Abstract
The goal of this study was to assess the ablation, coagulation, and carbonization characteristics of the holmium:YAG (Ho:YAG) laser and thulium fiber lasers (TFL). The Ho:YAG laser (100 W av.power), the quasi-continuous (QCW) TFL (120 W av.power), and the SuperPulsed (SP) TFL (50 W av.power) were compared on a non-frozen porcine kidney. To control the cutting speed (2 or 5 mm/s), an XY translation stage was used. The Ho:YAG was tested using E = 1.5 J and Pav = 40 W or Pav = 70 W settings. The TFL was tested using E = 1.5 J and Pav = 30 W or Pav = 60 W settings. After ex vivo incision, histological analysis was performed in order to estimate thermal damage. At 40 W, the Ho:YAG displayed a shallower cutting at 2 and 5 mm/s (1.1 ± 0.2 mm and 0.5 ± 0.2 mm, respectively) with virtually zero coagulation. While at 70 W, the minimal coagulation depth measured 0.1 ± 0.1 mm. The incisions demonstrated zero carbonization. Both the QCW and SP TFL did show effective cutting at all speeds (2.1 ± 0.2 mm and 1.3 ± 0.2 mm, respectively, at 30 W) with prominent coagulation (0.6 ± 0.1 mm and 0.4 ± 0.1 mm, respectively, at 70 W) and carbonization. Our study introduced the TFL as a novel efficient alternative for soft tissue surgery to the Ho:YAG laser. The SP TFL offers a Ho:YAG-like incision, while QCW TFL allows for fast, deep, and precise cutting with increased carbonization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fifty years passed since the introduction of lasers into urology [1]. Laser techniques stopped being just an interesting option for endourology and became its integral part. Currently, lasers are widely used in lithotripsy, benign prostatic hyperplasia (BPH) surgery, bladder cancer (BCa), and upper tract urinary carcinoma (UTUC) resection [2]. For the clinician, the first criterion for selecting a laser is to choose a wavelength that will be optimally absorbed by the components of the target tissue or compound. Obviously, for each application, different types of lasers are used. However, one laser—Ho:YAG (holmium:YAG) (emitting at 2.09 μm)—has been widely used for all causes. Such versatility of Ho:YAG is explained by its high absorption in water (absorption coefficient 26 cm−1) [3] and pulsed mode. Its potential rival in endoscopic tissue surgery is a Tm:YAG laser (emitting at 2.0 μm) which allows for even better water absorption (absorption coefficient of 52 cm−1) [3] and continuous wave (CW) mode which translates into longer pulse length and therefore decreased pulse power. Tm:YAG was shown to be at least comparable to Ho:YAG in BPH surgery and may even be more convenient for bladder surgery with some papers reporting better cutting and pathology achievable with Tm:YAG [4, 5]. The main drawback of the Tm:YAG laser is its continuous wave mode of operation, which leads to lower pulse power resulting in significant carbonization during surgery [3]. This may hinder visualization or affect cutting precision.
Thulium fiber lasers (TFL) emitting at 1.9 μm aimed to solve this problem, with the highest water absorption among endourological lasers (absorption coefficient of 114 cm−1) and quasi-continuous SuperPulsed mode enabling higher pulse power compared to Tm:YAG, presumably leading to improved cutting efficiency and reduced carbonization [6]. TFL is based on the new type of technology and might excel over solid-state (YAG) lasers. Much similar in many respects, all these lasers, still, are not at all identical in terms of laser-tissue interaction and differ in terms of depth of ablation, coagulation efficacy, and cutting speed. Currently, there is a lack of evidence in terms of TFL’s ablation and coagulation capabilities in endoscopy. In extensive use, none previously performed its comparison with other devices in endoscopy regimens and setups [7, 8].
The goal of this study was to assess the ablation, coagulation, and carbonization characteristics of the Ho:YAG laser and TFL during endoscopic surgery.
Materials and methods
Laser systems
Three lasers were compared in our experiment: TFL with a wavelength of 1.9 μm (NTO IRE-Polus, Fryazino, Russia/IPG Medical, Marlborough, MS, USA) in quasi-continuous (QCW) mode (Pav = 120 W, Pmax = 120 W), TFL with a wavelength of 1.9 μm (NTO IRE-Polus, Fryazino, Russia/IPG Medical, Marlborough, MS, USA) in SuperPulsed (SP) mode (Pav = 50 W, Pmax = 500 W), and Ho:YAG laser with a wavelength of 2.09 μm (Lumenis, USA) in pulsed mode (Pav = 100 W, Pmax = 2-10 kW). Each of them had two profile sets: for Ho:YAG, (a) Pav = 40 W and E = 1.5 J, (b) Pav = 70 W and E = 1.5 J; for QCW TFL, (a) Pav = 30 W and E = 1.5 J, (b) Pav = 60 W and E = 1.5 J; and for SP TFL, (a) Pav = 30 W and E = 1.5 J, (b) Pav = 50 W and E = 1.5 J. These parameters are the most commonly applied in EEP surgery [9]. Inexperienced surgeons who are not yet comfortable with a surgical laser typically select lower power settings and larger spot sizes to keep incision and ablation rates lower than those likely to be used by an experienced surgeon. Surgical fibers with core diameters of 550 and 600 μm were used for Ho:YAG and TFL, respectively.
The experimental setup
To test the cutting properties of Ho:YAG and TFL, a non-frozen porcine kidney was used as a model. Previously, it was shown that the kidney is representative of a prostate possessing a comparable specific absorption coefficient [10,11,12]. To allow robustness and repeatability, we cut the kidney into 8-mm-thick, 60-mm-long, and 20-mm-wide samples with an electric slicer. Slices were placed onto a metal platform with an 8-mm-deep cavity and covered by a 0.5-mm-thick metal plate with slots for cutting. Laser fiber was put into contact with the metal plate, which allowed for quasi-contact kidney cutting (Supplementary Figures 2, 3, and 4).
A motorized XY translation stage with a fixed fiber holder and a micrometer screw allowed for controlling the cutting speed and changing the gap between tissue and fiber with a ± 0.1-mm margin of error. Fiber speeds were 2 and 5 mm/s to imitate slow and fast cutting. Normal saline solution was used as the medium. All experiments were performed at room temperature. A total of 180 incisions were performed ex vivo (15 for each speed and power regimen).
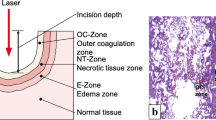
After ex vivo incision histological analysis was performed. The samples were sliced with a microtome (Leica RM2235, Leica Biosystems, Switzerland) to produce 300-μm-thick sagittal cryosections [13].
From each cut, at least three sections were examined with the Leica DM1000 B LED microscope, equipped with the Leica DFC 7000 T digital camera and the LAS V4.8 software (Leica Microsystems, Switzerland). Both pathologist (YuS) and urologist (MT) performed the laser wound analysis. Ablation depth = incision depth (mm) and axial coagulation depth (mm) were subjected to evaluation. Ablation and coagulation depths were presented as mean ± SD. Visual examination of the resection surface was performed to determine the tissue carbonization level. The carbonization grade (CG) was measured with a visual grading scale (from 0 (no carbonization) to 3 (extensive carbonization)) (Supplementary Figure 1).
Statistical analysis
Statistical analysis was performed using SPSS version 23 (IBM Corp., Armonk, NY, USA). The Mann-Whitney U test was used to determine the statistical differences of the independent parameters of the groups. Data were expressed as mean ± SD. A p value of ≤ 0.05 was considered to indicate statistical significance.
Results
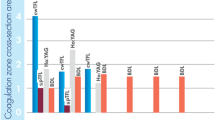
The experiment exposed pronounced differences. In the Ho:YAG, the deepest incision among all slices was found at Pav = 70 W and 2 mm/s; it demonstrated a tapering ablation zone (1.6 ± 0.2 mm) with a small coagulation area (up to 0.1 ± 0.1 mm) in certain cuts. The least incision depth in Ho:YAG was detected at Pav = 40 W and 5 mm/s with a conical ablation zone to 0.5 ± 0.2 mm with a deep tissue rupture at the apex of the cone, though without any visual coagulation zone in any of the slices.
In the QCW TFL, the highest incision depth was determined at Pav = 60 W and 2 mm/s; it demonstrated a coniform ablation zone (2.7 ± 0.2 mm) with a rounded apex surrounded by a marked coagulation zone (0.6 ± 0.1 mm); сarbonization was presented in every case with a mode of 2. The least incision depth in the TFL was observed at Pav = 30 W and 5 mm/s with the ablation zone (0.7 ± 0.2 mm) shaped rounded. A layer of the coagulation zone (0.3 ± 0.1 mm) was found around the periphery of the ablation crater, with the CG measuring 1–2.
Whereas QCW TFL outcomes differ from those of Ho:YAG, SP TFL yielded rather similar results with the deepest incision of 2.1 ± 0.3 mm at 2 mm/s and Pav = 50 W and no substantial carbonization (grades 1–2, mode 1). At Pav = 30 W, SP TFL provided cuts similar to Ho:YAG with a depth of 0.5 ± 0.2 mm at 5 mm/s. As with Ho:YAG, almost no coagulation and carbonization were observed (grades 0–1).
With the fiber speed changing (the fixed fiber holder of the XY translation stage), the incision and coagulation depths also varied considerably (Table 1). The Ho:YAG’s ablation capability was much higher at 2 mm/s than at 5 mm/s (1.1 ± 0.2 mm and 0.5 ± 0.2 mm, respectively, at 40 W) with the TFL at the same speeds ranging between 2.1 ± 0.2 and 0.7 ± 0.2 mm, respectively, at 30 W. Effects on tissues and data on lasers are summarized in Table 2.
Discussion
The main laser-tissue interaction in soft tissue surgery is photothermolysis, where radiant energy is converted into kinetic (thermal) energy for the purpose of ablation, coagulation, and carbonization. Heating tissue above 60 °C without exceeding 100 °C causes denaturation of proteins and beginning of pyrolysis, leading to thermal coagulation; the power density of the laser beam must not exceed the threshold of vaporization [14]. Thermal tissue ablation/vaporization refers to the destruction of tissue by extreme hyperthermia exceeding 100 °C; the power density of the laser beam must exceed the ablation threshold of vaporization to boil intracellular water [14]. A further increase in temperature leads to the onset of pyrolysis, which eventually terminates in carbonization.
We conducted a comparative study on ablation, coagulation, and carbonization characteristics of Ho:YAG versus TFL for the ablation of soft tissues. In general, the deepest incision among all slices was in the TFL—up to 2.7 mm. The previous study showed similar results, Fried [15] detected that the TFL is able to effectively ablate the tissue not only due to its better water absorption but also due to its quasi-continuous operation mode. Instead of CW mode used in Tm:YAG devices, when there are no pauses in laser firing, the QCW means that there are short pauses between laser pulses, allowing for thermal relaxation of the tissue. This is similar to the pulsed mode of operation of the Ho:YAG laser. This allows reducing the carbonization greatly.
For the past decade, the Ho:YAG has been the standard for prostate surgery. Though effective and safe, there are disadvantages, such as its steep learning curve. Shah et al. [16] found that surgeons inexperienced in the holmium laser enucleation of the prostate (HoLEP) require ~ 50 cases to efficiently perform the procedure. As most authors say, enucleation is challenged by proper regaining of the enucleation plane in case of its loss (it is a frontier line between the adenoma nodes and the surgical capsule) [7, 17]. Thus, the selection of a laser device with the most optimal physical properties to avoid this difficulty is an important issue [17].
The more the laser is cutting through small volumes of tissue, the more chances to regain the enucleation plane. The Ho:YAG is reputed for its low ablation depth at 0.4–0.7 mm [3, 18]. Our macro- and microscopic assessment of the Ho:YAG’s incisions resulted in the following: (1) no burning (carbonization) traced in the incision zone; (2) torn margins of the incisions witnessed; (3) a deep but narrow ablation crater observed; and (4) minimal-to-no coagulation evident. And all these are due to the Ho:YAG’s operational mode (thermomechanical tissue cutting with a shockwave generated at cavitation collapse) [19]. Each laser pulse has a peak power ranging between 2 and 10 kW, whereas the average power ranges between 40 and 70 W (depending on the regimen selection) [20]. Such high peak power generates a vapor bubble (from vaporized water) on the fiber tip [21, 22]. Once formed, it reaches tissue and ruptures it to produce deep and narrow incisions [6]. If ablating soft tissue, the bubble may impede precise incising and prevent efficient coagulation and hemostasis [23].
Searching for ways to effectively dissect and cut tissue resulted in the emergence of the continuous wave Tm:YAG. If the Ho:YAG’s pulse emissions cause tissue tearing, the Tm:YAG’s continuous emission, conversely, allows for smooth incising and vaporizing accompanied by excellent hemostasis. The downside of CW operation is high carbonization, which usually hampers the intraoperative navigation [23].
Developing a new laser generation has brought about a novel thulium fiber laser (TFL). Somewhat similar to the Ho:YAG laser, it produces a sequence of pulses. However, there are two significant differences. First, at equal pulse energy, the peak power of the TFL pulse is 10–200 times lower than that of Ho:YAG; second, the pulse length of TFL is much greater than that of Ho:YAG. As a result, the tissue cutting mechanism of TFL is predominantly photothermal, as opposed to the photothermomechanical mechanism of Ho:YAG. TFL’s advantage is its low peak power and longer pulse. It is exactly what allows for distributing energy equally during a single laser pulse and provides consistent and efficient tissue vaporization. The TFL’s vapor bubble forms slower with low mechanical impact to the tissue compared to the Ho:YAG’s one, and it implies that the TFL cuts tissue by absorbing laser energy in tissue and its vaporization. In contrast, Ho:YAG has significantly higher peak power and shorter pulse width with fast vaporization of water between fiber tips and tissue and formation bubble and cutting tissue primarily due to laser-induced mechanical pressure, bubble formation, and cavitation. At our trial, the TFL showed about 2.7 mm of incision depth at 60 W and 2 mm/s. The basic incision features are given here: (1) non-extensive carbonization; (2) clear-сut incision margins with no rupture; (3) widely based coniform ablation; and (4) extensive and marked coagulation (up to 0.6 ± 0.2 mm). These recordings are of special importance to us as they eventually testify to the fact that the TFL would secure better hemostasis than the Ho:YAG which demonstrated the lowest (0.1 ± 0.2 mm) or just zero coagulation. This aspect was not emphasized in previous clinical reports that may be due to its minimal intraoperative effect, yet we could consider that TFL has more preferable modality than Ho:YAG in patients on anticoagulant therapy. Novelty of TFL technology explains the relative scarcity of publications on the topic. Fried and Murray [6] in their report on the low-powered TFL system (40 W) previously showed that it allows for effective ablation with limited coagulation. In our study, the SuperPulsed TFL which had already proven its substantial benefits in lithotripsy in preclinical and clinical trials [24,25,26] was shown to share the ablation efficacy with Ho:YAG. This device, due to its increased peak power and shorter pulse duration, also separated tissue like Ho:YAG and yielded carbonization-free incisions. It should be noted that while SP TFL settings were similar to those of QCW TFL, the difference in outcomes was striking. The main reasons for such differences could be decreased pulse duration of SP TFL [27] which leads to lower energy fluence; therefore, it would decrease ablation, coagulation, and carbonization. However, like Ho:YAG, SP TFL causes thermomechanical damage to tissues which was observed as multiple ruptures and uneven incision margins. SP TFL seems to be a viable alternative to Ho:YAG, yet it does not allow for fast and effective cutting like QCW TFL.
It is also worth noting that ablation capabilities strongly depend on fiber speed, and therefore, there comes up a risk that an investigator bias could lead to favoring one laser over another one [8]. To avoid any bias in our ex vivo experiment, we employed an XY translation stage with a fixed fiber holder. With the speed rising from 2 to 5 mm/s, our Ho:YAG displayed a substantial decrease in tissue ablation (1.1 ± 0.2 mm and 0.5 ± 0.2 mm, respectively, at 40 W). Besides that, the Ho:YAG’s thermomechanical effect and its exposure speed are presumed to be interdependent: the lower the speed, the smaller the steam affection. These effects can potentially be mitigated by the Moses effect. Large et al. [28] conducted a study trying to prove that HoLEP with Moses could decrease blood loss. While they were able to prove that HoLEP with the Moses effect allows for lower postoperative hemoglobin drop and shorter time to achieve postoperative hemostasis, both of these findings have only limited clinical relevance.
The scope of future work on TFL includes exploring laser effects on blood-perfused kidney models. Despite minimal absorption of the energy of TFL and Ho:YAG in hemoglobin [29], blood could affect the thermal conductivity of tissue, thus altering the results. This was confirmed in a study by Bach et al. [8] on the effects of Tm:YAG on tissue. The authors used blood-perfused models not only to assess incision depth and characteristics but also to estimate how effective Tm:YAG could be in terms of hemostasis and, more importantly, what coagulation zone would be enough for effective and safe hemostasis. We believe that despite using a non-perfused model, we still obtained important data on the effects of TFL and Ho:YAG without any concomitant factors (e.g., kidney curvature, hand-held laser fiber, and difference in the blood supply of different kidney parts). Further research should shed light on how the effects of the two lasers would differ in perfused models.
Limitations
A limitation of the study is that the Ho:YAG was not complemented with the Moses effect. The Ho:YAG coupled with the Moses technology may cause marked coagulation. Another limitation is that the samples studied were from a non-blood-perfused porcine kidney. However, laser applications in medicine rely on light absorption by chromophores (water or hemoglobin). Both the Ho:YAG and the TFL generate emissions to be absorbed by water, and that is the reason why we deem our research results to be representative enough. The main goal of this particular study was to provide data which could be useful for daily clinical practice. For this, we used a clinically applicable surgical device. Unfortunately, this introduced a significant limitation as we were unable to precisely attune it to the different range of modes. Therefore, we only focused on regimens suitable for clinical needs.
Conclusions
Our study introduced the TFL as a novel efficient alternative for soft tissue surgery to the Ho:YAG laser. The SP TFL offers a Ho:YAG-like incision, while QCW TFL allows for fast, deep, and precise cutting with increased carbonization.
Data availability
On demand.
References
Parsons RL, Campbell JL, Thomley MW (1968) Carcinoma of the penis treated by the ruby laser. J Urol 100(1):38–39
Bruskewitz RC (2003) Quality of life and sexual function in patients with benign prostatic hyperplasia. Rev Urol 5(2):72–80
Rieken M, Bachmann A (2014) Laser treatment of benign prostate enlargement--which laser for which prostate? Nat Rev Urol 11(3):142–152
Kyriazis I, Swiniarski PP, Jutzi S, Wolters M, Netsch C, Burchardt M et al (2015) Transurethral anatomical enucleation of the prostate with Tm:YAG support (ThuLEP): review of the literature on a novel surgical approach in the management of benign prostatic enlargement. World J Urol 33(4):525–530
Li K, Xu Y, Tan M, Xia S, Xu Z, Xu D (2019) A retrospective comparison of thulium laser en bloc resection of bladder tumor and plasmakinetic transurethral resection of bladder tumor in primary non-muscle invasive bladder cancer. Lasers Med Sci 34(1):85–92
Fried NM, Murray KE (2005) High-power thulium fiber laser ablation of urinary tissues at 1.94 microm. J Endourol 19(1):25–31
Enikeev D, Glybochko P, Rapoport L, Gahan J, Gazimiev M, Spivak L et al (2018) A randomized trial comparing the learning curve of three endoscopic enucleation techniques (HoLEP, ThuFLEP and MEP) for BPH using mentoring approach - initial results. Urology 121:51
Bach T, Huck N, Wezel F, Hacker A, Gross AJ, Michel MS (2010) 70 vs 120 W thulium:yttrium-aluminium-garnet 2 microm continuous-wave laser for the treatment of benign prostatic hyperplasia: a systematic ex-vivo evaluation. BJU Int 106(3):368–372. https://doi.org/10.1111/j.1464-410X.2009.09059.x
Enikeev D, Glybochko P, Okhunov Z, Alyaev Y, Rapoport L, Tsarichenko D et al (2018) Retrospective analysis of short-term outcomes after monopolar versus laser endoscopic enucleation of the prostate: a single center experience. J Endourol 32(5):417–423
Cooper TE, Trezek GJ (1972) A probe technique for determining the thermal conductivity of tissue. J Heat Transf 94:133–140
Patch SK, Rao N, Kelly H, Jacobsohn K, See WA (2011) Specific heat capacity of freshly excised prostate specimens. Physiol Meas 32(11):N55–N64
Giering K, Lamprecht I, Minet O (1995) Determination of the specific heat capacity of healthy and tumorous human tissue. Thermochim Acta 251:199–205
Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH protocols. 2008;2008:pdb prot4986
Peavy GM (2002) Lasers and laser-tissue interaction. Vet Clin North Am Small Anim Pract 32(3):517–534 v-vi
Fried NM (2006) Therapeutic applications of lasers in urology: an update. Exp Rev Med Dev 3(1):81–94
Shah HN, Mahajan AP, Sodha HS, Hegde S, Mohile PD, Bansal MB (2007) Prospective evaluation of the learning curve for holmium laser enucleation of the prostate. J Urol 177(4):1468–1474
Netsch C, Bach T, Herrmann TR, Neubauer O, Gross AJ (2013) Evaluation of the learning curve for thulium vapoenucleation of the prostate (ThuVEP) using a mentor-based approach. World J Urol 31(5):1231–1238
Kuntz RM (2006) Current role of lasers in the treatment of benign prostatic hyperplasia (BPH). Eur Urol 49(6):961–969
Jung GI, Kim JS, Lee TH, Choi JH, Oh HB, Kim AH et al (2015) Photomechanical effect on type I collagen using pulsed diode laser. Technol Health Care 23(Suppl 2):S535–S541
Andreeva V, Vinarov A, Yaroslavsky I, Kovalenko A, Vybornov A, Rapoport L et al (2019) Preclinical comparison of superpulse thulium fiber laser and a holmium:YAG laser for lithotripsy. World J Urol 38:497
Kuntz RM (2007) Laser treatment of benign prostatic hyperplasia. World J Urol 25(3):241–247
Teichmann HO, Herrmann TR, Bach T (2007) Technical aspects of lasers in urology. World J Urol 25(3):221–225
Enikeev D, Glybochko P, Rapoport L, Gahan J, Gazimiev M, Spivak L et al (2018) A randomized trial comparing the learning curve of 3 endoscopic enucleation techniques (HoLEP, ThuFLEP, and MEP) for BPH using mentoring approach-initial results. Urology. 121:51–57
Enikeev D, Taratkin M, Klimov R, et al. Superpulsed thulium fiber laser for stone dusting: in search of a perfect ablation regimen-a prospective single-center study. J Endourol. 2020;https://doi.org/10.1089/end.2020.0519. [published online ahead of print, 2020 Jul 15]
Enikeev D, Taratkin M, Klimov R, et al. Thulium-fiber laser for lithotripsy: first clinical experience in percutaneous nephrolithotomy. World J Urol. 2020;https://doi.org/10.1007/s00345-020-03134-x. [published online ahead of print, 2020 Feb 27]
Taratkin M, Laukhtina E, Singla N, et al. How lasers ablate stones: in vitro study of laser lithotripsy (Ho:YAG and Tm-fiber lasers) in different environments [published online ahead of print, 2020 Jan 29]. J Endourol. 2020;https://doi.org/10.1089/end.2019.0441. doi:https://doi.org/10.1089/end.2019.0441
Fried NM (2018) Recent advances in infrared laser lithotripsy [Invited]. Biomed Opt Express 9(9):4552–4568. https://doi.org/10.1364/BOE.9.004552 Published 2018 Aug 30
Large T, Nottingham C, Stoughton C, Williams J Jr, Krambeck A (2020) Comparative study of holmium laser enucleation of the prostate with MOSES enabled pulsed laser modulation. Urology. 136:196–201. https://doi.org/10.1016/j.urology.2019.11.029
Enikeev D, Shariat SF, Taratkin M, Glybochko P (2020) The changing role of lasers in urologic surgery. Curr Opin Urol 30(1):24–29. https://doi.org/10.1097/MOU.0000000000000695
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
No ethics approval is necessary for this type of work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 259 kb)
Rights and permissions
About this article
Cite this article
Taratkin, M., Kovalenko, A., Laukhtina, E. et al. Ex vivo study of Ho:YAG and thulium fiber lasers for soft tissue surgery: which laser for which case?. Lasers Med Sci 37, 149–154 (2022). https://doi.org/10.1007/s10103-020-03189-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-020-03189-7