Abstract
Purpose
The present study examined the changes in and risk factors for body composition (BC) during the first postoperative month when dynamic biological reactions occur.
Methods
We retrospectively assessed 202 patients who underwent gastrectomy. The BC was assessed using a bioelectrical impedance analysis and evaluated within 1 month preoperatively, 1 week postoperatively, and 1 month postoperatively. Multiple regression analyses were performed to identify predictive factors for BC change.
Results
The mean reduction rate in BC at 1 month postoperatively was − 6.0, − 10.5, − 5.6, − 1.1, − 10.1, and + 1.2% for body weight, body fat, skeletal muscle, bone mineral, extracellular water/total body water, and the whole-body phase angle, respectively. A multiple regression analysis revealed that independent risk factors for weight loss were complications, operative time, and type of gastrectomy (P = 0.004, 0.011, 0.015, respectively), and those for skeletal muscle loss were complications and gastrectomy type (P = 0.002, 0.010, respectively). A segmental lean mass analysis revealed that the lower limbs were markedly reduced at 1 week postoperatively (− 8.0%), and these independent risk factors were the female sex and Stage II/III disease (P = 0.008, 0.036, respectively).
Conclusion
Detailed analyses of BC might help elucidate the mechanisms underlying postoperative physical changes, which might be useful for perioperative management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is a major cancer and the third leading cause of cancer-related death worldwide [1], and gastrectomy is the most effective treatment for its radical cure [2]. However, gastrectomy causes post-gastrectomy symptoms [3], including changes in digestive juice, gastric acid, and ghrelin [4, 5], leading to various disabilities, such as weight loss and a decreased quality of life.
Body composition changes in patients with gastric cancer frequently occur after gastrectomy and can be caused by various mechanisms, including poor food intake, immobility due to decreased activity, adjuvant chemotherapy, and hypermetabolism associated with the inflammatory response to surgery and complications [6,7,8]. Especially in the first month after gastrectomy, the body composition is dramatically altered by the rapid postoperative environmental variation. Surgical stress causes immune cells to produce cytokines, which act as mediators of both immune and systemic responses [9], and cause muscle catabolism characterized by a rapid decrease in protein content and accelerated release of amino acids [10]. Muscle tissue degradation is a key systemic response to invasion, generating amino acids related to glycogenesis, immune response, and tissue repair [10, 11]. Adequate nutrition and exercise during the recovery and anabolic phases are necessary to increase the total muscle protein lost due to surgical invasion. Therefore, body composition deterioration is closely related to deterioration in the nutritional status and postoperative quality of life [12, 13]. Furthermore, a previous study demonstrated that a loss of lean body mass over 5% at 1 month after gastrectomy, which is observed in approximately 30% of patients, was a significant risk factor for compliance with S-1 adjuvant chemotherapy, leading to a decreased survival [14, 15].
A bioelectrical impedance analysis (BIA) quantitatively measures the components of the human body based on the impedance generated when an electric current is applied to the human body. Body components are roughly divided into "fat," "muscle," "bone," and "water." However, past reports on body composition have focused only on body weight and lean body mass, with few reports examining other body composition details. A detailed analysis of body composition might help elucidate the mechanisms underlying postoperative physical changes, which might be useful for perioperative management, such as nutrition and rehabilitation.
The present study, therefore, examined the changes and risk factors in each body composition factor during the first postoperative month, when dynamic biological reactions occur.
Methods
Study population
This retrospective observational cohort study was conducted at Tottori Prefectural Central Hospital and Tottori University Hospital.
The inclusion criteria were as follows: (1) histologically proven gastric cancer; (2) pathological Stage I/II/III with R0 resection; (3) having undergone distal gastrectomy (DG), proximal gastrectomy (PG), or total gastrectomy (TG) with lymph node dissection as the primary treatment between January 2019 and April 2022; (4) no body weight loss of > 15% before surgery; and (5) a body composition analysis performed within 1 month preoperatively, 1 week postoperatively, and 1 month postoperatively.
The exclusion criteria included an omitted BIA examination, incomplete data, implanted cardiac pacemaker, ascites effusion, conspicuous edema, and other active primary malignancies. Patients with pathological tage IV disease, palliative gastrectomy, neoadjuvant chemotherapy, and an inability to receive oral nutrition at 1 month postoperatively were also excluded. Of the 280 patients who underwent gastrectomy during the study period, we included 202, while 78 patients were excluded. Cancer staging was based on the Japanese Classification of Gastric Carcinoma, 15th edition [16].
This study was approved by the ethics board of the Tottori Prefectural Central Hospital (approval number: 2020–23) and Tottori University Hospital (approval number: 22A036). The requirement for informed consent was waived due to the retrospective nature of this study.
Perioperative management
Perioperative management is generally performed according to the clinical pathway of our institutions based on enhanced recovery after surgery (ERAS). Postoperative patients were rehabilitated from the day after surgery, and nutritional guidance was provided twice until discharge. In 202 curative patients, standard radical gastrectomy and D1+ or D2 lymph node dissection were performed according to the Japanese Gastric Cancer Treatment Guidelines [2].
Body composition analyses
Body composition was assessed using a direct segmental multifrequency BIA with InBody S10® (InBody Co., Ltd., Seoul, Korea). The InBody S10® uses multifrequency body segmental measurements and an 8-point tactile electrode. The multifrequency measurement uses 1-, 5-, 50-, 250-, 500-, and 1,000-kHz frequencies for each body segment (trunk, upper, and lower limbs). InBody® automatically provides relative information regarding the amount of lean body mass in the trunk area and each limb and the overall body composition, such as the body weight, skeletal muscle mass, lean body mass, body fat mass, bone mineral content, extracellular water/total body water (ECW/TBW), and whole-body phase angle (WPA). Body composition was evaluated within 1 month preoperatively, 1 week postoperatively, and 1 month postoperatively (first, second, and third measurements, respectively). The rate of change in body composition was defined as each postoperative weight minus preoperative weight divided by preoperative weight and multiplied by 100. This analyzer is not based on statistical data from a specific population and can accurately assess people with varying physiques, such as the obese, elderly, and athletic [17, 18].
Modified frailty index
The modified frailty index (mFI) is based on 11 physiological deficits derived from the original 70-item Canadian Study of the Health and Aging Frailty Index [19]. Patients were assigned 1 point for each of the 11 physiological deficits, and the total points assigned to each patient were divided by 11. A higher score indicated increased frailty [20]. Patients were subsequently divided into the mFIHigh (≥ 0.272) and mFILow (< 0.272) groups.
Statistical analyses
Continuous and categorical variables are expressed as the mean and standard deviation (SD) and numbers (percentages), respectively. The Dunn–Bonferroni multiple comparisons, Mann–Whitney U tests, Kruskal–Wallis test, and Dunn's multiple were performed for statistical analyses. Multiple regression analyses were performed to identify predictive factors for significant variations in the change in body composition. The significance threshold was set at P < 0.05. The GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA) and SPSS Statistics, version 24 (IBM Corp., Armonk, NY, USA) software programs were used for the statistical analyses.
Results
Study population
Table 1 presents the clinicopathologic characteristics of the participants in this study. The number of elderly patients ≥ 75 years old was 75 (37.1%). DG, PG, and TG were performed in 137 (67.9%), 23 (11.4%), and 42 patients (20.8%), respectively. Laparoscopic surgery was performed in 187 patients (92.6%), of whom 116 underwent conventional laparoscopic surgery and 71 underwent robotic surgery. The pathological stages were Stage I in 116 (57.4%), Stage II in 40 (19.8%), and Stage III in 46 (22.8%) patients. Using the first quartile of the operative time and blood loss distribution as the cutoff points, the operative time and blood loss were 371 min and 70 mL, respectively, and the patients were classified into two groups.
Table 2 presents the details of complications. Grade ≥ II complications, according to the Clavien–Dindo classification, occurred in 37 patients (18.3%), including Grade II in 27, Grade IIIa in 8, Grade IVa in 1, and Grade IVb in 1.
Table 3 shows the comorbidities that might affect the results of postoperative body composition evaluations.
Body composition
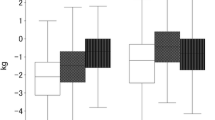
The change in the body composition at 1 week and 1 month postoperatively were − 2.93% ± 3.0% and − 6.00% ± 3.5% for body weight, − 1.78% ± 13.8% and − 10.53% ± 14.7% for body fat, − 3.49% ± 4.8% and − 5.57% ± 5.2% for skeletal muscle, 1.03% ± 7.4% and − 1.07% ± 7.2% for bone mineral, − 0.25% ± 1.7% and 1.27% ± 1.6% for ECW/TBW, and 0.88% ± 11.6% and − 10.09% ± 11.9% for WPA, respectively (Fig. 1). Figure 1 illustrates the changes in body composition according to the Dunn–Bonferroni multiple comparison test for the preoperative, 1-week postoperative, and 1-month postoperative time points. Regarding the proportions of changes, the body weight and skeletal muscle decreased immediately postoperatively and continued to decrease 1 month postoperatively, whereas body fat did not decrease markedly immediately postoperatively but then decreased rapidly 1 month postoperatively. Bone mineral remained almost unchanged from preoperative to 1 month postoperative, and ECW/TBW decreased immediately postoperatively but increased at 1 month postoperatively. WPA decreased sharply between 1 week and 1 month postoperatively.
Segmental lean mass
Segmental lean mass was also analyzed. The percentage change in lean body mass at 1 week and 1 month postoperatively was − 3.09% ± 4.8% and − 4.31% ± 4.9% for the whole body, − 1.52% ± 8.0% and − 7.22% ± 7.0% for upper limbs, − 0.99% ± 4.7% and − 5.18% ± 4.4% for the truncus, and − 7.98% ± 8.5% and − 5.72% ± 8.7% for the lower limbs, respectively (Fig. 2). All segmental lean mass components and all time periods revealed significant differences concerning changes. The most specific changes were in the lower limbs, which were markedly reduced at 1 week postoperatively and improved moderately at 1 month postoperatively.
Individual body composition factors
The results of the first postoperative week are described first. Body weight loss was more significant in Stage II/III than in Stage I (− 3.4 and − 2.6%, respectively, P = 0.038) and in D2 lymphadenectomy than in D1/D1 + lymphadenectomy and (− 3.6 and − 2.5%, respectively, P = 0.005). The WPA was significantly lower in the group that underwent D1/D1 + lymphadenectomy than in the group that underwent D2 lymphadenectomy (− 0.8 and 3.7%, respectively, P = 0.006). ECW/TBW was significantly lower in the bleeding ≤ 70 mL group than in the bleeding > 70 mL (− 0.4 and 0.3%, respectively, P = 0.009). The other factors were not significantly different and changed uniformly, indicating that each factor had a minimal effect on body composition 1 week after surgery (data not shown).
Table 4 shows the results of simple and multiple regression analyses for each body composition factor at 1 month postoperatively. Multiple regression analyses revealed that independent risk factors for weight loss were Grade ≥ II complications, operative time, and type of gastrectomy (P = 0.004, 0.011, 0.015, respectively), and for skeletal muscle loss, these factors were Grade ≥ II complications and type of gastrectomy (P = 0.002, 0.010, respectively). Other risk factors were the type of gastrectomy for bone mineral, female sex for ECW/TBW, and operative time ≤ 371 min for WPA (P = 0.005, 0.013, 0.011, respectively). In the subgroup analysis of laparoscopic surgery, there was no significant difference between conventional laparoscopic surgery and robotic surgery for each body composition factor at 1 month postoperatively (data not shown). In contrast, in the subgroup analysis of complications, there was a significant difference between Grade ≥ III and Grade II complications in body weight at 1 month postoperatively (− 9.3 and − 6.8%, respectively, P = 0.044) (Table 5).
Lean body mass of the lower limbs at 1 week postoperatively
Table 6 presents the results of simple and multiple regression analyses for lean body mass of the lower limbs at 1 week postoperatively. A multiple regression analysis revealed that the female sex and Stage II/III disease were independent risk factors for lean body mass of the lower limbs at 1 week postoperatively (P = 0.009, 0.036, respectively).
Body composition according to each type of gastrectomy
Figure 3 illustrates the change in body composition according to each type of gastrectomy according to the Kruskal–Wallis test and Dunn's multiple comparisons test for the preoperative, 1-week postoperative, and 1-month postoperative timepoints. One week postoperatively, there were no significant differences among body composition factors. However, 1 month postoperatively, there were significant differences in the body weight, skeletal muscle, and bone mineral between DG and TG (P = 0.006, 0.033, 0.027, respectively), and in WPA, there was a significant difference between DG and PG (P = 0.040).
Discussion
This study demonstrated the body composition details during the first postoperative month when dynamic biological reactions occur. First, body weight and skeletal muscle decreased immediately postoperatively and continued to decrease 1 month postoperatively. In contrast, body fat did not decrease significantly immediately after surgery and decreased rapidly 1 month postoperatively. Second, gastrectomy type and complications were significant risk factors for body weight and skeletal muscle loss at 1 month postoperatively. Third, lean body mass of the lower limbs was markedly reduced in the first postoperative week, and the risk factors for this reduction were female sex and Stage II/III disease. These findings allow us to explore different approaches to postoperative management, including nutrition and rehabilitation.
In the present study, the mean reduction amount and rate in body composition at 1 month postoperatively was − 3.6 kg (− 6.0%) for body weight, − 1.7 kg (− 10.5%) for body fat, − 1.3 kg (− 5.6%) for skeletal muscle, and − 0.03 kg (− 1.1%) for bone mineral. Body weight loss was largely attributable to skeletal muscle (or lean body mass) at 1 week postoperatively and body fat at 1 month postoperatively. This phenomenon has been shown in previous studies [21] and can be explained by biological responses in recovery from surgical invasion. When tissue damage occurs due to surgical invasion, various hormones and cytokines are mobilized, and systemic responses are triggered to maintain homeostasis. Muscle tissue degradation is a key systemic response to the invasion, which provides amino acids related to glycogenesis, immune response, and tissue repair. As energy deficiency persists, the energy source shifts from glucose to fat, and fatty acids and ketone bodies become the energy sources through lipolysis. The metabolic rate decreases as lipolysis proceeds, conserving energy sources, inhibiting muscle collapse, and preserving body proteins [22].
Recent reports have revealed that advances in ERAS have reduced weight loss in the early postoperative period [23], with an approximate − 6% body weight loss rate at 1 month after gastrectomy in patients with gastric cancer, which was very similar to the present study [21]. Regarding body composition at 1 month postoperatively, Segami et al. reported that the risk factors for weight loss of ≥ 10% were total gastrectomy and postoperative complications [24], while Aoyama et al. reported that the risk factors for lean body mass loss of ≥ 5% were total gastrectomy, postoperative complications, and male sex [3]. Similar results were observed in our study, which revealed that independent risk factors for weight loss and skeletal muscle were the type of gastrectomy and Grade ≥ II complications. In our study, changes in body composition according to each type of gastrectomy at 1 month postoperatively were as expected, with DG showing the least amount of change, TG the greatest, and PG in between. However, only WPA was significantly reduced in the PG group. WPA is widely used as an indicator of cellular nutritional status and aging, and is affected by muscle mass, cellular aging, and body water content. Therefore, female patients, the elderly, and those with edema show a reduced WPA. However, PG showed no sex differences; no significant decrease in body weight, muscle mass, or ECW/TBW at 1 month postoperatively; and no significant differences in complications when compared to DG or TG. Why only WPA was significantly reduced in the PG group is unclear, but it may be due to variance in the small number of PG cases. Further studies with a larger sample size are, therefore, needed.
Laparoscopic and robotic surgeries reduced surgical invasiveness [25,26,27]; however, there was no significant difference between laparoscopic and open surgery in body composition at 1 month postoperatively, nor was there a significant difference between conventional laparoscopic surgery and robotic surgery. Minimally invasive surgery minimizes abdominal wall destruction, reduces pain, and contributes to an early postoperative recovery; nonetheless, tissue damage from intraperitoneal manipulation, such as gastrectomy and lymph node dissection, remains largely unchanged, and the proportion of abdominal wall tissue damage regarding the overall tissue damage is low. Therefore, minimally invasive surgery might not have resulted in a biological response leading to a significant reduction in body weight or muscle mass. In contrast, Grade ≥ III complications significantly reduced body weight compared with Grade II complications. Furthermore, although not significantly different, Grade ≥ III complications tended to have higher reduction rates in skeletal muscle and bone mineral loss than Grade II complications, thus suggesting that the intensity of invasion and inflammation due to complications might be destructive to the body. Therefore, efforts to avoid complications, regardless of the approach, are essential.
Despite various surgical approaches, such as avoiding TG [28, 29] and performing minimally invasive surgery [30], body weight loss remains a major complication of gastrectomy and is yet unsolved. Several studies have reported that body weight loss is associated with a significant deterioration in the quality of life [12, 31] and a decreased survival of patients with gastric cancer from intolerance to adjuvant chemotherapy [14]. A large-scale randomized controlled trial demonstrated that BWL at 1, 3, and 6 months was significantly lower in the oral nutrition supplements group than in the control group. However, the difference in percentage weight loss between both groups at 1 month postoperatively was < 1% [32]. From this evidence, approaches to prevent further body weight loss have reached their limits. Therefore, postoperative management, including nutrition and rehabilitation, should be conducted with these limitations and be understood by the patients.
To our knowledge, this is the first report of prominent lean body mass wasting of lower limbs in patients with gastric cancer 1 week after gastrectomy. Zudin et al. demonstrated that the rectus femoris cross-sectional area using ultrasonography decreased significantly from days 1–7 (− 12.5%; P = 0.002) in intensive care unit patients [33]. It is essential to note that disuse atrophy and other factors associated with surgical invasion, like immobility, increased catabolism, nutritional and metabolic disorders, and inflammation, are involved. In our study, one risk factor for lean body mass wasting of lower limbs was female sex. One reason for this is that anabolic steroids, which enhance muscle anabolism, are secreted more frequently by males than by females. Therefore, it is vital to strengthen anabolism while maintaining adequate nutritional intake to prevent muscle wasting after gastrectomy. Furthermore, administering beta-hydroxy-beta-methylbutyrate, a leucine metabolite with strong anabolic and catabolic inhibitory effects, and rehabilitation may strengthen anabolism [34]. Further studies are needed to confirm methods for improving postoperative skeletal muscle.
Several limitations associated with the present study warrant mention. First, this was a retrospective study conducted at two hospitals. Different perioperative management approaches in ERAS may yield different results. However, our institutions have performed standard and aggressive ERAS, which is by no means markedly inferior to approaches in other institutions. Second, the sample size was small; accordingly, further large-scale trials are required to confirm the results. Third, body composition values measured via a BIA are affected by the measuring instrument type, edema, and dehydration. However, InBody® uses multifrequency, body segmental measurement method, and an 8-point tactile electrode. This analyzer is not based on statistical data from a specific population and can accurately assess people with different physiques.
In conclusion, body weight and skeletal muscle continued to decrease during the first postoperative month, and the risk factor was the type of gastrectomy and complication. Lean body mass of the lower limbs decreased markedly during the first postoperative week. Therefore, surgeons should apply these findings to postoperative management, including nutrition and rehabilitation.
Data availability
The data that support the findings of this study are available on request from the corresponding author, [T.O]. The data are not publicly available due to [restrictions e.g. their containing information that could compromise the privacy of research participants].
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Association JGC. Gastric cancer treatment guidelines 2021. LTD, Tokyo: KANEHARA & CO.; 2021.
Aoyama T, Sato T, Segami K, Maezawa Y, Kano K, Kawabe T, et al. Risk factors for the loss of lean body mass after gastrectomy for gastric cancer. Ann Surg Oncol. 2016;23:1963–70.
Catarci M, Berlanda M, Grassi GB, Masedu F, Guadagni S. Pancreatic enzyme supplementation after gastrectomy for gastric cancer: a randomized controlled trial. Gastric Cancer. 2018;21:542–51.
Doki Y, Takachi K, Ishikawa O, Miyashiro I, Sasaki Y, Ohigashi H, et al. Ghrelin reduction after esophageal substitution and its correlation to postoperative body weight loss in esophageal cancer patients. Surgery. 2006;139:797–805.
Davis JL, Selby LV, Chou JF, Schattner M, Ilson DH, Capanu M, et al. Patterns and predictors of weight loss after gastrectomy for cancer. Ann Surg Oncol. 2016;23:1639–45.
Yang W, Xia F, Wang J, Zhou M, Li G, Shen L, et al. Quantifying skeletal muscle wasting during chemoradiotherapy with Jacobian calculations for the prediction of survival and toxicity in patients with gastric cancer. Eur J Surg Oncol. 2020;46:1254–61.
Aoyama T, Yoshikawa T, Maezawa Y, Segami K, Kano K, Numata M, et al. Influence of postoperative surgical complications after gastrectomy on body weight and body composition changes in patients with gastric cancer. Anticancer Res. 2019;39:1073–8.
Cruickshank AM, Fraser WD, Burns HJ, Van Damme J, Shenkin A. Response of serum interleukin-6 in patients undergoing elective surgery of varying severity. Clin Sci (Lond). 1990;79:161–5.
Tiao G, Hobler S, Wang JJ, Meyer TA, Luchette FA, Fischer JE, et al. Sepsis is associated with increased mRNAs of the ubiquitin-proteasome proteolytic pathway in human skeletal muscle. J Clin Invest. 1997;99:163–8.
Mansoor O, Beaufrere B, Boirie Y, Ralliere C, Taillandier D, Aurousseau E, et al. Increased mRNA levels for components of the lysosomal, Ca2+-activated, and ATP-ubiquitin-dependent proteolytic pathways in skeletal muscle from head trauma patients. Proc Natl Acad Sci USA. 1996;93:2714–8.
Tanabe K, Takahashi M, Urushihara T, Nakamura Y, Yamada M, Lee SW, et al. Predictive factors for body weight loss and its impact on quality of life following gastrectomy. World J Gastroenterol. 2017;23:4823–30.
Aoyama T, Yoshikawa T, Shirai J, Hayashi T, Yamada T, Tsuchida K, et al. Body weight loss after surgery is an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol. 2013;20:2000–6.
Aoyama T, Sato T, Maezawa Y, Kano K, Hayashi T, Yamada T, et al. Postoperative weight loss leads to poor survival through poor S-1 efficacy in patients with stage II/III gastric cancer. Int J Clin Oncol. 2017;22:476–83.
Aoyama T, Kawabe T, Fujikawa H, Hayashi T, Yamada T, Tsuchida K, et al. Loss of lean body mass as an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol. 2015;22:2560–6.
Association JGC. Japanese classification of gastric carcinoma (the 15th edition). Tokyo: KANEHARA & CO. LTD; 2017.
Cha K, Chertow GM, Gonzalez J, Lazarus JM, Wilmore DW. Multifrequency bioelectrical impedance estimates the distribution of body water. J Appl Physiol. 1985;1995(79):1316–9.
Kim M, Shinkai S, Murayama H, Mori S. Comparison of segmental multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for the assessment of body composition in a community-dwelling older population. Geriatr Gerontol Int. 2015;15:1013–22.
Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J. 2001;1:323–36.
Velanovich V, Antoine H, Swartz A, Peters D, Rubinfeld I. Accumulating deficits model of frailty and postoperative mortality and morbidity: its application to a national database. J Surg Res. 2013;183:104–10.
Aoyama T, Kawabe T, Hirohito F, Hayashi T, Yamada T, Tsuchida K, et al. Body composition analysis within 1 month after gastrectomy for gastric cancer. Gastric Cancer. 2016;19:645–50.
Dulloo AG, Jacquet J. Adaptive reduction in basal metabolic rate in response to food deprivation in humans: a role for feedback signals from fat stores. Am J Clin Nutr. 1998;68:599–606.
Yamada T, Hayashi T, Cho H, Yoshikawa T, Taniguchi H, Fukushima R, et al. Usefulness of enhanced recovery after surgery protocol as compared with conventional perioperative care in gastric surgery. Gastric Cancer. 2012;15:34–41.
Segami K, Aoyama T, Kano K, Maezawa Y, Nakajima T, Ikeda K, et al. Risk factors for severe weight loss at 1 month after gastrectomy for gastric cancer. Asian J Surg. 2018;41:349–55.
Wang JF, Zhang SZ, Zhang NY, Wu ZY, Feng JY, Ying LP, et al. Laparoscopic gastrectomy versus open gastrectomy for elderly patients with gastric cancer: a systematic review and meta-analysis. World J Surg Oncol. 2016;14:90.
Shibasaki S, Suda K, Nakauchi M, Nakamura K, Kikuchi K, Inaba K, et al. Non-robotic minimally invasive gastrectomy as an independent risk factor for postoperative intra-abdominal infectious complications: a single-center, retrospective and propensity score-matched analysis. World J Gastroenterol. 2020;26:1172–84.
Kinoshita T, Sato R, Akimoto E, Tanaka Y, Okayama T, Habu T. Reduction in postoperative complications by robotic surgery: a case–control study of robotic versus conventional laparoscopic surgery for gastric cancer. Surg Endosc. 2022;36:1989–98.
Furukawa H, Kurokawa Y, Takiguchi S, Tanaka K, Miyazaki Y, Makino T, et al. Short-term outcomes and nutritional status after laparoscopic subtotal gastrectomy with a very small remnant stomach for cStage I proximal gastric carcinoma. Gastric Cancer. 2018;21:500–7.
Jiang X, Hiki N, Nunobe S, Nohara K, Kumagai K, Sano T, et al. Laparoscopy-assisted subtotal gastrectomy with very small remnant stomach: a novel surgical procedure for selected early gastric cancer in the upper stomach. Gastric Cancer. 2011;14:194–9.
Abdiev S, Kodera Y, Fujiwara M, Koike M, Nakayama G, Ohashi N, et al. Nutritional recovery after open and laparoscopic gastrectomies. Gastric Cancer. 2011;14:144–9.
Huang DD, Ji YB, Zhou DL, Li B, Wang SL, Chen XL, et al. Effect of surgery-induced acute muscle wasting on postoperative outcomes and quality of life. J Surg Res. 2017;218:58–66.
Miyazaki Y, Omori T, Fujitani K, Fujita J, Kawabata R, Imamura H, et al. Oral nutritional supplements versus a regular diet alone for body weight loss after gastrectomy: a phase 3, multicenter, open-label randomized controlled trial. Gastric Cancer. 2021;24:1150–9.
Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591–600.
Deutz NE, Matheson EM, Matarese LE, Luo M, Baggs GE, Nelson JL, et al. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: a randomized clinical trial. Clin Nutr. 2016;35:18–26.
Acknowledgements
We thank Editage Group (https://www.editage.jp/services/english-editing) for the English language editing of the manuscript.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Written informed consent was not obtained from the patients, but all patients were previously informed in the form of an opt-out option.
Ethics approval
This study was approved by the ethics boards of the Tottori Prefectural Central Hospital (approval number: 2020–23) and Tottori University Hospital (approval number: 22A036).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Osaki, T., Matsunaga, T., Makinoya, M. et al. Impact of gastrectomy on body composition within 1 month in patients with gastric cancer. Surg Today 53, 834–844 (2023). https://doi.org/10.1007/s00595-022-02633-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-022-02633-1