %. BWL was significantly greater in the early period than in the late period (−2.1 kg vs −1.2 kg, p < 0.001). In the early period, loss of lean body mass was significantly greater than loss of fat mass (−1.5 kg vs −0.6 kg, p < 0.001). The same trend was observed when the subset of patients who had surgical morbidities was excluded.
Conclusion
BWL during the first week after surgery was significantly greater than that during the subsequent 3 weeks. Furthermore, loss of lean body mass accounted for a significant part of the BWL during the first week.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the fourth commonest human malignant disease and the second commonest cause of cancer-related death worldwide [1]. Complete resection is essential for the cure of gastric cancer. Body weight loss (BWL) is a common problem after gastrectomy for gastric cancer, and occurs during the initial 1–3 months after surgery [2]. Previous studies demonstrated that BWL after gastrectomy was approximately 10–20 % of the preoperative body weight [3].
Recently, we found that more than 5 % loss of lean body mass at 1 month after surgery, observed in about 30 % of the patients, was a significant risk factor for the continuation of S-1 adjuvant chemotherapy [4]. This prompted us to analyze in greater detail how the lean body mass is lost during the early postoperative period and to explain, if possible, why such a loss could ultimately influence compliance with chemotherapy. Generally, the body can be divided into two or more compartments on the basis of its anatomic, fluid, or chemical components [5]. The most commonly used model of body composition, evaluable by a body composition analyzer, is a two-component model, in which the body is divided into fat mass and lean body mass representing muscle mass. Once the surgical stress occurs, immune cells produce cytokines, which, as well as acting as mediators of both immune and systemic responses to injury [6], cause muscle catabolism characterized by a rapid decrease in the protein content and accelerated amino acid release [7, 8]. The levels of these cytokines reportedly peak and decrease to normal levels by day 7 [9]. We therefore hypothesized that a loss of lean body mass is the predominant cause of BWL during the early postoperative phase, and that this is due mainly to the surgical stress rather than or in addition to the generally conceived causes such as lack of exercise and decrease in food intake.
To confirm our hypothesis, we performed serial evaluations of body composition for each patient using a bioelectrical impedance analyzer, and compared the degree of loss in the two body components between the early postoperative period (until 1 week after surgery) and the late postoperative period (from 1 week to 1 month after surgery).
Patients and methods
Patients
This is a retrospective cohort study. Patients’ records were retrieved from a prospectively collected database of Kanagawa Cancer Center from May 2010 to October 2013. The inclusion criteria were as follows: (1) received distal or total gastrectomy with lymph node dissection for gastric cancer as primary treatment, (2) R0 resection was achieved, (3) did not experience weight loss of over 15 % before surgery, and (4) body composition analysis was performed within 1 week before surgery, at 1 week after surgery, and at 1 month after surgery. Of the 295 patients who were treated by either an open or a laparoscopic approach during the study period, 16 patients were excluded because of noncurative resection and 35 patients were excluded because of missing data in the body composition analysis. No patients were excluded because of overt weight loss before surgery. Consequently, the remaining 244 patients were included in this study. Among them, 55 patients received laparoscopy-assisted distal gastrectomy and 28 patients received laparoscopy-assisted total gastrectomy.
Surgical procedure
All patients received distal or total gastrectomy with nodal dissection for gastric cancer. In principle, a D1 or a D1+ lymphadenectomy is indicated for cT1N0 tumors, and D2 lymphadenectomy is applied for cN+ or cT2–cT4 tumors regardless of the approach [10]. Spleen-preserving D2 total gastrectomy was permitted in this study.
Perioperative care
The patients received an enhanced recovery after surgery (ERAS) protocol after gastrectomy. The details of this protocol have been previously reported [11]. In brief, the patients were allowed to eat until midnight on the day before the surgery and were required to drink 1000 ml of rehydration solution until 3 h before surgery. The nasogastric tube was removed immediately after surgery. Oral intake was initiated on postoperative day 2, beginning with water and an oral nutritional supplement. The patients began to eat solid food on postoperative day 3, starting with rice gruel and soft food on postoperative day 3 and advancing in three steps to regular food intake on postoperative day 7. The patients were discharged when they had achieved adequate pain relief and soft food intake, had recovered sufficiently so as to resume rehabilitation at home, and exhibited normal laboratory data on postoperative day 7.
Evaluation of operative morbidity and mortality
The surgical and nonsurgical complications were assessed prospectively and classified according to the Clavien–Dindo classification [12]. Operative mortality was defined as postoperative death from any cause within 30 days after surgery or during the same hospital stay.
Body composition analysis
The segmental body composition was analyzed using an MC-190EM bioelectrical impedance analyzer (Tanita, Tokyo, Japan), which provides relative information regarding the amount of lean and fat tissue in the trunk area and each limb, as well as the overall body composition and hydration status. Body weight and composition were evaluated by the bioelectrical impedance analyzer within 1 week before surgery (first measurement), at 1 week after surgery (second measurement), and at 1 month after surgery (third measurement). The changes in the early postoperative period were defined as the differences until the second measurement, and those in the late postoperative period were defined as the differences from the second to the third measurement.
Evaluation, statistical analyses, and ethics
The values are expressed as the median and the range. The statistical analyses were performed using the chi-square test or the Wilcoxon signed-rank test. A P value of less than 0.05 was considered to indicate statistical significance. The SPSS software package (version 12.0 J Win; SPSS, Chicago, IL, USA) was used for all statistical analyses. R category and extent of dissection were determined by the Japanese Classification of Gastric Carcinoma, third English edition and the Japanese Gastric Cancer Association guidelines [13]. The study was approved by the Institutional Review Board of Kanagawa Cancer Center.
Results
Background
Baseline demographics and disease characteristics are shown in Table 1. Laparoscopic surgery was selected in 34.0 % of patients. Thirty-one patients had surgical morbidities. The details of the surgical morbidities are shown in Table 2. Among the 31 patients who developed morbidity, 21 patients dropped out from the ERAS protocol because of surgical morbidity. No patients died.
Body composition
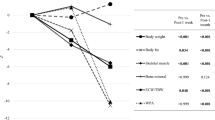
Total BWL at 1 month was −3.4 kg (range −13.0 to +3.1 kg) in the whole cohort. For the laparoscopic approach it was −3.2 kg (range −8.9 to 1.6 kg), for the open approach it was −3.5 kg (range −13.0 to 3.1 kg), for distal gastrectomy it was −2.9 kg (range −13.0 to 1.6 kg), and for total gastrectomy it was −4.2 kg (range −9.9 to 3.1 kg). The rate of body weight at 1 month to the preoperative body weight was 94.1 % (range 84.5–105.3 %) in the whole cohort. Figure 1 shows the changes of body compositions between the early and the late postoperative period in all cases. BWL was significantly greater in the early period than in the late period (−2.1 kg, with a range from −8.5 to 1.0 kg, vs −1.2 kg, with a range from −9.4 to 3.8 kg, p < 0.001). In the early period, the loss of lean body mass was significantly greater than the loss of fat mass (−1.5 kg, with a range from −8.3 to 7.7 kg, vs −0.7 kg, with a range from −7.1 to 6.1 kg, p < 0.001).
The same trend was observed in the subset which excluded 31 patients who developed surgical morbidity (Fig. 2). BWL was significantly greater in the early period than in the late period (−2.1 kg, with a range from −6.7 to 1.0 kg, vs −1.2 kg, with a range from −6.1 to 3.8 kg, p < 0.001). Moreover, the loss of lean body mass was significantly greater in the early period than in the late period (−1.5 kg, with a range from −8.3 to +7.7 kg, vs −0.3 kg, with a range from −12.8 to 4.9 kg, p < 0.001).
Discussion
This study indicated that the loss of lean body mass within 1 week was greater than that during the subsequent 3 weeks. Furthermore, loss of lean body mass rather than fat mass accounted for a significant part of the BWL during the early period. These findings can be explained in terms of surgical stress and cytokine production, which usually continues for about 1 week. Once the surgical stress occurs, immune cells produce cytokines, which act as mediators of both immune and systemic responses to surgical stress [6]. These cytokines also cause muscle catabolism. Iida et al. [14] reported that postoperative muscle weakness was associated with IL-6 production immediately after cardiac surgery in 154 consecutive patients who had undergone coronary artery bypass grafting. In addition, Bautmans et al. [15] demonstrated that surgery-induced changes in IL-6 levels were significantly related to changes in grip work in 66 elective abdominal surgery patients. Furthermore, in animal experiments, it was reported that a significant increase in muscle proteolysis was induced at 2 h after injection of endotoxin [16]. Considering these reports, the postoperative lean body mass loss may be due to enhanced catabolism induced by cytokine production after surgery.
In the present study, the median total amount of BWL at 1 month was less than 4 kg, and the rate of BWL at 1 month to the preoperative body weight was around 6 % in the whole cohort. Previous studies reported that BWL after gastrectomy was approximately 10–20 % of the preoperative weight [3]. For example, Noguchi et al. [17] evaluated 97 patients who had undergone gastrectomy, and found that the mean body weight at discharge was 79 ± 7 % in the total gastrectomy group and 84 ± 7 % in the distal gastrectomy group This discrepancy could be explained by the following factors. First, the starting date for the oral intake was the second postoperative day in the present study, whereas the patients started oral intake on seventh or eighth postoperative day in the previous study. The longer starvation could cause the severer weight loss. Second, the postoperative course was different. The patients received ERAS programs in the present study, whereas the old conventional care was selected in the previous studies. Yamada et al. [11] found that the ratio of the postoperative (1 week) body weight to the preoperative body weight was significantly greater for ERAS care than for conventional care in gastric cancer surgery.
The present study demonstrated that BWL within 1 week was significantly greater than that observed during the subsequent 3 weeks. Abdiev et al. [18] evaluated the change in body composition during 1 and 6 months after gastrectomy using a body composition analyzer, and found that both fat and muscle mass reductions were responsible for the BWL during the first 1 month after surgery, and that the fat mass and body weight continue to decrease until 6 months after surgery. The present study confirmed that the major component of BWL during the first month is the lean mass and that this tendency is even more striking during the first week. These two studies together suggest that the patient first loses muscle mass because of the characteristic pattern of metabolism under surgical stress in a very early phase after surgery. Loss of muscle mass due to the lack of physical activity may also be a relatively early event, whereas the effect of decrease in food intake would be a late event and is more likely reflected in the loss of fat mass.
The present study has some limitations. First, we did not measure surgical stress and oral intake calories after surgery. The exact mechanisms of BWL thus remain speculative. Second, this study was a retrospective study from a single institution. Third, there are concerns regarding the accuracy of body composition at 1 week after surgery. Measurements by a body analyzer at this time point could have suffered from several confounding factors, such as postoperative fever and fluid therapy. However, frequent measurement of alternative parameters such as volumetry by computerized tomography images was considered unethical and was not used at this time. Therefore, the results of this study need to be validated in another cohort.
In conclusion, BWL during the first week after surgery was significantly greater than that during the subsequent 3 weeks. In addition, the loss of lean body mass accounted for a significant part of the BWL during this period. To gain further insight into body composition in early postoperative periods, further studies focusing on surgical stress and oral intake calories are warranted.
References
Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
Adams JF. The clinical and metabolic consequences of total gastrectomy. I. Morbidity, weight, and nutrition. Scand J Gastroenterol. 1967;2:137–49.
Fein M, Fuchs KH, Thalheimer A, et al. Long-term benefits of Roux-en-Y pouch reconstruction after total gastrectomy: a randomized trial. Ann Surg. 2008;247:759–65.
Aoyama T, Kawabe T, Fujikawa H, et al. Loss of lean body mass as an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol. 2014. doi:10.1245/s10434-014-4296-z.
Kiyama T, Mizutani T, Okuda T, et al. Postoperative changes in body composition after gastrectomy. J Gastrointest Surg. 2005;9:313–9.
Cruickshank AM, Fraser WD, Burns HJ, et al. Response of serum interleukin-6 in patients undergoing elective surgery of varying severity. Clin Sci. 1990;79:161–5.
Mansoor O, Beaufrere B, Boirie Y, et al. Increased mRNA levels for components of the lysosomal, Ca2+-activated, and ATP-ubiquitin-dependent proteolytic pathways in skeletal muscle from head trauma patients. Proc Natl Acad Sci U S A. 1996;93:2714–8.
Tiao G, Hobler S, Wang JJ, et al. Sepsis is associated with increased mRNAs of the ubiquitin-proteasome proteolytic pathway in human skeletal muscle. J Clin Invest. 1997;99:163–8.
Kragsbjerg P, Holmberg H, Vikerfors T. Serum concentrations of interleukin-6, tumour necrosis factor-alpha, and C-reactive protein in patients undergoing major operations. Eur J Surg. 1995;161:17–22.
Association Japanese Gastric Cancer. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113–23.
Yamada T, Hayashi T, Cho H, et al. Usefulness of enhanced recovery after surgery protocol as compared with conventional perioperative care in gastric surgery. Gastric Cancer. 2012;15:34–41.
Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96.
Association Japanese Gastric Cancer. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12.
Iida Y, Yamada S, Nishida O, Nakamura T. Body mass index is negatively correlated with respiratory muscle weakness and interleukin-6 production after coronary artery bypass grafting. J Crit Care. 2010;25(172):e1–8.
Bautmans I, Njemini R, De Backer J, De Waele E, Mets T. Surgery-induced inflammation in relation to age, muscle endurance, and self-perceived fatigue. J Gerontol A Biol Sci Med Sci. 2010;65:266–73.
Chai J, Wu Y, Sheng ZZ. Role of ubiquitin-proteasome pathway in skeletal muscle wasting in rats with endotoxemia. Crit Care Med. 2003;31:1802–7.
Noguchi Y, Tsuburaya A, Makino T, et al. Metabolic alteration in totally gastrectomised patients—caloric intake and energy consumption. Asian J Surg. 1992;15:97–102.
Abdiev S, Kodera Y, Fujiwara M, et al. Nutritional recovery after open and laparoscopic gastrectomies. Gastric Cancer. 2011;14:144–9.
Acknowledgments
This work was supported, in part, by the nongovernmental organization Kanagawa Standard Anti-cancer Therapy Support System. The authors express their sincere gratitude to Rika Takahashi for her excellent data management in this study.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
T. Aoyama and T. Yoshikawa contributed to this article equally.
Rights and permissions
About this article
Cite this article
Aoyama, T., Kawabe, T., Hirohito, F. et al. Body composition analysis within 1 month after gastrectomy for gastric cancer. Gastric Cancer 19, 645–650 (2016). https://doi.org/10.1007/s10120-015-0496-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-015-0496-x