Abstract
Background
Gastric perforation is a rare presentation of gastric cancer and is thought to be a predictor of advanced disease and, thus, poor prognosis. Guidelines do not exist for the optimal management strategy. We aimed to identify, review, and summarize the literature pertaining to perforation in the setting of gastric cancer.
Methods
A qualitative, systematic review of the literature was performed from January 1, 1985, to January 1, 2010. Searches of MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials were performed using search terms related to gastric cancer surgery. Abstracts were examined by two independent reviewers and a standardized data collection tool was used to extract relevant data points. Summary tables were created.
Results
Nine articles were included. Perforation was reported to occur in fewer than 5% of gastric cancer patients. Preoperative diagnosis of a gastric cancer was rated and occurred in 14–57% of patients in the papers reviewed. Mortality rates for emergency gastrectomy ranged from 0 to 50% and for simple closure procedures the rates ranged from 8 to 100%. Patients able to receive an R0 gastrectomy demonstrated better long-term survival (median 75 months, 50% 5-year) compared with patients who had simple closure procedures.
Conclusions
Gastric cancer patients presenting with a gastric perforation demonstrate improved overall survival with an R0 resection; however, implementation of this management technique is complicated by infrequent preoperative gastric cancer diagnosis, and inability to perform an oncologic resection due to patient instability and intra-abdominal contamination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exceptional cases of gastric cancer manifest with gastric perforation and necessitate emergent management. The mechanism of spontaneous perforation is not as well described or as clearly understood as perforation secondary to diagnostic or interventional procedures; however, it may be similar to that of all hollow, viscous malignancies: transmural penetration of the cancer or ischemic changes. Patients may also have concomitant ulcer disease and experience perforation secondary to the gastric ulcer, rather than via transmural invasion by the cancer. Patients experiencing gastric perforation most frequently exhibit acute onset of abdominal pain with evidence of free air on abdominal X-ray [1, 2]. Patients with both a benign perforated gastric ulcer and malignancy will display similar symptoms, and as this is a surgical emergency, patients are often taken for laparotomy with little investigation. Even with a thorough examination, it is often difficult to distinguish between malignant and benign causes of perforation. The goals of conventional surgical intervention for a benign gastric perforation are management of sepsis, closure of the perforation, and definitive operation to decrease the possibility of recurrence and/or complications from the ulcer [1, 2]. In cases of perforation from a gastric cancer, diagnosis and management of the malignancy must also be achieved. Consequently, the management of a gastric perforation in the setting of a cancer poses several challenges to the surgeon. Given that patients with benign and malignant gastric perforations exhibit similar symptoms, the diagnosis of gastric cancer is often made post-operatively. Surgeons seldom have the opportunity for pathologic confirmation of malignancy until after the surgical intervention [1, 2]. Surgical management of a gastric perforation is also associated with high mortality (10–40%), regardless of the presence or absence of malignancy [1, 2]. Worse outcomes are reported for delayed time to operative intervention, extent of peritoneal contamination, and overall health of the patient [1, 2]. Finally, the optimal strategy for managing patients with gastric perforation in the presence of malignancy remains unclear. Options available to the surgeon include an oversew operation aimed at closing the perforation; resection of the ulcer; or if malignancy is suspected, gastrectomy with or without a formal lymphadenectomy [1, 2]. With a presentation of perforation, a definitive R0 resection and lymphadenectomy may not be possible due to the hemodynamic instability of the patient.
Gastric ulceration, and ultimately perforation from benign disease, has become less common since the development of proton pump inhibitors and treatment of Helicobacter pylori infection [1, 2], and this likely increases the relative risk of a gastric perforation having a malignant cause. Therefore, we undertook a systematic qualitative approach to summarize the existing published literature on perforation in the setting of gastric cancer and report on any critical themes or trends in clinical presentation, management patterns, or outcomes to guide clinical decision-making.
Methods
Data sources
Electronic literature searches were conducted in MEDLINE and EMBASE from January 1, 1985, to January 1, 2010. The a priori decision was made to identify all articles that related to management of perforation in the setting of gastric malignancy as part of a larger search strategy for surgical outcomes in gastric cancer. Search terms included: [exp Stomach Cancer/or (((gastric or stomach) adj1 cancer$) or ((gastric or stomach) adj1 carcinoma) or ((gastric or stomach) adj1 adenocarcinoma) or ((gastric or stomach) adj1 neoplasm$)).mp.] and [((negative or resection) adj2 margin$).mp. or exp frozen section/or exp GASTRECTOMY/or ((gastric or stomach) adj2 resect$).mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] or omentectom$.mp. or multivisceral resection$.mp.] and [clinical trial/or controlled clinical trial/or exp comparative study/or meta-analysis/or multicenter study/or exp practice guideline/or randomized controlled trial/] not [Case Report/or review]. A separate search of the Cochrane Central Register of Controlled Trials (1998–2009) was performed using the search term gastric cancer. No attempt was made to locate unpublished material.
Study selection and review process
To be eligible, studies had to report procedure-related morbidity, mortality, and/or survival in perforated gastric cancer cases. Studies in the following formats were excluded: reviews, meta-analyses, systematic reviews, abstracts, editorials or letters, case reports, and guidelines. Searches were limited to English-language and primary reports. All electronic search titles, selected abstracts, and full-text articles were independently reviewed by a minimum of two reviewers (NC, AM, and LH). Reference lists from review papers and relevant articles were also examined for additional studies that met our inclusion criteria. Disagreements on study inclusion/exclusion were resolved with a consensus meeting.
Data extraction
A systematic approach to data extraction was used to produce a descriptive summary of participants, interventions, and study findings. The first reviewer (AM) independently extracted the data and a second reviewer (NC) checked the data extraction. No attempt was made to contact authors for additional information.
Results
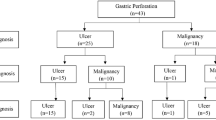
We identified 3608 articles from the original search strategy. Of those, nine articles were included in this qualitative review of perforated gastric cancer (Table 1) [3–11]. Two of the articles report outcomes on the same series of patients; therefore, results are reported together to avoid duplication [6, 7]. Characteristics of the nine articles and their patient populations are described in detail in Table 1. This review encompasses 127 patients from 7 countries. All studies employed retrospective methodology. All studies included fewer than 35 patients and as such there was considerable heterogeneity with respect to patient characteristics. Perforated gastric cancer represented ≤3% of institutional cases of gastric cancer in all studies. The mean or median age was reported in 8 of the 9 papers and ranged from 61–72 years. The stage of gastric cancer was reported by 7 of the 9 papers. The majority of patients presented with advanced disease: 19–62% had stage III disease and 8–57% had stage IV disease (Table 1). In 8 of the 9 studies, a preoperative diagnostic status was reported by the authors. On average, fewer than half of the patients presenting with perforation and receiving subsequent treatment had a preoperative or intra-operative diagnosis of gastric cancer [3–7, 9–11]. Jwo et al. [9] reported one patient who experienced perforation during chemotherapy for metastatic disease. Gertsch et al. [4] reported information on preoperative investigations, citing roentgenology and laboratory tests for diagnosis of perforation.
Initial simple repair of the defect or closure of the perforation and treatment of peritonitis was performed in 35 patients. Of the 35 initial closures, nine patients went on to receive a gastrectomy as a second operation. Poor overall health, high clinical risk, advanced and/or metastatic disease, and unresectable tumor were reasons provided for simple closure only as the management strategy. Radical gastrectomy including the dissection of lymph nodes was reported in few patients and indication for more extensive surgery was not outlined in the studies. Subtotal gastrectomy was performed in 41 patients, total gastrectomy was performed in 15 patients, and 7 received a gastrectomy not otherwise specified. Remaining operative approaches included wedge resection and local excision of the tumor. The status of the operation (emergent vs elective) was classified by 5 of the studies and 100% were executed emergently [5, 7–9, 11]. Gertsch et al. [4] reported that 21% of patients were in preoperative shock at presentation while Ozmen et al. [3] reported 7/14 patients were in preoperative shock at presentation.
Short-term outcomes
Procedure-related morbidity and mortality rates are reported in Table 2. Procedure-related morbidity was measured for the overall group of perforated patients in four studies and rates ranged from 15 to 57% (Table 2). Overall operative mortality rates ranged from 8 to 40%. Mortality rates for simple closure of the perforation ranged from 8 to 100% and for patients managed with a form of resection, operative mortality ranged from 0 to 50%. The high procedure-related mortality for simple closure likely reflects the worse health status and increased cancer burden of patients receiving this management strategy.
Long-term outcomes
Long-term survival was measured for the different surgical management strategies and is reported in Table 3. Different treatment categories and survival outcomes were reported among the studies. A trend towards improved survival was demonstrated among studies that compared patients receiving curative intent or R0 resections with patients receiving non-curative or R1 or R2 procedures. None of the studies reported the long-term survival in patients receiving simple closure of the perforation without a subsequent attempt at resection (Table 3). Interpretation of survival differences is made difficult by the significant bias in patient selection for R0 gastrectomy that likely exists. Patient co-morbid status and intra-operative status were not reported by management strategy and this information would have aided understanding.
Two-stage operations for improved oncologic outcomes were discussed in 5 studies [3, 4, 6, 7, 11]. Citing extreme shock and peritonitis as precluding initial extensive surgery, Ozmen et al. [3] performed secondary cancer-related gastrectomy following improved health status of the patient. Lehnert et al. [11] also reported primary surgery for control of the perforation and suspected ulcer disease and then secondary radical cancer-directed gastrectomy after recovery from the initial operation. Kasakura et al. [6, 7] performed second surgeries following initial simple repair cases. As well, in cases where limited lymph node dissection was initially performed, and on confirmation of malignancy, a second more extensive dissection was undertaken [6, 7].
Discussion
Many strategies for the elective surgical management of gastric cancer exist; however, there are limited data on the emergent management of perforation in the setting of gastric cancer. In this paper, we reviewed nine studies examining the management of 127 patients with perforated gastric cancer to aid in the decision-making for these rare cases. These studies represent institution case series in Asian and European populations and may be representative of the patient population worldwide with this rare complication. Patient characteristics demonstrated heterogeneity among study populations with respect to age, gender, and stage. Both management strategies and outcomes of the patients treated for perforated gastric cancer varied substantially. Importantly, long-term survival was reported for patients receiving R0 resection, with a median survival of 75 months and 50% 5-year survival. The review of these studies provides an opportunity to assess the presentation of patients with perforation in the setting of gastric cancer, to consider the different surgical approaches available, and to attempt to understand the differences observed among studies and interventions.
Patients with gastric perforation in the setting of malignancy present with signs and symptoms that are generally indistinguishable from those of benign peptic ulcer disease and in all cases are managed emergently. Ergul et al. [12] reported that perforations occurring in older patients, with a larger diameter of perforation, a perforation site in the middle or upper third of the stomach, or a longer duration of symptoms at presentation were more likely to be related to gastric cancer. These criteria may be important in stratifying which patients are more likely to have gastric cancer, but are not definitive for diagnosis of a malignancy. Preoperative diagnosis of a gastric cancer was rare and occurred in 14–57% of patients in the papers reviewed. For those patients with a pre-perforation diagnosis of gastric cancer, chemotherapy was only discussed as being the cause of the perforation in one case [9]. Intra-operative diagnosis can also be challenging, as inflammation may mimic invasion [7]. As these operations are undertaken emergently, pathologists may be unavailable for consultation and examination of frozen section specimens. Jwo et al. [9] reported diagnosing 31% of cases as malignant on intra-operative frozen sections and this allowed one-stage curative resection for these patients, avoiding a second operation.
The majority of patients with a gastric perforation in the setting of malignancy presented with advanced, non-curative disease; however, stage I or II patients made up 0–36% of patients with perforation in the series [3–11]. Adachi et al. [13] reviewed 155 patients from the Japanese literature and also found that 31% of patients were stage I or II. The accuracy of the reported stage must be interpreted carefully, as inadequate lymph node retrieval would be expected in emergency resections without formal lymphadenectomy and thus patients may be under-staged [4]. Differences in the number of early-stage gastric cancers in each study may account for some of the differences in outcomes, especially in long-term survival, as stage is recognized as a significant predictor of outcome [4, 9]. Additionally, the mechanism of perforation for ulcer disease concomitant with an early-stage gastric cancer compared to necrotic perforation of an advanced cancer is likely different and was only addressed by Gertsch et al. [4] through exclusion of patients with concomitant ulcers if the tumor was not responsible for the perforation. As stage of the cancer likely contributes to mechanism of perforation, it will influence both prognosis and management.
Treatment decisions for the surgeon confronted with a gastric perforation revolve around the management of life-threatening sepsis and the extent of intervention. Surgical management requires consideration of patient factors, such as hemodynamic stability and pre-existing co-morbidities, as well as the extent of disease and contamination. The surgeon must choose between non-oncologic procedures, such as simple closure and emergent simple resection, and more extensive oncologic resections that may not be well tolerated in the setting of perforation.
Simple closure of the perforation has historically been associated with poor results [4]. A high rate of secondary leaks is thought to occur in these patients, given the difficulty of handling inflamed and tumor-infiltrated tissues [3]. In the literature, mortality for patients receiving simple closure ranged from 12.5 to 100%. Although high procedure-related mortality (10–40%) is also reported in non-malignant gastric perforation cases, bias from patient selection may explain the differences in outcomes for the available interventions [1, 2]. Patients undergoing simple closure of perforation likely represent the sickest patients in these series; therefore, simple closure may be the only appropriate option in the setting of multiple co-morbidities, extensive peritonitis, hemodynamic instability, or in the presence of overt metastatic disease.
For carefully selected patients, a formal gastric resection likely represents the optimal strategy. A survival benefit appears to exist for those patients eligible for complete R0 resection of the tumor even in the face of perforation [7, 9]. This curative resection may take place emergently or as a separate operation following the initial surgical management performed prior to knowledge of a malignant diagnosis. Pathologic confirmation of cancer prior to radical resection with extensive lymphadenectomy is necessary to minimize unnecessary morbidity and mortality associated with gastrectomy and allows for patients to be selected for radical surgery by performance status and stage of disease [9]. Delaying extensive surgery may also be necessary due to the poor health status of the patient following peritonitis.
A two-stage surgical approach for perforation in the setting of gastric cancer was identified in the literature, which includes simple closure or gastrectomy as the first stage, followed by definitive gastric cancer resection in an elective setting [3, 4, 6, 7, 11]. The need for a second operation with an extended lymphadenectomy is uncertain, as a long-term survival benefit is also seen for patients receiving R0 resections with limited lymphadenectomy at the initial, emergent operation [5, 8]. Jwo et al. [9] found that there was no difference in survival between patients who underwent radical surgery with lymphadenectomy and patients who underwent resection without radical lymphadenectomy. However, Jwo et al. [9] do support the selective use of a two-staged approach with an extended lymphadenectomy for those patients with T3 tumors or an R1/R2 resection.
Importantly, these series demonstrate that long-term survival can be achieved with curative-intent resection despite the presence of perforation at presentation. Half of the curatively resected patients survived to 5 years [11] and a median survival of up to 75 months was reported [6, 7, 9]. While many patients who present with perforation are diagnosed with stage IV or non-curable disease, a significant number of patients with stage I–III disease may benefit from curative-intent resection. Spillage of tumor into the peritoneal cavity as a result of perforation was thought to carry a poor prognosis, similar to that of peritoneal disease [14, 15]; however, several of the publications in this review argue that peritoneal contamination did not adversely affect survival in these patients [4, 9, 11].
Selection bias is likely responsible for variation in both the short-term and long-term outcomes between treatment strategy groups. All authors reported that patients who received only simple closure were sicker than those patients who received more extensive treatment, making it more likely that differences in both in-hospital mortality and long-term survival are related to disease factors outside the treatment strategy selected. Several factors were identified in the studies as predictors of poor outcomes, including age [4, 8], duration of symptoms [3], preoperative shock [3, 8], and multiple co-morbidities [10]. The presence of long-term survivors in this review supports the premise that perforation alone may not be predictive of poor outcome and, consequently, surgical management should not only be focused on management of the perforation but also on primary treatment for the gastric cancer.
Conclusion
Gastric cancer presenting with a gastric perforation is a rare event that has a poor overall prognosis. Carefully selected patients may benefit from aggressive surgical intervention that addresses both the perforation and the malignancy in a single or multi-step process. Given the scarcity of these cases for evaluating outcomes, further research in this patient population should include prospective pooled multi-institutional studies to investigate the effectiveness of available approaches for management, with attention to identifying both preoperative predictive factors for a cancer diagnosis and best preoperative diagnostic and staging investigations.
References
Soybel DI. Gastric outlet obstruction, perforation and other complications of gastroduodenal ulcer. In: Wolfe MM, editor. Therapy of digestive disease disorders: a companion to Sleisenger and Fordtran’s gastrointestinal and liver disease. Philadelphia: WB Saunders Company; 2000. p. 153.
Pappas TN, Lapp JA. Complications of peptic ulcer disease: perforation and obstruction. In: Taylor MB, editor. Gastrointestinal emergencies. 2nd ed. Baltimore: Williams and Wilkins: A Waverly Company; 1997. p. 87.
Ozmen MM, Zulfikaroglu B, Kece C, Aslar AK, Ozalp N, Koc M. Factors influencing mortality in spontaneous gastric tumour perforations. J Int Med Res. 2002;30(2):180–4.
Gertsch P, Yip SK, Chow LW, Lauder IJ. Free perforation of gastric carcinoma results of surgical treatment. Arch Surg. 1995;130(2):177–81.
Roviello F, Rossi S, Marrelli D, De Manzoni G, Pedrazzani C, Morgagni P, et al. Perforated gastric carcinoma: a report of 10 cases and review of the literature. World J Surg Oncol. 2006;4:19.
Kasakura Y, Ajani JA, Fujii M, Mochizuki F, Takayama T. Management of perforated gastric carcinoma: a report of 16 cases and review of world literature. Am Surg. 2002;68(5):434–40.
Kasakura Y, Ajani JA, Mochizuki F, Morishita Y, Fujii M, Takayama T. Outcomes after emergency surgery for gastric perforation or severe bleeding in patients with gastric cancer. J Surg Oncol. 2002;80(4):181–5.
So JB, Yam A, Cheah WK, Kum CK, Goh PM. Risk factors related to operative mortality and morbidity in patients undergoing emergency gastrectomy. Br J Surg. 2000;87(12):1702.
Jwo SC, Chien RN, Chao TC, Chen HY, Lin CY. Clinicopathological features, surgical management and disease outcome of perforated gastric cancer. J Surg Oncol. 2005;91(4):219–25.
Pyrc J, Kersting S, Dobrowolski F, Denz A, Kuhlisch E, Saeger HD. Tumor-related bleeding, perforation, and stenosis as prognostic factors of gastric cancer. Adv Clin Exp Med. 2006;15(6):1015–22.
Lehnert T, Buhl K, Dueck M, Hinz U, Herfarth C. Two-stage radical gastrectomy for perforated gastric cancer. Eur J Surg Oncol. 2000;26(8):780–4.
Ergul E, Gozetlik EO. Emergency spontaneous gastric perforations: ulcus versus cancer. Langenbecks Arch Surg. 2009;394(4):643–6.
Adachi Y, Mori M, Maehara Y, Matsumata T, Okudaira Y, Sugimachi K. Surgical results of perforated gastric carcinoma: an analysis of 155 Japanese patients. Am J Gastroenterol. 1997;92(3):516–8.
Bonenkamp JJ, Songun I, Hermans J, van de Velde CJ. Prognostic value of positive cytology findings from abdominal washings in patients with gastric cancer. Br J Surg. 1996;83(5):672–4.
Boku T, Nakane Y, Minoura T, Takada H, Yamamura M, Hioki K, et al. Prognostic significance of serosal invasion and free intraperitoneal cancer cells in gastric cancer. Br J Surg. 1990;77(4):436–9.
Acknowledgments
This study was funded by the Canadian Cancer Society (Grant # 019325). Dr. Coburn is supported by a Ministry of Health and Long Term Care Career Scientist Award. Dr. Law is supported by the Hanna Family Chair in Surgical Oncology Chair.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahar, A.L., Brar, S.S., Coburn, N.G. et al. Surgical management of gastric perforation in the setting of gastric cancer. Gastric Cancer 15 (Suppl 1), 146–152 (2012). https://doi.org/10.1007/s10120-011-0095-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-011-0095-4