Abstract
The epidermal growth factor receptor (EGFR) signaling pathway may be involved in cell activation and may influence the neuronal microenvironment, microglia activation, and production of proinflammatory cytokines. Arginase and nitric oxide synthase (NOS) both use l-arginine as a common substrate. Decreasing the arginase expression may increase l-arginine consumption by NOS and increase nitric oxide (NO) synthesis. Intravenous laser blood irradiation (ILBI) is an effective systemic treatment for different pathologies including diabetes mellitus. Previous studies have shown that low-level laser therapy can have an effect on the release of certain cytokines and growth factors. The aim of this study was to evaluate the effects of ILBI on the expression of arginase and epidermal growth factor receptor in type 2 diabetic patients. We used 630 nm red laser light, 1.5 mW, continuous mode, intravenously for 30 min in 13 type 2 diabetic patients and compared their blood samples using the flow cytometry technique, before and after ILBI. The difference between the percentage of cells before and after therapy was analyzed using repeated-measures ANOVA, and the relationship between EGFR and arginase expression in blood and tissue was evaluated by calculating the Pearson correlation coefficient. We found a significant decrease in the expression of both arginase- and EGFR-positive cells after laser therapy (P < 0.01). In conclusion, laser therapy may have a beneficial effect for diabetic patients via decreasing arginase expression and activation of the NOS/NO pathway which increases NO production and vasodilation, and decreasing EGFR expression which may reduce neuroinflammation and its secondary damages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic diseases including diabetes and cardiovascular diseases are one of the main public health problems [1], and atherosclerosis is a hallmark in the development of myocardial infarction, stroke, and other cardiovascular disorders [1]. Micro- and macrovascular angiopathy is one of the main complications in long-term-duration diabetes mellitus [2]. Since 40 years ago, low-level laser therapy (LLLT) or laser photobiomodulation has been used in experimental and clinical fields as an adjuvant therapy. LLLT can stimulate or inhibit disturbed biological functions and normalize them. LLLT has been reported to accelerate wound healing, collagen production, and modulation of the immune system. It has been used for various medical conditions including wound healing, dermatological disorders, pain, inflammation, neurologic damage, blood disorders, and musculoskeletal complications and can modulate chronic stress including stress due to chronic disease [3–7]. This technique is non-thermal and non-cytotoxic, using continuous or pulsed red or near-infrared light (600–1100 nm) with output power of 1–500 mW [8]. One of the methods for laser irradiation is intravenous or intravascular laser blood irradiation (ILBI) which has been used in Russia, China, Germany, and Iran since more than 20 years ago [7, 9–12]. This is a safe and cost-benefit treatment with systemic effects. In this method, laser light directly irradiates blood via a sterile disposable catheter. Two millimeters of the fiber optic enters the vein and irradiates the circulating blood. This procedure is just like serum injection. As ILBI can produce systemic effects, it can be effective in pathologies like diabetes which has generalized complications. Many functions in diabetic patients are impaired and laser irradiation may affect these impairments [9, 10, 13].
Laser light can activate microcirculation, angiogenesis, and regeneration. It can regulate the expression of growth factors including epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF) [14–16]. It seems that laser light can increase production of arginine and nitric oxide [4] and affects several signaling pathways [17]. He-Ne laser can increase the endothelial NO synthase gene and protein expression and NO secretion in humans [18].
Most studies highlight the role of nitric oxide on endothelial dysfunction as the main mechanism in developing cardiovascular disease [19]. Nitric oxide plays a key role in decreasing vascular tone [2]. Hyper production of free radicals decreases the production of nitric oxide and is suggested to be responsible for endothelial dysfunction in diabetes [20]. l-Arginine is an amino acid which participates in the wound-healing process. This semi-essential amino acid is a common substrate for arginase enzyme and nitric oxide synthase (NOS) [21]. It can be metabolized by one of these two pathways, arginase or nitric oxide synthase. If l-arginine is metabolized by the NOS enzyme, the result is nitric oxide (NO) and l-citrolline formation. NO is a free radical which is involved in endothelium-dependent relaxation of vascular smooth muscle [22]. l-Arginine can be metabolized by arginase too. Arginase is a manganese-metallo-enzyme that hydrolyzes l-arginine to urea and l-ornithine [23]. Arginase uses the same substrate for the known NOS isoforms: endothelial NOS, neuronal NOS, and inducible NOS [24]. Increasing the arginase expression may increase l-arginine consumption and convert it to l-ornithine and urea; thus, available l-arginine for NO synthesis by NOS reduces. Several studies show increasing activity of arginase in diabetes mellitus type 2 and hypertension, which proposes the critical role of this enzyme in the pathogenesis of cardiovascular disorders [25]. Studies showed that NOS/NO has an important role in many VEGF-induced functions. VEGF induces the release of NO from vascular endothelial cells [26, 27]. A balance in the NOS/l-arginine/arginase axis is necessary for maintenance of NO homeostatic levels. There are two main mechanisms for reducing bioactive NO: (1) reduction of its synthesis by NOS and (2) increased oxidative reactivation of NO by reactive oxygen species (ROS) which leads to cardiovascular impairment [28]. Laser irradiation can affect both pathways according to the body condition and balance the axis [29]. Studies show that laser light 350, 420, 470, and 760 nm can affect the turnover of arginine to NO [29].
One of the most important endogenous growth factors in the wound-healing process is epidermal growth factor (EGF) [30]. Signaling through epidermal growth factor receptors is essential for fundamental cellular functions including differentiation, growth, migration, and proliferation. The EGF-related peptides bind to ErbB receptors and induce the formation of homo- and hetero-dimers, which stimulates activation of intrinsic kinase and induces intracellular signaling pathways. In addition, Erbs has an important role in embryogenesis; development of cardiomyocytes; muscle regeneration; development of skin, hair, and eyes; and also cancer [31]. EGF-related peptide binds to the ErbB receptor, activates intrinsic kinase, and stimulates intrinsic signaling pathways [31]. Activation of the tyrosine kinase of the receptor induces signal transduction epidermal growth factor receptor (EGFR) and activates downstream signaling molecules [32].
EGFR has key roles in regulating cell activation. Downregulation of EGFR decreases the EGFR/MAPK cascade and regulates inflammation. EGFR signaling plays roles in several central nervous system disorders and ischemia [33]. Reduction of EGFR expression can decrease microglia activation and IL-1β and TNF-α production [34].
EGFR overexpression also has been reported in some cancers including breast cancer, small cell lung cancer, and glioblastoma. It seems that an overexpression can induce a transformation of the cell line into malignant phenotype [35, 36].
Our previous studies showed significant effects of LLLT on wound healing, expression of genes involved in wound healing, neuropathy, and metabonomics of the blood in diabetes [7, 9, 10, 15, 37]. In this study, for the first time, we investigate the effects of low-level laser irradiation on the expression of arginase and EGFR after intravenous laser irradiation in diabetic patients.
Materials and methods
After approval of the ethics committee of Tehran University of Medical Sciences, 13 type 2 diabetic patients who were referred to the laser clinic of Milad Hospital and who agreed to participate in our study were selected. After consent was given by the patients, non-fasting venous blood samples were collected in standard 5-ml sodium heparin tubes for flow cytometry study before and after the laser therapy. ILBI was applied intravenously (the laser fiber was inserted into the cubital vein in the forearm using a catheter, the catheter enters 2–3 cm2 into the vein, and 2 mm of the fiber is exposed at the tip of the catheter) with a 1.5-mW, continuous, 630-nm laser therapy apparatus (Mulat, Tecknica Co., Moscow, Russia) for 30 min. The power output was 1.5 mW and the spot size was 0.01 cm2. This protocol was designed according to our previous experiments [7]. Another venous blood sample was collected in a standard 5-ml sodium heparin tube 30 min after ILBI. The samples were stored at −20 °C until analysis and thawed prior to use.
Flow cytometry
For cell extraction, tissue of foot debris and lymphocytes from whole blood were, shortly, cultured and incubated with RPMI media (Sigma-Aldrich, St. Louis, MO, USA) for 30 min at 37 °C. Cells were washed twice in KCl 0.075 M and fixed in methanol (Merck KGaA, Darmstadt, Germany).
Staining of the cells was performed by monoclonal anti-arginase liver type, isotype IgG1 (US Biological, Swampscott, MA 01907 USA), and EGFR antibody (528) as a mouse monoclonal IgG2a (Santa Cruz Biotechnology Inc., 69115, Heidelberg, Germany).
The cells were incubated at 4 °C for 25 min and were washed twice by PBS. For the next step, secondary antibody goat anti-mouse IgG2a/R-Pe (AbDSerotec, MorphoSys UK Ltd, Oxford, UK) and antibody anti-mouse IgG1/Cy5 (Rockland, Gilbertsville, PA 19525 USA) were added and incubated for 25 min; then, the cells were washed with PBS. Mouse IgG2a/PE-Cy5 and IgG1/R-Pe (Dakocytomation, Glostrup, Denmark) were used as negative control for the same population.
The Partec-Denmark flow cytometry apparatus (Sysmex and Partec, Denemark, CyFlow® Cube) and LEICA, DM RXA2-fluorescence microscope (Wetzlar and Mannheim, Germany) were used for cellular assays.
Results
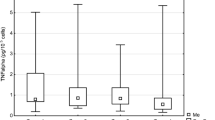
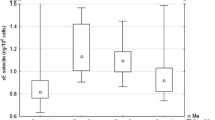
The number of cells with each marker was expressed and analyzed as percent (Tables 1 and 2). The difference between the percentage of cells before and after laser therapy and the effect of sex and age group were assessed using repeated-measures ANOVA (The age of patients was entered into the equation as a two-level categorized variable: <60 and ≥60 years) (Table 3). The relationship between percentage of EGFR- and arginase-positive cells in blood and tissue was evaluated by calculating the Pearson correlation coefficient (Figs. 1, 2, 3, and 4). All statistical analyses were conducted by SPSS version 22, and an error probability of P < 0.05 was regarded as significant.
The effect of sex and age in the reduction of the percentage of positive cells; a (left figure) arginase-positive cells before and after laser therapy in male and female patients below 60 years old, b (right figure) arginase-positive cells before and after laser therapy in male and female patients above 60 years old, c (left figure) EGFR-positive cells before and after laser therapy in male and female patients below 60 years old, d (right figure) EGFR-positive cells before and after laser therapy in male and female patients above 60 years old
Flow cytometry of the peripheral blood from a patient affected with diabetes before laser therapy. FL2 the cells conjugated with Pe-cy5 and reflect protein expression of arginase. FL3 the cells conjugated with R-Pe and reflect protein expression of epidermal growth factor. Particles, 695; R1, 572 (82.30 %); FL2 (arginase), 35 (1.92 %); FL3 (EGFR), 15 (2.62 %)
Two of 13 patients were smokers (20 %). Four of 10 patients had A+ blood type (40 %), four of them had O+ blood type (40 %), just one patient was A−, and one was AB+ (10). The blood type data was not available for other patients. The mean difference between the percentage of positive cells before and after treatment was supposed to be 2. Taking a standard deviation of 2, the power of statistical analysis for a sample size of 13 was calculated using open epi version 3.03a. The power for this sample size was approximately 72 %.
Discussion
The main finding of this study is that intravenous laser treatment in patients with diabetes mellitus type 2 affects significantly arginase and EGFR at the level of gene expression and downregulates them, using the flow cytometry technique. Decreasing arginase expression may activate the NOS/NO pathway and induce vasodilatation in patients with macro- and microangiopathy including patients with diabetes. It is suggested that some diabetic complications including endothelial, cardiovascular, and erectile dysfunction are related to increased arginase gene expression and reduction of NO production [38, 39]. Important factors that decrease NO production in these patients are reduction of l-arginine, activity of eNOS [40], and upregulation of arginase. Decreasing EGFR expression may influence the neuronal microenvironment, microglia activation, and production of proinflammatory cytokines [34]. EGFR regulates cell activation and its downregulation regulates inflammation by decreasing the EGFR/MAPK cascade. EGFR signaling plays roles in several central nervous system disorders and ischemia [33]. Our previous study showed that LLLT had a significant effect on diabetic neuropathy, in which this mechanism may be involved [37]. Our study showed that LLLT affects the expression of genes involved in wound healing in diabetic mice. The results showed a significant increase in fibroblast growth factor (FGF) and a downregulation of vascular endothelial growth factor which was not statistically significant, which may be due to an insufficient sample size [15]. Our previous clinical studies showed that LLLT is an effective therapeutic method for diabetic foot ulcer, neuropathy, and regulating metabolism and blood sugar in these patients [7, 10, 37]. These observations suggest that gene expression regulation is a key mechanism in treating diabetic complications using LLLT.
Our results in this study also showed that the basic level of arginase and EGFR is higher in diabetic women below age 60; however, after age 60, the basic level of both of these markers is higher in men. Although we did not find any reason for this finding in the literature review, the results showed that laser irradiation has a greater effect on the group with the higher basic level of these markers and a more gentle effect on the lower basic levels. It may suggest that laser can modulate cell pathways and bring them to normal functions through a remarkable manner.
Romero et al. reported that arginase I competes with NOS for l-arginine and could cause coronary vascular dysfunction in diabetic patients [41]. Shemyakin et al. reported that arginase inhibition significantly improved endothelial function in patients with diabetes mellitus type 2 and coronary artery disease [39]. Trinity et al. compared the expression of arginase in cavernosal tissue of healthy people and diabetic patients with erectile dysfunction. They reported that arginase II gene expression is higher in diabetic patients. They suggested that elevated expression of arginase II in cavernosal tissue of diabetic patients may play a role in erectile dysfunction and other complications related to NO production in diabetic patients [38].
Qu and colleagues showed that EGFR blockade inhibited the EGFR/MAPK cascade, reduced production of cytokines in microglia, and modulated inflammatory response after spinal cord injury (SCI). They reported that inhibition of EGFR phosphorylation decreased the production of TNF-α and IL-1β in activated microglia [34]. Erschbamer and colleagues reported that in experimental spinal cord injury, inhibition of EGFR improves motor and sensory functions and improves bladder function [33]. Koprivica and colleagues suggested that EGFR inhibition promotes nerve regeneration in optic nerve injury [42]. Liu et al. reported that upregulation of EGFR promotes NOS-2 in astrocytes of the human optic nerve and excessive NO causes pressure damage in the optic nerve [42]. Furthermore, cancer studies showed that EGFR inhibitors may normalize vascular function [43].
Several studies suggested the effects of LLLT on gene expression. Ogita and colleagues studied protein expression and cell proliferation induced by low-level Er:YAG laser irradiation in human gingival fibroblasts by proteomics analysis. They showed that LLLT can promote human gingival fibroblast proliferation and induce protein expression and upregulation of galectin-7 which contributes to cell proliferation [44]. Peplow and colleagues studied the effects of laser irradiation on the release of growth factors, cytokines, and gene expression in animal and human cell cultures in a systemic review article. They concluded that laser therapy can modulate gene expression and the release of growth factors and cytokines in cell culture; however, further clinical studies are needed [45]. Zhang and colleagues used the cDNA microarray technique to evaluate the effects of laser irradiation on the gene expression profile in human fibroblasts. They reported that irradiation of an optimum dose of red light can affect the expression of 111 genes. Most of these genes play roles in enhancement of cell proliferation, directly or indirectly [46]. Conlan and colleagues showed in a review article that red light laser can enhance basic fibroblast growth factor [47]. In our previous study, we evaluated the effect of laser therapy on the expression of VEGF, PDGF, and FGF in diabetic mice. The results showed that laser therapy can significantly increase FGF expression. It decreased VEGF and increased PDGF but these changes were not statistically significant [15].
Our previous studies indicated the significant effect of LLLT in complications including neuropathy and wound healing and blood sugar regulation in diabetes [7, 9, 10, 15, 37]. Our results showed that using LLLT for treating diabetic foot ulcers was significantly effective (Fig. 5, adopted from reference [10], Figs. 6 and 7) [7].
Flow cytometry of the peripheral blood from a patient affected with diabetes after laser therapy. FL2 the cells conjugated with Pe-cy5 and reflect protein expression of arginase. FL3 the cells conjugated with R-Pe and reflect protein expression of epidermal growth factor. Particles, 5001; R1, 3118 (62.35 %); FL2 (arginase), 29 (0.93 %); FL3 (EGFR), 37 (1.19 %)
Flow cytometry of the foot debris from a patient affected with diabetes before laser therapy. FL2 the cells conjugated with Pe-cy5 and reflect protein expression of arginase. FL3 the cells conjugated with R-Pe and reflect protein expression of epidermal growth factor. Particles, 2270; R1, 2111 (93.00 %); FL2 (arginase), 348 (16.49 %); FL3 (EGFR), 471 (22.31 %)
In another clinical trial, we did a metobonomics study on the serum of the blood of the same patient who participated in the present study (“Modifying Effect of Intravenous Laser Therapy on the Protein Expression of Arginase and Epidermal Growth Factor Receptor in Type 2 Diabetic Patients”). Our results showed that ILBI significantly decreased glucose, glucose 6 phosphate, dehydroascorbic acid, R-3-hydroxybutyric acid, l-histidine, and l-alanine and significantly increased the l-arginine level in blood. Blood sugar (BS) in these patients also reduced significantly (P < 0.05) [9]. We also compared the effects of blue and red intravenous laser on the blood sugar level in diabetic patients. Both red and blue laser lights decreased the BS level significantly (P < 0.0001), but we did not find a significant difference between them [10]. Our clinical trial on the efficacy of LLLT in the neuropathy of diabetic patients clearly demonstrated the significant effect of LLLT on the improvement of nerve conduction velocity in diabetic distal symmetric polyneuropathy [37]. These findings support the therapeutic potential of low-level lasers in diabetic patients.
Our novel findings in this research were as follows: (1) Laser therapy has significant effects on downregulation of arginase and EGFR expression; (2) the basic level of arginase and EGFR is higher in women below age 60; however, after age 60, the basic level of both of these markers is higher in men; (3) as Fig. 4 illustrates, laser irradiation has a greater effect on the group with a higher basic level of these markers and a more gentle effect on lower basic levels. It may suggest that laser can modulate cell pathways and bring them to normal functions through a remarkable manner.
Conclusion
In summary, we report that intravenous laser therapy significantly decreases the expression of arginase and EGFR in diabetic patients. Downregulation of arginase and increasing the metabolism of l-arginine by NOS which induces NO production may be one of the mechanisms of vasodilation that occurs after laser therapy. Our results also showed a decrease of EGFR expression which can explain the effects of laser therapy on the improvement of neuropathy in diabetic patients. We also found a relationship between basic levels of studied markers and the intensity of laser effects that may be explained by regulatory effects of laser therapy. As literature review showed, arginase and EGFR upregulation has a significant role in diabetic complications including endothelial, cardiovascular, and sexual disorders and laser therapy may regulate the expression of these genes. Future studies for measuring clinical effects are needed.
References
Libby P, Ridker PM, Hansson GK (2011) Progress and challenges in translating the biology of atherosclerosis. Nature 473(7347):317–325
Group UPDS (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352(9131):837–853
Abergel RP, Meeker CA, Lam TS, Dwyer RM, Lesavoy MA, Uitto J (1984) Control of connective tissue metabolism by lasers: recent developments and future prospects. J Am Acad Dermatol 11(6):1142–1150
Lyons RF, Abergel RP, White RA, Dwyer RM, Castel JC, Uitto J (1987) Biostimulation of wound healing in vivo by a helium-neon laser. Ann Plast Surg 18(1):47–50
Rochkind S, Rousso M, Nissan M, Villarreal M, Barr‐Nea L, Rees D (1989) Systemic effects of low‐power laser irradiation on the peripheral and central nervous system, cutaneous wounds, and burns. Lasers Surg Med 9(2):174–182
Liu TC-Y, Liu Y-Y, Wei E-X, Li F-H (2012) Photobiomodulation on stress. Int J Photoenerg
Kazemi-Khoo N (2006) Successful treatment of diabetic foot ulcers with low-level laser therapy. Foot 16(4):184–187
AlGhamdi KM, Kumar A, Moussa NA (2012) Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med Sci 27(1):237–249
Khoo NK, Iravani A, Arjmand M, Vahabi F, Lajevardi M, Akrami S, Zamani Z (2013) A metabolomic study on the effect of intravascular laser blood irradiation on type 2 diabetic patients. Lasers Med Sci 28(6):1527–1532
KazemiKhoo N, Ansari F (2015) Blue or red: which intravascular laser light has more effects in diabetic patients? Lasers Med Sci 30(1):363–366
Chenzhong Y, Daping F, Xiaoning Z, Zhengrong H, Zuxuan Z (1998) Biological effects of intravascular low level laser irradiation with five different wave length. Appl Laser 2:012
Weber M, Fußgänger-May T, Wolf T (2007) The intravenous laser blood irradiation. Introduction of a new therapy. Ger J Acupunct Relat Tech 50(3):12–23
Gasparyan L (2003) Laser irradiation of the blood. Laser Partner-Clinixperience-All Volumes-2003:1–4
Gasparyan LV, Brill G, Makela AM Activation of angiogenesis under influence of red low level laser radiation. In: Laser Florence 2004, 2005. Int Soc Optics Photonics:596806-596806-596806
Khoo NK, Shokrgozar MA, Kashani IR, Amanzadeh A, Mostafavi E, Sanati H, Habibi L, Talebi S, Abouzaripour M, Akrami SM (2014) In vitro therapeutic effects of low level laser at mRNA level on the release of skin growth factors from fibroblasts in diabetic mice. Avicenna J Med Biotechnol 6(2):113
Kassab KN, Hassan AI, Khater MS, Elfouly SA (2013) Evaluating the regenerative potential of low level laser and/or epidermal growth factor therapies in cisplatin induced acute renal failure in rats. Int J Med Med Sci 46(1):1134
Gao X, Xing D (2009) Molecular mechanisms of cell proliferation induced by low power laser irradiation. J Biomed Sci 16(4):1–16
Chen C-H, Hung H-S, S-h H (2008) Low-energy laser irradiation increases endothelial cell proliferation, migration, and eNOS gene expression possibly via PI3K signal pathway. Lasers Surg Med 40(1):46
Lakin RO, Zhu W, Feiten L, Kashyap VS (2013) Techniques to harvest diseased human peripheral arteries and measure endothelial function in an ex vivo model. J Vasc Surg 58(2):470–477
Tesfamariam B, Cohen RA (1992) Free radicals mediate endothelial cell dysfunction caused by elevated glucose. Am J Phys Heart Circ Phys 263(2):H321–H326
Durante W, Johnson FK, Johnson RA (2007) Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol 34(9):906–911
Appleton I, Tomlinson A, Willoughby DA (1996) Induction of cyclo-oxygenase and nitric oxide synthase in inflammation. Adv Pharmacol (San Diego, Calif) 35:27
Ash DE, Cox JD, Christianson DW (2000) Arginase: a binuclear manganese metalloenzyme. Met Ions Biol Syst 37:407–428
Sikka G, Pandey D, Bhuniya AK, Steppan J, Armstrong D, Santhanam L, Nyhan D, Berkowitz DE (2013) Contribution of arginase activation to vascular dysfunction in cigarette smoking. Atherosclerosis 231(1):91–94
Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, Thunnissen FB, Farver C, Hazen SL, Jennings C (2004) Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J 18(14):1746–1748
van der Zee R, Murohara T, Luo Z, Zollmann F, Passeri J, Lekutat C, Isner JM (1997) Vascular endothelial growth factor/vascular permeability factor augments nitric oxide release from quiescent rabbit and human vascular endothelium. Circulation 95(4):1030–1037
Parenti A, Morbidelli L, Cui X-L, Douglas JG, Hood JD, Granger HJ, Ledda F, Ziche M (1998) Nitric oxide is an upstream signal of vascular endothelial growth factor-induced extracellular signal-regulated kinase½ activation in postcapillary endothelium. J Biol Chem 273(7):4220–4226
Harrison DG (1997) Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Investig 100(9):2153
Makela AM Role of L-arginine in the biological effects of blue light. In: Laser Florence 2004, 2005. Int Soc Optics and Photonics, pp 596805-596805-596813
Sharma K, Babu PC, Sasidhar P, Srinivas V, Mohan VK, Krishna E (2008) Recombinant human epidermal growth factor inclusion body solubilization and refolding at large scale using expanded-bed adsorption chromatography from Escherichia coli. Protein Expr Purif 60(1):7–14
Holbro T, Hynes NE (2004) ErbB receptors: directing key signaling networks throughout life. Annu Rev Pharmacol Toxicol 44:195–217
Kassenbrock CK, Hunter S, Garl P, Johnson GL, Anderson SM (2002) Inhibition of Src family kinases blocks epidermal growth factor (EGF)-induced activation of Akt, phosphorylation of c-Cbl, and ubiquitination of the EGF receptor. J Biol Chem 277(28):24967–24975
Erschbamer M, Pernold K, Olson L (2007) Inhibiting epidermal growth factor receptor improves structural, locomotor, sensory, and bladder recovery from experimental spinal cord injury. J Neurosci 27(24):6428–6435
Qu W, Tian D, Guo Z, Fang J, Zhang Q, Yu Z, Xie M, Zhang H, Lü J, Wang W (2012) Inhibition of EGFR/MAPK signaling reduces microglial inflammatory response and the associated secondary damage in rats after spinal cord injury. J Neuroinflammation 9(178):2094–2099
Ge H, Gong X, Tang CK (2002) Evidence of high incidence of EGFRvIII expression and coexpression with EGFR in human invasive breast cancer by laser capture microdissection and immunohistochemical analysis. Int J Cancer 98(3):357–361
VanMeter AJ, Rodriguez AS, Bowman ED, Jen J, Harris CC, Deng J, Calvert VS, Silvestri A, Fredolini C, Chandhoke V (2008) Laser capture microdissection and protein microarray analysis of human non-small cell lung cancer differential epidermal growth factor receptor (EGPR) phosphorylation events associated with mutated EGFR compared with wild type. Mol Cell Proteomics 7(10):1902–1924
Khamseh ME, Kazemikho N, Aghili R, Forough B, Lajevardi M, Dabaghian FH, Goushegir A, Malek M (2011) Diabetic distal symmetric polyneuropathy: effect of low-intensity laser therapy. Lasers Med Sci 26(6):831–835
Bivalacqua TJ, Hellstrom WJ, Kadowitz PJ, Champion HC (2001) Increased expression of arginase II in human diabetic corpus cavernosum: in diabetic-associated erectile dysfunction. Biochem Biophys Res Commun 283(4):923–927
Shemyakin A, Kövamees O, Rafnsson A, Böhm F, Svenarud P, Settergren M, Jung C, Pernow J (2012) Arginase inhibition improves endothelial function in patients with coronary artery disease and type 2 diabetes mellitus. Circulation 126(25):2943–2950
Förstermann U, Münzel T (2006) Endothelial nitric oxide synthase in vascular disease from marvel to menace. Circulation 113(13):1708–1714
Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, Caldwell RB, Caldwell RW (2008) Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res 102(1):95–102
Koprivica V, Cho K-S, Park JB, Yiu G, Atwal J, Gore B, Kim JA, Lin E, Tessier-Lavigne M, Chen DF (2005) EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science 310(5745):106–110
Cerniglia GJ, Pore N, Tsai JH, Schultz S, Mick R, Choe R, Xing X, Durduran T, Yodh AG, Evans SM (2009) Epidermal growth factor receptor inhibition modulates the microenvironment by vascular normalization to improve chemotherapy and radiotherapy efficacy. PLoS One 4(8):e6539
Ogita M, Tsuchida S, Aoki A, Satoh M, Kado S, Sawabe M, Nanbara H, Kobayashi H, Takeuchi Y, Mizutani K (2014) Increased cell proliferation and differential protein expression induced by low-level Er: YAG laser irradiation in human gingival fibroblasts: proteomic analysis. Lasers Med Sci:1–12
Peplow PV, Chung T-Y, Ryan B, Baxter GD (2011) Laser photobiomodulation of gene expression and release of growth factors and cytokines from cells in culture: a review of human and animal studies. Photomed Laser Surg 29(5):285–304
Zhang Y, Song S, Fong C-C, Tsang C-H, Yang Z, Yang M (2003) cDNA microarray analysis of gene expression profiles in human fibroblast cells irradiated with red light. J Investig Dermatol 120(5):849–857
Conlan MJ, Rapley JW, Cobb CM (1996) Biostimulation of wound healing by low‐energy laser irradiation. A review. J Clin Periodontol 23(5):492–496
Acknowledgments
This work was financially supported by Research Affairs, Tehran University of Medical Sciences. We also thank Behsaz Institute for providing laser apparatus and the staff of the laser clinic and laboratory of Milad Hospital for their support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Kazemikhoo, N., Sarafnejad, A.F., Ansari, F. et al. Modifying effect of intravenous laser therapy on the protein expression of arginase and epidermal growth factor receptor in type 2 diabetic patients. Lasers Med Sci 31, 1537–1545 (2016). https://doi.org/10.1007/s10103-016-2012-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-016-2012-x