Abstract
This study investigated the local effect of photobiomodulation (PBM) for the treatment of periodontal pockets in patients with periodontitis and type 2 diabetes. Thirty-eight periodontal pockets presenting probing depth (PD) and clinical attachment level (CAL) ≥ 5 mm were selected from 19 patients (two pockets/patient). The selected periodontal pockets were randomly assigned to receive mechanical debridement only (control group) or mechanical debridement with PBM (PBM group). Clinical measures, such as PD, CAL, bleeding on probing (BoP), and presence of supragingival biofilm (PI), were collected and compared at baseline, 3, 6, and 12 months. After 12 months, no statistically difference was observed for mean PD and mean CAL when control and PBM groups were compared. The frequency of pockets with PD 5–6 mm was significantly lower for the PBM group at 6 months when compared to the control group. Pockets with PD ≥ 7 mm changed significantly between baseline and 3, 6, and 12 months for the PBM group, while for the control group, statistical significance was only observed between baseline and 6 months. The PBM protocol used in this study did not provide significant changes for PD and CAL in periodontal pockets when compared to mechanical therapy only. However, PBM was more effective in reducing the percentage of moderate periodontal pockets at 6 months in patients with type 2 DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontitis is an inflammatory disease, initiated by dental biofilm, that leads to attachment loss, bone resorption, and, eventually, tooth loss [1]. The purpose of periodontal therapy is to remove supra- and subgingival dental biofilm to reduce periodontal inflammatory burden, thus re-establishing tissue homeostasis and stopping the progression of periodontal disease. The gold standard for the treatment of periodontitis is mechanical periodontal therapy, which can be performed by either traditional manual scaling and root planing or ultrasonic debridement [2]. Both the disease initiation or progression and treatment effectiveness of periodontitis can be negatively influenced by systemic, genetic, and behavioral modifiers. [3].
Evidence shows that diabetes mellitus (DM) increases the risk for the initiation and progression of periodontitis [4]. Patients with poor metabolic control frequently present impaired wound healing. The inflammatory mechanisms involved in this process greatly affect diseased periodontal tissues in patients with DM [5, 6]. Several studies show that the expression of local inflammatory factors in the crevicular fluid is increased in patients with poor metabolic control and periodontitis [7,8,9].
The outcomes of studies that investigated mechanical therapy for the treatment of periodontitis in patients with type 2 DM show that periodontal conditions improve when mechanical periodontal therapy is performed [10,11,12,13]. However, impaired wound healing, a significant phenomenon in patients with DM [6], may lead to unfavorable results when conventional periodontal therapy is used in those patients. For this reason, an additional therapy that enhances wound healing, by modulating the host response toward bacterial injury, is of great interest for treating periodontitis in patients with type 2 DM [14,15,16].
Photobiomodulation (PBM), or low-level laser therapy (LLLT), has been used for a wide range of purposes, including wound healing acceleration [17, 18], tissue biostimulation [19, 20], and pain reduction [21]. This therapy involves the placement of a light source at the visible and near infrared of the spectrum at low density with the purpose of stimulating biological activities at the receptive tissue. Differently from other laser applications that result in ablative and thermal injury, PBM focuses on photon activity to stimulate chemical changes related to the mitochondrial respiratory chain [22]. Cytochrome c oxidase (Cox) is the terminal enzyme of the electron transport chain responsible for electron transfer from cytochrome c to molecular oxygen. This enzyme acts as a photoacceptor and transducer of photosignals in red-to-near-infrared range (620–1100 nm) [23]. The interaction between light and this chromophore increases electron transport, mitochondrial membrane potential, and adenosine triphosphate (ATP) production [24].
PBM has been proposed as an adjunct therapy to mechanical debridement for the treatment of periodontitis to reduce gingival inflammation and promote wound healing [25, 26]. Clinical investigations concerning the additional use of PBM to mechanical periodontal debridement reported improved clinical periodontal parameters and decreased pro-inflammatory markers in normoglycemic patients and controlled diabetic patients [27,28,29,30]. Moreover, favorable results were reported when PBM was applied to enhance wound healing on the oral mucosa of diabetic animals [31, 32]. Nevertheless, there is no consensus in the literature regarding the efficacy of PBM in the treatment of periodontitis in patients with type 2 DM.
The aim of this study was to compare the local effects of periodontal debridement with and without adjunct PBM for the treatment of periodontal pockets in patients with periodontitis and type 2 DM through a 1-year randomized clinical trial.
Materials and methods
Sample size calculation
Sample size calculation considered a mean probing depth (PD) reduction of 1 mm between groups, with a standard deviation of 0.8 mm. Seventeen periodontal pockets in each group would provide 95% power with a 5% significance level. With a hypothetical attrition rate of 15%, 19 periodontal pockets were included in each group [33].
Study population
Individuals with type 2 DM and moderate to severe generalized periodontitis [1], who volunteered to receive periodontal treatment, were selected from the population referred to the Periodontal Clinic at São Paulo State University (Unesp), Institute of Science and Technology (São José dos Campos, SP, Brazil). Detailed dental and medical records were obtained. Individuals who met the following inclusion criteria were selected: diagnosis of type 2 DM for ≥ 5 years; treatment of diabetes with oral hypoglycemic agents or insulin supplementation; glycated hemoglobin (HbA1c) levels from 6.5 to 11%; age ≥ 35; at least 15 teeth (excluding third molars and teeth indicated for extraction); and moderate to severe generalized periodontitis [1]. The exclusion criteria were medical conditions that required antibiotic prophylaxis, periodontal therapy in the previous 6 months, antimicrobial and/or anti-inflammatory therapies in the previous 6 months, systemic conditions other than diabetes that could affect the progression of periodontitis, the current use of medication that could interfere with the periodontal response to treatment, pregnancy or lactation, current smoking. Informed consent was obtained from all individual participants included in the study after a thorough explanation of the nature, risks, and benefits of the clinical investigations. The Institutional Review Board at São Paulo State University (Unesp), Institute of Science and Technology approved the study protocol (CAAE 28968714.7.0000.0077). The ClinicalTrials.gov identifier of the present study is NCT02817152.
Randomization and allocation concealment
In this split-mouth double-blind randomized clinical trial, two periodontal pockets with PD and clinical attachment level (CAL) ≥ 5 mm in single-rooted teeth from different quadrants were selected in each patient. The study coordinator (MPS) used a computer program to randomize the selected 38 periodontal pockets to a control group (ultrasonic periodontal debridement only) or a PBM group (ultrasonic periodontal debridement + photobiomodulation). For blinding, one investigator (NMRBA) was responsible for the treatment of the patients and another investigator (NCCS) was responsible for the clinical measurements. The randomization parameters and blinding were performed according to the CONSORT statement 2010 [34].
Treatment protocol
All patients received supragingival biofilm and calculus removal, extraction of hopeless teeth, dental decay removal, and provisional restoration, as well as oral hygiene instructions. After the initial therapy phase, all patients received full-mouth ultrasonic periodontal debridement (Cavitron, Dentsply, York, PA, USA) with specific inserts (UI25KSF10S, Hu-Friedy). All diseased sites were instrumented in one session and were checked with a periodontal probe for complete subgingival biofilm and calculus removal. After periodontal debridement, one previously selected periodontal pocket received additional PBM.
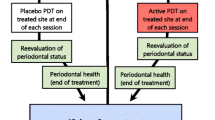
One previously randomly selected periodontal pocket with a PD and CAL ≥ 5 mm received PBM. The selected periodontal pocket received 20 s of continuous wave diode laser irradiation with a fiber optic of 600 μm in diameter (TheraLase, DMC Ltda, São Carlos, SP, Brazil), a wavelength of 660 nm, power of 0.03 W, fluency of 22 J/cm2, an area of 0.028 cm2, irradiation of 1.1 W/cm2, and total energy of 0.6 J. The application was performed in two points of laser irradiation (one buccal and one lingual) using punctual contact to reduce reflection, with the tip perpendicular to the gingival tissue [19] (Fig. 1).
Clinical parameters
Periodontal clinical measures were performed by one calibrated operator (NCCS), who was blinded to the treatment allocation. The examiner participated in a calibration exercise in which the PD and CAL of 10 patients were measured twice in a 24-h interval. Then, the measurement was submitted to an intraclass correction test. The agreement for variables was > 90%.
Clinical measures were performed before treatment (baseline), 3, 6, and 12 months after treatment. The primary outcome variable was mean PD. Secondary outcome variables were mean CAL, PD reduction, CAL gain, bleeding on probing (BoP), presence of supragingival biofilm (PI), and frequency of distribution of sites according to PD. All of the clinical measures were assessed using a manual probe (North Carolina-Hu-Friedy).
Statistical analysis
The clinical parameters were computed per subject and per selected pocket. The mean and standard deviation were calculated for each parameter. The normality of the data was analyzed according to the Shapiro–Wilk test. As the data did not present normal distribution for any of the evaluated variables, non-parametric tests were performed for each outcome. The Friedman test was used to compare differences in periodontal pocket parameters, such as mean PD, mean CAL, percentage of sites with BoP, percentage of sites with supragingival biofilm accumulation, PD reduction, and CAL gain. For the frequency of distribution of sites, Kruskal-Wallis test with Dunn’s multiple comparisons was performed for intragroup comparisons and Chi-square test was used to compare the differences between groups.
Results
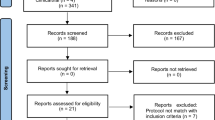
Thirty-eight periodontal pockets were selected in patients with periodontitis and type 2 DM. All the patients included in this study completed the follow-up of 12 months. Figure 2 shows the flowchart representation of the study. Patients were questioned about the adverse effects of laser therapy, such as discomfort, burning sensation, and pain. No adverse effect was reported.
Demographic data and full-mouth clinical parameters are presented in Table 1. The study population was composed of adults with generalized moderate to severe periodontitis and type 2 DM. Mean glycated hemoglobin (HbA1c) indicated that, on average, patients presented poor metabolic control at baseline, which increases the risk of periodontal disease progression. Full-mouth periodontal parameters showed that patients presented advanced stages of periodontitis and increased inflammatory profile, with more than 50% of sites presenting BoP.
The PBM group presented statistically significant changes in mean PD between baseline and all time points (p < 0.05), while for the control group, significant differences were observed at 6 and 12 months, but not at 3 months (p > 0.05). No statistically significant difference was observed when comparing the study groups (p > 0.05). Mean CAL showed statistically significant changes between baseline and 6 and 12 months for both groups (p < 0.05). PD reduction and CAL gain did not present statistically significant difference between groups (p > 0.05) (Table 2). Percentages of sites with BoP and Pl accumulation decreased over time for both groups (p < 0.01) with no statistically significant difference between groups (p > 0.05).
The frequency of distribution of sites according to PD was analyzed using Kruskal-Wallis test (intragroup) and Chi-square test (intergroup). Statistically significant differences between groups were observed for the percentage of pockets with PD 5–6 mm at 6 months (p < 0.01). For the intragroup analysis of deep pockets (PD ≥ 7 mm), the PBM group presented statistically significant reduction in the percentage of pockets at 3 months (p < 0.001), and this was also observed at 6 and 12 months (p < 0.001) (Table 3).
Discussion
The modest results obtained with current gold standard procedures for adjunctive therapies in the treatment of periodontal conditions have led to the investigation of PBM as a possible alternative. The use of PBM for the treatment of periodontitis has been discussed in clinical studies and reviews [25,26,27,28,29,30,31,32, 35,36,37,38,39]. Our study did not detect statistically significant differences between groups for mean PD, the primary outcome of this investigation. Intergroup difference in mean CAL, PD reduction, and CAL gain were also not observed. Although no significant differences were detected between groups, the control and PBM groups presented changes in PD and CAL over time, indicating that both treatments were effective in treating moderate and deep periodontal pockets in patients with type 2 DM. In a systematic review concerning the use of PBM as an adjunctive treatment for periodontitis in patients with type 2 DM, Abduljabbar et al. reported that all the clinical trials using PBM showed improved results in periodontal parameters [39]. However, the follow-up of these studies was up to 12 weeks. In addition, their analyses were based on full-mouth patient-centered parameters, while here we investigated variables related to periodontal pockets.
The frequency of distribution of PD analysis showed that the additional use of PBM decreased the percentage of moderate periodontal pockets at 6 months, with statistically significant difference between groups. This result indicates that the adjunctive use of PBM may be beneficial in the short term. However, at the 12-month follow-up, the significant differences were not sustained. This may be due to the immediate wound healing properties of laser therapy. With the purpose of evaluating tissue biostimulation for root coverage, Fernandes-Dias et al. compared the use of PBM as an adjunct treatment to connective tissue graft (CTG) to CTG alone. In a 6-month follow-up, the authors reported that PBM increased the percentage of complete root coverage when associated with CTG. However, when these patients were reevaluated in a 2-year follow-up, PBM did not present additional benefits for root coverage. The results of these studies indicate that there are additional benefits for low-level laser biostimulation in periodontal soft tissues in the short-term, but satisfactory outcomes seem to be equally achieved with or without PBM in longitudinal observation [20, 21]. Although these clinical trials aimed to treat a different periodontal condition than the present study, their findings are in agreement with our outcomes concerning long-term periodontal soft tissue stability.
For the initially deep pockets (PD ≥ 7 mm), PBM greatly decreased the percentage of periodontal pockets, from 47.37% at baseline to 5.26%, at 3 months. Interestingly, the three-month result was maintained at 6 and 12 months. In the control group, there was no statistically significant difference between baseline and 3 months, with 36.84% and 15.79% of pockets, respectively. At 6 months, the percentage of deep pockets decreased to 5.26%, but increased again to 10.53% at 12 months. These results suggest that PBM may provide additional benefits for the treatment of deep periodontal pockets and that these benefits may offer periodontal stability over time. This finding could be explained by in vitro and in vivo observations of fibroblast proliferation and collagen formation when PBM was applied to samples of diabetic cells and tissues [40, 41]. Obradović et al. [42] performed a histological evaluation of human periodontal tissue from diabetic and non-diabetic patients who received adjunct PBM to periodontal therapy. The authors reported that, after PBM application, diabetic periodontal tissue showed less edema, fewer inflammatory cells, and distinguished collagenization. Therefore, wound healing of deep periodontal pockets may be enhanced with PBM, decreasing the percentage of sites with deep periodontal pockets in the short term, and providing periodontal stability in the long-term.
Despite the number of clinical studies investigating the use of PBM for the treatment of periodontal disease already published, the literature still lacks full consensus on the subject. Different laser protocols, including laser parameters and delivery, play a central role in the divergence in study results [25,26,27,28]. Kreisler et al. [26] compared scaling and root planing (SRP) associated or not with PBM through a split-mouth clinical study. Twenty-two patients received PBM + SRP in one pocket and SRP alone in another pocket in the contralateral jaw. Laser protocol, as performed, used a GaAlAs diode laser; a wavelength of 809 nm; power of 1 W; for 10 s; and an optic fiber of 600 μm introduced into the periodontal pocket. An intergroup statistically significant difference was found for the PD and CAL, but not for the BoP, GI, and PI. On the other hand, Dukic et al. [27] investigated the effects of PBM through a split-mouth clinical trial. Thirty-five patients each received the treatments in two different periodontal pockets. The laser protocol was a diode laser; a wavelength of 980 nm; power of 2 W; for 20 s; with an optic fiber of 300 μm introduced into the periodontal pocket. PBM was performed in three sessions: days 1, 3, and 7 after SRP. No intergroup statistically significant difference was observed for any of the analyzed clinical parameters.
To evaluate the additional use of PBM for nonsurgical periodontal therapy in patients with type 2 DM, Koçak et al. [35] performed a parallel clinical trial in which 60 patients were randomly allocated to SRP or SRP + PBM. The laser protocol was a pulsed InGaAlP diode laser; a wavelength of 940 nm; power of 1.5 W; a fluency of 15 (pulse length) and 20 J/cm2 (pulse interval); for 20 s; with an optic fiber of 300 μm introduced into the periodontal pocket. PBM was performed in one single session. Although the PD, CAL, PI, and GI did not present intergroup statistically significant differences for the whole-mouth analysis, the PD and CAL showed statistically significant changes in deep pockets at 3 months. Patients were controlled diabetics with a mean HbA1c of 6.54 ± 0.66% (SRP) and 6.91 ± 0.79% (SRP + PBM) at baseline, which significantly reduced at 3 months.
A systematic review [38] pointed out that PBM tends to be more effective when the laser is applied on the oral epithelium, which could be observed in some clinical trials [19, 22, 27, 28]. Makhlouf et al. [28] investigated the use of adjunct PBM for SRP through a split-mouth clinical trial. Sixteen patients received PBM + SRP in one site and SRP alone in the contralateral site. The laser protocol was performed as described: a wavelength of 830 nm; power of 0.10 W; an area of 0.03 cm2; a fluency of 3 J/cm2; for 30 s; perpendicularly to the gingival tissue. PBM was performed in 10 sessions. An intergroup statistically significant difference was found for PD at 5 weeks and 12 weeks, but no statistically significant difference was found at any time point for the GI.
A recurrent question concerning PBM is the contribution of heat as secondary action of lasers, since thermal variance could be the true influencer on cell metabolism in the target tissue. To evaluate the role of this confounding factor, Wang et al. [43] performed a study in humans in which they measured time-dependent temperature increases by laser and by thermal stimulation. No relation could be determinate between hemodynamic and metabolic tissue effects when pure thermal stimulation was performed, whereas significant hemodynamic and metabolic responses were observed when laser was applied, demonstrating that PBM therapeutic properties did not result from tissue-heat interaction.
To the best of our knowledge, this was the first clinical trial with a follow-up of 12 months that evaluated the effects of PBM in periodontal pockets of patients with periodontitis and type 2 DM. The split-mouth design was chosen for this investigation so that the differences inherent to host response were suppressed, prioritizing differences between treatments. This measure is particularly relevant for patients who are likely to present systemic variations that may impact periodontal tissue and that cannot be managed or controlled by periodontists (alterations due to medication, weight gain/loss, etc.). The main weakness of this study is that analyses regarding inflammatory markers were not performed, which limits our understanding of periodontal tissue response to PBM in patients with periodontitis and DM. Future comparisons should focus on other variations of the promising protocols presented in the literature, such as an increased number of application sessions or multiple application points.
In conclusion, the results showed that the PBM protocol used in this study did not provide significant changes PD and CAL in periodontal pockets when compared to mechanical therapy only in patients with periodontitis and type 2 DM. However, PBM was more effective in reducing the percentage of moderate periodontal pockets at 6 months. Additionally, PBM decreased the percentage of deep pockets at 3 months, and this result was maintained at 6 and 12 months.
References
Armitage GC (1999) Development of a classification system for periodontal diseases and conditions. Ann Periodontol 4:1–6
Cobb CM (1996) Non-surgical pocket therapy: mechanical. Ann Periodontol 1:443–490. https://doi.org/10.1902/annals.1996.1.1.443
Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS (1997) Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Peiodontol 2000 14:216–248
Chapple ILC, Genco R, on behalf of the working group 2 of the joint EFP/AAP workshop (2013) Diabetes and periodontal diseases: consensus report of the joint EFP/AAP workshop on periodontitis and systemic diseases. J Periodontol 84:S106–S112. https://doi.org/10.1902/jop.2013.1340011
Lalla E, Lamster IB, Stern DM, Schmidt AM (2001) Receptor for advanced glycation end products, inflammation, and accelerated periodontal disease in diabetes: mechanisms and insights into therapeutics modalities. Ann Periodontol 6(1):113–118
Nassar H, Kantarci A, Van Dyke TE (2007) Diabetic periodontitis: a model for activated innate immunity and impaired resolution of inflammation. Periodontol 2000 43:233–244
Duarte PM, Bezerra JP, Miranda TS, Feres M, Chambrone L, Shaddox LM (2014) Local levels of inflammatory mediators in uncontrolled type 2 diabetic subjects with chronic periodontitis. J Clin Periodontol 41(1):11–18. https://doi.org/10.1111/jcpe.12179
Costa PP, Trevisan GL, Macedo GO, Palioto DB, Souza SL, Grisi MF, Novaes AB Jr, Taba M Jr (2010) Salivary interleukin-6, matrix metalloproteinase-8, and osteoprotegerin in patients with periodontitis and diabetes. J Periodontol 81(3):384–391. https://doi.org/10.1902/jop.2009.090510
Bastos MF, Tucci MA, de Siqueira A, de Faveri M, Figueiredo LC, Vallim PC, Duarte PM (2016) Diabetes may affect the expression of matrix metalloproteinase and their inhibitors more than smoking in chronic periodontitis. J Periodontal Res. https://doi.org/10.1111/jre.12394
Westfelt E, Rylander H, Blohmé G, Jonasson P, Lindhe J (1996) The effect of periodontal therapy in diabetics. Results after 5 years. J Clin Periodontol 23:92–100
Kiran M, Arpak N, Unsal E, Erdogan MF (2005) The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. J Clin Periodontol 32:266–272. https://doi.org/10.1111/j.1600-051X.2005.00658.x
Singh S, Kumar V, Kumar S, Subbappa A (2008) The effect of periodontal therapy on the improvement of glycaemic control in patients with type 2 diabetes mellitus: a randomized controlled clinical trial. Int J Diabetes Dev Ctries 28:38–44. https://doi.org/10.4103/0973-3930.43097
Koromantzos PA, Makrilakis K, Dereka X, Katsilambros N, Vrotsos IA, Madianos PN (2011) A randomized, controlled trial on the effect of non-surgical periodontal therapy in patients with type 2 diabetes. Part I: effect on periodontal status and glycaemic control. J Clin Periodontol 38:142–147. https://doi.org/10.1111/j.1600-051X.2010.01652.x
Santos VR, Lima JA, Miranda TS, Gonçalves TE, Figueiredo LC, Faveri M, Duarte PM (2013) Full-mouth disinfections a therapeutic protocol for type-2 diabetic subjects with chronic periodontitis: twelve-month outcomes. A randomized clinical trial. J Clin Periodontol 40(2):155–162. https://doi.org/10.1111/jcpe.12040
Castro dos Santos NC, Andere NM, Araujo CF, de Marco AC, Dos Santos LM, Jardini MA, Santamaria MP (2016) Local adjunct effect of antimicrobial photodynamic therapy for the treatment of chronic periodontitis in type 2 diabetics: split-mouth double-blind randomized controlled clinical trial. Lasers Med Sci 31(8):1633–1640. https://doi.org/10.1007/s10103-016-2030-8
Tamashiro NS, Duarte PM, Miranda TS, Maciel SS, Figueiredo LC, Faveri M, Feres M. Amoxicillin plus metronidazole therapy for patients with periodontitis and type 2 diabetes: a 2-year randomized controlled trial. J Dent Res 2016;95(7):829–836. doi: 10.1177/0022034516639274
Dias SB, Fonseca MV, Dos Santos NC, Mathias IF, Martinho FC, Junior MS, Jardini MA, Santamaria MP (2015) Effect of GaAIAs low-level laser therapy on the healing of human palate mucosa after connective tissue graft harvesting: randomized clinical trial. Lasers Med Sci 30(6):1695–1702. https://doi.org/10.1007/s10103-014-1685-2
da Silva Neves FL, Silveira CA, Dias SB, Santamaria Júnior M, de Marco AC, Kerbauy WD, de Melo Filho AB, Jardini MA, Santamaria MP (2016) Comparison of two power densities on the healing of palatal wounds after connective tissue graft removal: randomized clinical trial. Lasers Med Sci 31(7):1371–1378. https://doi.org/10.1007/s10103-016-1988-6
Fernandes-Dias SB, de Marco AC, Santamaria M Jr, Kerbauy WD, Jardini MA, Santamaria MP (2015) Connective tissue graft associated or not with low laser therapy to treat gingival recession: randomized clinical trial. J Clin Periodontol 42(1):54–61. https://doi.org/10.1111/jcpe.12328
Santamaria MP, Fernandes-Dias SB, Araújo CF, da Silva Neves FL, Mathias IF, Rebelato Bechara Andere NM, Neves Jardini MA (2017) 2-year assessment of tissue bioestimulation with low-level laser on the outcomes of connective tissue graft in the treatment of single gingival recession. Randomized clinical trial. J Periodontol 88(4):320-328. https://doi.org/10.1902/jop.2016.160391
Carrillo JS, Calatayud J, Manso FJ, Barberia E, Martinez JM, Donado M (1990) A randomized double-blind clinical trial on the effectiveness of helium-neon laser in the prevention of pain, swelling and trismus after removal of impacted third molars. Int Dent J 40(1):31–36
Hennessy M, Hamblin MR (2017) Photobiomodulation in the brain: a new paradigm. J Opt 19(1):013003. https://doi.org/10.1088/2040-8986/19/1/013003
Wang X, Tian F, Soni SS, Gonzalez-Lime F, Liu H Interplay between up-regulation of cytochrome-c-oxidase and hemoglobin oxygenation induced by near-infrared laser. Sci Rep 3(6):30540. https://doi.org/10.1038/srep30540
de Freitas LF, Hamblin MR (2016) Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron 22(3). https://doi.org/10.1109/JSTQE.2016.2561201
Moritz A, Schoop U, Goharkhay K, Schauer P, Doertbudak O, Wernisch J, Sperr W (1998) Treatment of periodontal pockets with a diode laser. Lasers Surg Med 22(5):302–311
Kreisler M, Al Haj H, d'Hoedt B (2005) Clinical efficacy of semiconductor laser application as an adjunct to conventional scaling and root planing. Lasers Surg Med 37(5):350–355
Dukić W, Bago I, Aurer A, Roguljić M (2013) Clinical effectiveness of diode laser therapy as an adjunct to non-surgical periodontal treatment: a randomized clinical study. J Periodontol 84(8):1111–1117. https://doi.org/10.1902/jop.2012.110708
Makhlouf M, Dahaba MM, Tunér J, Eissa SA, Harhash TA (2012) Effect of adjunctive low-level laser therapy (LLLT) on nonsurgical treatment of chronic periodontitis. Photomed Laser Surg 30(3):160–166. https://doi.org/10.1089/pho.2011.3069
Saglam M, Kantarci A, Dundar N, Hakki SS (2014) Clinical and biochemical effects of diode laser as an adjunct to nonsurgical treatment of chronic periodontitis: a randomized, controlled clinical trial. Lasers Med Sci 29(1):37–46. https://doi.org/10.1007/s10103-012-1230-0
Koçak E, Sağlam M, Kayış SA, Dündar N, Kebapçılar L, Loos BG, Hakkı SS (2016) Nonsurgical periodontal therapy with/without diode laser modulates metabolic control of type 2 diabetics with periodontitis: a randomized clinical trial. Lasers Med Sci 31(2):343–353. https://doi.org/10.1007/s10103-016-1868-0
Fekrazad R, Mirmoezzi A, Kalhori KA, Arany P (2015) The effect of red, green and blue lasers on healing of oral wounds in diabetic rats. J Photochem Photobiol B 148:242–245. https://doi.org/10.1016/j.jphotobiol.2015.04.018
Fahimipour F, Houshmand B, Alemi P, Asnaashari M, Tafti MA, Akhoundikharanagh F, Farashah SE, Aminisharifabad M, Korani AS, Mahdian M, Bastami F, Tahriri M (2016) The effect of He-Ne and Ga-Al-As lasers on the healing of oral mucosa in diabetic mice. J Photochem Photobiol B 159:149–154. https://doi.org/10.1016/j.jphotobiol.2016.03.020
Julius SA (2004) Sample sizes for clinical trials with Normal data. Stat Med 23:1921–1986
Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ et al (2010) CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol 63:e1–e37. https://doi.org/10.1016/j.jclinepi.2010.03.004
Koçak E, Sağlam M, Kayış SA, Dündar N, Kebapçılar L, Loos BG, Hakkı SS (2016) Nonsurgical periodontal therapy with/without diode laser modulates metabolic control of type 2 diabetics in chronic periodontitis: a randomized clinical trial. Lasers Med Sci 31(2):343–353. https://doi.org/10.1007/s10103-016-1868-0
Schwarz F, Aoki A, Sculean A, Becker J (2009) The impact of laser application on periodontal and peri-implant wound healing. Periodontol 2000 51:79–108. https://doi.org/10.1111/j.1600-0757.2009.00301.x
Cobb MC (2017) Lasers and the treatment of periodontitis: the essence and the noise. Periodontol 2000 75(1):205–295. https://doi.org/10.1111/pdr.12137
Cheng Y, Chen JW, Ge MK, Zhou ZY, Yin X, Zou SJ (2016) Efficacy of adjunctive laser in non-surgical periodontal treatment: a systematic review and meta-analysis. Lasers Med Sci 31(1):151–163. https://doi.org/10.1007/s10103-015-1795-5
Abduljabbar T, Javed F, Shah A, Samer MS, Vohra F, Akram A (2017) Role of lasers as an adjunct to scaling and root planing in patients with type 2 diabetes mellitus: a systematic review. Lasers Med Sci 32(2):449–459. https://doi.org/10.1007/s10103-016-2086-5
Maiya GA, Kumar P, Rao L (2005) Effect of low intensity helium-neon (He-Ne) laser irradiation on diabetic wound healing dynamcs. Photomed Laser Surg 23(2):187–190
Reddy GK, Stehno-Bittel L, Enwemeka CS (2001) Laser photostimulation accelerates wound healing in diabetic rats. Wound Repair Regen 9(3):248–255
Obradović R, Kesić L, Mihailović D, Jovanović G, Antić S, Brkić Z (2012) Low-level lasers as an adjunct in periodontal therapy in patients with diabetes mellitus. Diabetes Technol Ther 14(9):799–803. https://doi.org/10.1089/dia.2012.0027
Wang X, Reddy DD, Nalawade SS, Pal S, Gonzalez-Lima F, Liu H (2018) Impact of heat on metabolic and hemodynamic changes in transcranial infrared laser stimulation measured by broadband near-infrared spectroscopy. Neurophotonics. 5(1):011004. https://doi.org/10.1117/1.NPh.5.1.011004
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Conflict of interest
The authors declare that they have no conflict of interest. This study did not receive any funding.
Declarations of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Castro dos Santos, N., Andere, N.M.R.B., Miguel, M.M.V. et al. Photobiomodulation for the treatment of periodontal pockets in patients with type 2 diabetes: 1-year results of a randomized clinical trial. Lasers Med Sci 34, 1897–1904 (2019). https://doi.org/10.1007/s10103-019-02799-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-019-02799-0