Abstract
The biological effects of local therapy with laser on bone repair have been well demonstrated; however, this possible effect on bone repair outside the irradiated field has not been evaluated. The aim of this study was to investigate the effect of low-level laser therapy (LLLT) (λ = 830 nm) on repair of surgical bone defects outside the irradiated field, in rats. Sixty Wistar rats were submitted to osteotomy on the left femur and randomly separated into four groups (n = 15): group I, control, bone defect only; group II, laser applied on the right femur (distant dose); group III, laser applied locally on the bone defect and also on the right femur (local and distant doses); and group IV, laser applied locally on the left femur (local dose). Laser groups received applications within a 48-h interval in one point per session of density energy (DE) = 210 J/cm2, P = 50 mW, t = 120 s, and beam diameter of 0.028 cm. Five animals of each group were euthanized 7, 15, and 21 days after surgery. Histologic analysis in all groups showed new bone formation in the region of interest (ROI) at 7 days. After 15 days, bone remodeling with a decrease of bone neoformation in the marrow area was observed in all groups. After 21 days, advanced bone remodeling with new bone mostly located in the cortical area was observed. The histomorphometric analysis showed at 7 days a significant increase of bone formation in groups III and IV compared to groups I and II. At days 15 and 21, histomorphometric analysis showed no significant differences between them. Laser therapy presented a positive local biostimulative effect in the early stage of bone healing, but the LLLT effect was not observed a long distance from the evaluated area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tissue repair is a phenomenon that occurs to reconstitute injured areas and involves numerous cells and chemical mediators [1]. In recent years, low-level laser therapy (LLLT) has gained importance among treatment modalities for various medical problems including bone repair processes [2], musculoskeletal complications [3], and pain control [4].
Considering the variety of laser protocols (Table 1), cells, and study types, the exact effects of low-level laser therapy need to be investigated. The use of lasers in biomodulation of bone repair has been studied in bone defects [1, 2] because it increases osteoblast activity [5], vascularization [6], and organization of collagen fibers [7]. In addition to local action, the possible effects from LLLT outside the irradiated field have been reported in soft tissue healing [8–10]. Based on these data, most of the studies evaluating LLLT on bone healing used different animals for experimental and control groups because of the possibility of systemic/distant effects [2, 6]. Some authors have suggested that these distant effects may explain the absence of laser biomodulatory effects in studies that used the same animal as experimental and control subject [8, 9]. However, there are studies that used internal control and also had positive results of LLLT on bone [1, 11] and cutaneous healing [12]. Considering these conflicting results, the aim of this study was to investigate the effect of low-level laser therapy (λ = 830 nm) on bone healing distant to the irradiation site.
Materials and methods
Study samples
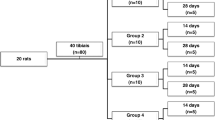
Sixty male healthy Wistar rats, weighing 300 to 400 g, were randomly selected and distributed into four groups of 15 animals (Table 2): control group (GI), distant LLLT group (GII), local and distant LLLT group (GIII), and local LLLT group (GIV). Animals were maintained under a light-dark period of 12 h and controlled temperature conditions (22 ± 2 °C), with balanced diet and water drinking ad libitum. This study was approved by the Science and Ethics Committee from Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, Brazil (Protocol 037/2009).
Surgery
All animals were submitted to osteotomy on the left femur for bone defect creation. All of them were anesthetized with an intraperitoneal injection of 100 mg/kg ketamine and 3 mg/kg xylazine hydrochloride. They were positioned on the right lateral decubitus, and the bone access was achieved through a 2-cm continuous longitudinal incision exposing the mid-diaphysis. A standardized 2.3-mm-diameter osteotomy was performed with a round bur under saline solution irrigation. The depth of drilling limit was the disruption of femur cortical bone. Then, soft tissues were repositioned and the suture was performed in the muscular and cutaneous layers using nylon 4-0. Animals with bone defects (BD) only are the control group.
Laser protocol
The animals of groups II, III, and IV were submitted to sessions of laser therapy. The animals of group II were irradiated on the right femur (distant dose), those of group III in both femurs (local and distant doses), and those of group IV on the left femur (local dose). The equipment used was gallium-aluminum-arsenide (GaAlAs) infrared laser diode (Flash Lase III, DMC Equipamentos, São Carlos, SP, Brazil) with a continuous wavelength of 830 nm, 50 mW of potency, and 0.028 cm beam diameter. The application was punctual, with a 6-J (density energy (DE) 210 J/cm2) dose per session during 2 min. The first session was applied immediately after drilling and before soft tissue repositioning in the defect area (local dose). The distant dose was applied in the sequence in the contralateral femur with the laser tip positioned over and perpendicular to the long axis of the bone. In the postoperative period, applications were taken every 48 h for 7, 15, and 21 days, resulting in 4, 8, and 11 sessions, according to each subgroup (Table 3). Five animals of each group were euthanatized at 7, 15, and 21 days postoperatively using saturated potassium chloride associated with general anesthesia.
Histological procedure
The bone defect area and the attached soft tissue were removed and immediately fixed in 10 % phosphate-buffered formaldehyde solution during 48 h. Thereafter, the tissue blocks were decalcified in EDTA 10 % along 4 weeks, dehydrated with graded alcohols, and embedded in paraffin. To confirm that the tissue blocks were completely decalcified, we used a solution of ammonium hydroxide 5 % with ammonium oxalate 5 % (in a 1:1 proportion). From the central region of the defect, histological sections of 5 μm were obtained and stained in hematoxylin and eosin and Mallory trichrome.
Histomorphometric analysis
The percentage of bone neoformation was quantified by the same examiner in a blind study. Histological images of the bone defect were captured at ×4 magnification, using an Olympus BX 40 binocular microscope (Shinjuku-ku, Tokyo, Japan) coupled with an OLY 200 camera (Center Valley, PA, USA). The histological sections of the whole bone defect area were digitalized using the HL Image 2005 program (Western Vision, Salt Lake City, UT, USA). The screenshots were merged and areas of soft tissue were erased using Photoshop CS2 software (Adobe®, Adobe System Inc., San Jose, CA, USA) and finally converted to binary images with HL Image 2005. The region of interest (ROI) within the bone defect was delineated with four straight lines (Fig. 1). The percentage of bone formation within the ROI was obtained.
Statistical analysis
The results were submitted to normality test and analyzed using analysis of variance (ANOVA) and Bonferroni. Differences were considered statistically significant if p < 0.05.
Results
Histological results
Histologic analysis in all groups showed new bone formation in the ROI extending through the medullar until the opposite cortical. The new bone tissue was primary type with trabecular arrangement delimiting small cavities, filled with cells, blood vessels, and collagen fibers. After 15 days, bone remodeling was observed, with a decrease of bone neoformation in the marrow area, when compared with the 7-day period in all groups. After 21 days, advanced bone remodeling was observed, with new bone mostly located in the cortical area. The cortical bone defect was almost filled by secondary bone. Little or no bone was observed in the marrow area (Fig. 2).
The histomorphometric analysis revealed a significant increase in the percentage of bone formation in groups III (39.66 ± 3.29) and IV (40.30 ± 3.54) in comparison to group II (26.24 ± 5.58) and the control group (26.20 ± 4.57), at 7 days (p < 0.05). There was no difference between group II and the control and also between groups III and IV at this period. At 15 and 21 days, respectively, the histomorphometric analysis revealed no significant differences of new bone formation between group I (19.86 ± 2.90; 18.10 ± 5.58), group II (21.02 ± 2.20; 18.00 ± 4.18), group III (22.34 ± 2.10; 17.68 ± 6.82), and group IV (24.23 ± 3.54; 17.05 ± 5.12) (Fig. 3).
Regarding bone healing in the course of time, histomorphometric analysis showed a significant decrease of bone percentage in all groups when compared between 7 and 21 days (p < 0.05), being more evident between groups III and IV (p < 0.005). Groups III and IV also showed a significant decrease of bone when compared between 7 and 15 days (p < 0.005) (Fig. 3).
Discussion
This study evaluated the possible induction of effects of LLLT on bone repair distant to the irradiation site in an animal model. The study of laser therapy should consider aspects such as wavelength and radiation dose. The wavelength of 830 nm (used in the present study) penetrates the tissue surface (skin), reaching the underlying bone (femur); thus, it is more appropriate for bone-related applications as in the present case. The application of 6 J (210 J/cm2), although considered a high dose, is similar to previous studies that found positive results on bone healing with doses of 178 J/cm2 per session [13]. In our study, the local positive effect of laser therapy was well evidenced in animals with laser applied directly over the bone defect area (groups III and IV) at 7 days, as observed by Batista et al. [2].
The observed increase in bone formation at this period may be due to local effects of laser stimulating the differentiation of mesenchymal cells and the proliferation of osteoblasts and fibroblasts. These events may explain the extensive bone neoformation, invading the medullar area and extending beyond the defect, which was frequently observed at 7 days in group III. This corroborates with other studies such as those of Gál et al. [12] and Batista et al. [2] that evaluated the effect of laser in wound repair and reported that the most significant morphological changes occurred during the first 7 days of healing. Also, Garavello-Freitas et al. [14] found maximal laser-stimulated bone growth after 7 days of irradiation.
An interesting fact observed was that the bone remodeling in groups III and IV was faster than that of groups I and II. The greater initial bone formation (7 days) was followed by accelerated bone resorption and remodeling, so no difference was found in the percentage of bone between all the groups at 15 and 21 days. This interpretation is also based on the significant reduction (p < 0.005) in the percentage of bone in those groups between 7 and 15 days and also between 7 and 21 days. These findings suggest that low-level laser irradiation would stimulate osteoclast activity to promote bone resorption and remodeling. Garavello-Freitas et al. [14] that evaluated LLLT in a rat model also observed a smaller area of trabeculae in the 14-day group compared to the 7-day-irradiated rat group submitted to the same dose of laser.
The present study did not observe a systemic effect of low-level laser therapy in bone healing, considering that laser application distant from the defect did not interfere on bone wound healing. The assessment of the distant effect on bone tissue is not well described in literature because most studies only assessed this effect in the healing of soft tissue wounds [8, 10] and with conflicting results [8]. Braverman et al. [8] that evaluated the distant effect of laser in cutaneous wound repair in rabbits showed a significant effect only on tensile strength evaluation of wounds; however, no statistically significant difference was observed in the histological evaluation of samples.
Schindler et al. [10] reported that the distant effect of LLLT is due to the release of cytokines and growth factors into the systemic bloodstream, causing vasodilation and neoangiogenesis. In our current study, the laser application point was the contralateral leg and, possibly, the released cytokines and growth factors did not reach the bone defect in sufficient concentration to interfere on the repair of bone tissue distant to the irradiation site, although this was not evaluated in our study. This leads us to think that spatial proximity of cells or direct cell-to-cell contacts may be crucial for transmitting the signals from irradiated cells to neighboring non-irradiated cells. Another important issue is that the laser scope is wider than the application site, causing what could be called regional effect. In fact, many studies that evaluated the distant effect of LLLT on soft tissues used relatively close cutaneous injuries (control and experimental) [8, 15] which could interfere with the results.
Coelho et al. [16] found distant effects when evaluating LLLT on bone repair; however, the authors used different experiment designs and laser protocols. Additionally, the implantation of PLLA-PGA screws modifies the evaluated microenvironment. Considering that it is a consensus that different forms of LLLT do not produce the same biological effects, even if all of the components of the irradiated bone are directly affected by laser, they would have different degrees of sensibility, and this makes it difficult to compare the results of different experimental models. Our present work provides relevant data about the potential efficacy of local LLLT, but not systemic, even with higher doses. However, the reasons for the laser stimulatory effect and also the absence of definite parameters to clinical use warrant further investigation.
Conclusion
LLLT exerts a biostimulatory effect and may be helpful to improve bone healing after surgical procedures. However, the results did not demonstrate any changes in bone repair after the application of LLLT a long distance from the evaluated area.
References
Merli LA, Santos MT, Genovese WJ, Faloppa F (2005) Effect of low-intensity laser irradiation on the process of bone repair. Photomed Laser Surg 23:212–5
Batista JD, Zanetta-Barbosa D, Cardoso SV, Dechichi P, Rocha FS, Pagnoncelli RM (2014) Effect of low-level laser therapy on repair of the bone compromised by radiotherapy. Lasers Med Sci 29(6):1913–8
Law D, McDonough S, Bleakley C, Baxter GD, Tumilty S (2015) Laser acupuncture for treating musculoskeletal pain: a systematic review with meta-analysis. J Acupunct Meridian Stud 8(1):2–16
Eslamian L, Borzabadi-Farahani A, Hassanzadeh-Azhiri A, Badiee MR, Fekrazad R (2014) The effect of 810-nm low-level laser therapy on pain caused by orthodontic elastomeric separators. Lasers Med Sci 29(2):559–64
Migliario M, Pittarella P, Fanuli M, Rizzi M, Renò F (2014) Laser-induced osteoblast proliferation is mediated by ROS production. Lasers Med Sci
Khadra M, Kasem N, Haanaes HR, Ellingsen JE, Lyngstadaas SP (2004) Enhancement of bone formation in rat calvarial bone defects using low-level laser therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 97:693–700
Pinheiro AL, Gerbi ME (2006) Photoengineering of bone repair processes. Photomed Laser Surg 24:169–78
Braverman B, McCarthy RJ, Ivancolich AD et al (1989) Effect of helium-neon and infrared laser on wound healing in rabbits. Lasers Surg Med 9:50–58
Rochkind S, Rousso M, Nissan M et al (1989) Systemic effects of low-power laser irradiation on the peripheral and central nervous system, cutaneous wounds and burns. Lasers Surg Med 9:174–182
Schindler A, Heinze G, Schindl M, Pernerstorfer-Schön H, Schindl L (2002) Systemic effects of low-intensity laser irradiation on skin microcirculation in patients with diabetic microangiopathy. Microvasc Res 64:240–6
Nissan J, Assif D, Gross MD, Yaffe A, Binderman I (2006) Effect of low intensity laser irradiation on surgically created bony defects in rats. J Oral Rehabil 33:619–924
Gál P, Vidinský B, Toporcer T et al (2006) Histological assessment of the effect of laser irradiation on skin wound healing in rats. Photomed Laser Surg 24:480–8
Pretel H, Lizarelli RF, Ramalho LT (2007) Effect of low-level laser therapy on bone repair: histological study in rats. Lasers Surg Med 39(10):788–96
Garavello-Freitas I, Baranauskas V, Joazeiro PP, Padovani CR, Dal Pai-Silva M, da Cruz-Höfling MA (2003) Low-power laser irradiation improves histomorphometrical parameters and bone matrix organization during tibia wound healing in rats. J Photochem Photobiol B 70:81–9
Rodrigo SM, Cunha A, Pozza DH, Blaya DS, Moraes JF, Weber JB, de Oliveira MG (2009) Analysis of the systemic effect of red and infrared laser therapy on wound repair. Photomed Laser Surg 27:929–35
Coelho RC, Zerbinati LP, de Oliveira MG, Weber JB (2014) Systemic effects of LLLT on bone repair around PLLA-PGA screws in the rabbit tibia. Lasers Med Sci 29(2):703–8
AboElsaad NS, Soory M, Gadalla LM, Ragab LI, Dunne S, Zalata KR, Louca C (2009) Effect of soft laser and bioactive glass on bone regeneration in the treatment of bone defects (an experimental study). Lasers Med Sci 24(4):527–33
Nascimento SB, Cardoso CA, Ribeiro TP, Almeida JD, Albertini R, Munin E, Arisawa EA (2010) Effect of low-level laser therapy and calcitonin on bone repair in castrated rats: a densitometric study. Photomed Laser Surg 28(1):45–9
Pires-Oliveira DA, Oliveira RF, Amadei SU, Pacheco-Soares C, Rocha RF (2010) Laser 904 nm action on bone repair in rats with osteoporosis. Osteoporos Int 21(12):2109–14
Pinheiro AL, Aciole GT, Cangussú MC, Pacheco MT, Silveira L Jr (2010) Effects of laser photherapy on bone defects grafted with mineral trioxide aggregate, bone morphogenetic proteins, and guided bone regeneration: a Raman spectroscopic study. J Biomed Mater Res A 95(4):1041–7
Fávaro-Pípi E, Ribeiro DA, Ribeiro JU, Bossini P, Oliveira P, Parizotto NA, Tim C, de Araújo HS, Renno AC (2011) Low-level laser therapy induces differential expression of osteogenic genes during bone repair in rats. Photomed Laser Surg 29(5):311–7
Acknowledgments
The authors would like to thank FAPEMIG for the research grants (APQ-0565-11).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Batista, J.D., Sargenti-Neto, S., Dechichi, P. et al. Low-level laser therapy on bone repair: is there any effect outside the irradiated field?. Lasers Med Sci 30, 1569–1574 (2015). https://doi.org/10.1007/s10103-015-1752-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-015-1752-3