Abstract
The aim of the present study was to compare the effectiveness of four different laser wavelengths used for low-level laser therapy(LLLT) on healing of mucositis in an animal model of wound healing, by investigating expression of transient receptor potential melastatin(TRPM) ion channels. Forty-five rats were intraperitoneally injected with 100 mg/kg 5-fluorouracil on day 1 and 65 mg/kg on day 3. Superficial scratching on left cheek pouch mucosa was performed on days 3 and 5. After ulcerative mucositis was clinically detected, LLLT was started (660 nm, HELBO; 810 nm, Fotona-XD; 980 nm, ARC-Fox; and 1064 nm, Fidelis-Plus3) at 8 J/cm2/day from days 1 to 4. Oval excisional biopsy was performed at the wound site, and expression of TRPM2 to TRPM8 was evaluated. Student’s t test was used for evaluation of significance of TRPM gene expression according to “0” value (α = 0.05). In 980-nm group, TRPM4, TRPM6, and TRPM7 were significantly higher than in the control group (p < 0.005). In 660, 810, and 1064 nm groups, only TRPM6 was significantly higher than in control group (p < 0.005). There were no significant differences between control and sham groups (p > 0.05). These findings suggest that expression of TRPM6 gene was significantly affected by irradiation with lasers at different wavelengths, whereas the TRPM4 and TRPM7 genes were only expressed in the 980-nm diode laser group. TRPM6 gene was highly expressed during LLLT, which may lead to accelerated wound healing and tissue repair. In contrast, there was some evidence that the 980-nm diode laser caused increased expression of TRPM4, TRPM6, and TRPM7 which are responsible for stimulation of Ca2+ and Mg2+ metabolism, as well as apoptotic pathways of controlled cell death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laser technology has developed rapidly during the past decade. It has a huge range of uses in many different situations and operations. Many studies about the utilization of laser techniques in dentistry have been published recently [1–5]. These studies have shown the benefits of laser applications on hard bone tissues, enamel and dentinal surfaces, and oral mucosal lesions.
Nowadays, laser therapy is also being used for oral tissue repair. The initial interaction of laser with the mucosal tissues causes release of some preformed substances such as histamine, serotonin, and bradykinin. The sodium–potassium pump system increases its efficiency after ATP production, resulting in better maintenance of the electrical potential between the inside and outside of the cell [6]. During the first stage of tissue repair, inflammatory responses comprise the initial reactions that are essential to conserve the integrity of the tissues. After this stimulation, a cascade begins, including several mediators such as histamine, prostaglandin E2, leukotriene D4, interleukin (IL)-1 and IL-6, and NO. These lead to tissue repair and enhance the wound healing mechanism (WHM) [7]. Different types of laser are being used for low-level laser therapy (LLLT), such as Nd:YAG, diode, helium–neon (He–Ne), indium gallium arsenide phosphide (InGaAlP), aluminum gallium arsenide (GaAlAs), indium gallium arsenide (InGaAs), CO2, and infrared [8].
Recent studies have focused on calcium metabolism and its effects on apoptosis and its relations to WHM [9–11]. Xu et al. have highlighted that one of the earliest steps of WHM is an increase in intracellular calcium mediated by a transient receptor potential (TRP) channel [11]. Those investigations have revealed that this calcium flash is dependent on epidermal expression of the TRP channel, especially transient receptor potential melastatin (TRPM) (GTL-2 in worms).
A common complication occurring during cancer chemotherapy and radiotherapy is oral mucositis. The cytotoxic agents used in chemotherapy and the application of radiation to the local areas, such as the oral mucosa, may play a role in causing inflammation of the oral mucosa. This irritation may appear as redness initially and severe ulceration as the treatment progresses. Many patients complain about these ulcerations and suffer from pain. Digestion, daily habits, and quality of life of the patients are affected negatively. It may also affect the patient’s endurance for cancer treatment as well [12, 13]. Sonis et al. declared that patients undergoing hematopoietic stem cell transplantation and having little or no oral mucositis showed a mortality rate of 1 %, whereas the same group of patients with severe ulceration had a mortality rate of 40 % [14]. Hence, these findings revealed that the morbidity and mortality are potentially affected by oral mucositis lesions during cancer therapy [15]. Many treatment protocols and agents have been used to prevent ulcerative lesions and mucositis, but to date, there is no method that is accepted worldwide for the management of this condition. Currently, the most frequent care of oral mucositis comprises basic oral hygiene and extensive patient education. Many different ways have been sought for preventing oral mucositis, such as mouthwashes, antibiotics, analgesics, local anesthetics, amifostine, hematologic growth factors, pentoxifylline, glutamine, LLLT, and cryotherapy [16]. New treatment protocols are of interest because of the lack of an efficient treatment modality for oral mucositis.
Many researchers have studied the molecular mechanism of LT [17–20] during wound healing. However, few studies have been set up to observe the apoptotic mechanism of LT, and no study has demonstrated the effects of TRPM channel activity, which plays a key role in apoptotic pathways [9]. Therefore, the aim of the present study was to evaluate the activity of TRPM channels 2–8 after LLLT for oral mucositis lesions.
Methods
Animals
Forty-five male Wistar albino rats weighing 250–300 g and aged 5 months were used. All animals were kept in the laboratory in order to orientate them to the laboratory conditions for at least 5 days prior to the study.
Mucosal wounding
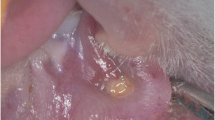
All except five rats were intraperitoneally injected with 100 mg/kg 5-fluorouracil on day 1 and 65 mg/kg on day 3. The tip of an 18-gauge needle was used to create a superficial scratch on the left cheek pouch mucosa, by dragging twice in a linear movement on days 3 and 5. This procedure was repeated to develop ulcerative mucositis, which is similar to human oral mucositis. The animals were anesthetized with xylazine hydrochloride (XylazineBio) 3 mg/kg and ketamine hydrochloride (Ketasol) 90 mg/kg prior to performing these procedures. After ulcerative mucositis was clinically detected on the left cheek pouch mucosa, LLLT was started.
Irradiation protocol
The animals were randomly divided into six groups: four test groups (n = 8), one control group (n = 8), and one sham group (n = 5). Laser irradiation was performed with the laser handpiece kept perpendicular to the area of mucositis at a distance of 1 cm in the test groups. Two clinicians performed the irradiation protocol. One of them held the rat tightly when the other was both retracting the cheek and irradiated the laser device to the mucosa. The laser applications were performed by the same clinician. Group 1 (control): in this group, no laser irradiation was applied to the mucositis. Group 2 (810 nm): diode laser (810 nm; Fotona XD-2 diode laser; Fotona, Slovenia) was used for the daily treatment of mucositis from days 1 to 4 (continuous mode, beam area 0.28 cm2, application time 9 s, total energy per session 2.24 J, energy density 8 J/cm2). Group 3 (980 nm): diode laser (980 nm; ARC Fox, Germany) was used for daily treatment of mucositis from days 1 to 4 (continuous mode, beam area 0.12 cm2, application time 10 s, total energy per session 0.99 J, energy density 8.3 J/cm2). Group 4 (1064 nm): Nd:YAG laser (1064 nm; Fidelis Plus 3; Fotona, Slovenia) was used for daily treatment of mucositis from days 1 to 4 (pulsed mode, average power 0.25 W, beam area 0.28 cm2, application time 9 s, total energy per session 2.24 J, energy density 8 J/cm2). Group 5 (660 nm): diode laser (660 nm; HELBO, Bredent, Germany) was used for daily treatment of mucositis from days 1 to 4 (average power 100 mW, beam area 0.75 cm2, application time 60 s, total energy per session 6 J, energy density 8 J/cm2). Group 6 (sham): in this sham group, neither mucositis was established on the oral tissues nor laser irradiation was applied to the mucosa.
Thirty minutes after completion of LLLT, all of the rats were sacrificed. Oval excisional biopsy specimens were taken from the wound site, and frozen samples were used for future laboratory procedures, and the specimens were kept in a nitrogen tank. All the specimens were taken to the genetic laboratory for evaluation of TRPM2–TRPM8 gene expression, except for TRPM5.
RNA extraction
RNA extraction was conducted using binding buffer OBB [20 mM Tris–HCl (pH 7.5), 1 M NaCl, 2 mM EDTA, 0.2 % SDS]. One hundred microliters of frozen sample was mixed with 200 μl 10 % (w/v) Oligotex Suspension in 10 mM Tris–HCl (pH 7.5), 500 mM NaCl, 1 mM EDTA, 0.1 % SDS, and 0.1 % NaN3 (QIAGEN Hilden, Germany) and incubated for 24 h at room temperature. Chloroform (50 μl) was added and centrifuged at 12,000 rpm for 15 min at 4 °C. Total RNA was ethanol-precipitated and dissolved in 10 μl of diethyl-pyrocarbonate-treated water.
cDNA preparation for mRNA expression
The reverse transcription kit (QIAGEN Sciences, San Diego, CA) enabled the transcription of 25 mg RNA into cDNA. In a standard assay of 20 ml with 0.2–2 mg RNA, the synthesis rates were directly proportional to the amount of RNA. The reagents were allowed to thaw, mixed well, and centrifuged briefly at 1000 rpm to collect the solutions at the bottom of the vials. The reagents were kept on ice while performing the assay and then stored at −20 °C after the experiment. For separation of the incorporated and free nucleotides, spin columns were used. For analysis of the reaction products denaturing, alkaline agarose gels were used.
Reverse-transcriptase polymerase chain reaction
Total RNA was isolated from 25 mg of the solid tissue samples each of which was collected from the rats after the application of laser therapy. Then, 20 mM Tris-Cl (pH 7.5), 1 M Nacl, 2 mM EDTA, and 0.2 % SDS were added on the sample tissue (QIAGEN cat. no. 79000). After 24 h of incubation at room temperature, 50 μl of chloroform was added. Fifteen minutes of centrifuge was performed to each sample at 12,000 rpm (+4 °C). To obtain total RNA, after precipitation was done with ethanol and the samples were dried down to the room temperature, 50 μl DEPCT water was added on to the RNA precipitation at the bottom of the tubes for dissolution of the mixture. Following this, each sample was measured one by one at 260 and 280 nm wavelengths in spectrophotometer, and the purity and quantity of the RNA was determined according to the ratio of A260/A280. Calculations were performed for the cDNA conversion after determining the amount in 1 μl of a mixture of RNA DEPCT solution. Total RNA samples were reverse-transcribed by AMV Reverse Transcription Kit (Roche, Germany) according to the procedures provided by the supplier.
The amounts of the solutions were prepared as 2 mg of RNA in each sample that were settled to 25 μl. Two microliters of 10× reverse-transcriptase buffer was added. Then, 4 μl of MgCl2 solution, 2 μl of deoxynucleotide, 2 μl of oligo-T primers, and 2 μl of RNase inhibitor were included. Following the addition of 0.8 μl of AMV reverse transcriptase, all the mixture was filled up with double-distilled water until the total volume had reached to 25 μl. An incubation protocol was followed for the enzyme in the prepared mixture to be activated and to produce cDNA. According to this protocol at first stage, after the incubation of the mixture at 45 °C for 45 min, the enzyme was denaturated at 94 °C for 3 min and tubes were then put on ice for cooling for 5 min. At the end of cooling, the cDNA’s were ready to use.
PCR protocol
After all these steps, PCR protocol was completed. Special primers arranged for messenger RNA (mRNA) expression were used in this process. cDNA was denatured at 94 °C for 3 min, annealed at 60 °C for 45 s (TRPM2), at 60 °C for 30 s (TRPM4), at 55.6 °C for 30 s (TRPM6), at 60.4 °C for 30 s (TRPM7), and at 62.4 °C for 45 s (NaV1.9) and extended at 94 °C for 3 min and at 72 °C for 30 s. GAPDH was utilized for housekeeping gene.
Electrophoresis
After PCR, all the products were run under electrophoresis process for 45 min in 2 % of agarose gel at 100 V. The bands were detected and the photographs of the products were taken by using UV screening. Data were transferred to the computer and to evaluate the magnitude of gene expression, the relative density of cDNA bands in the display gels were graded on a scale according to intensity. The integer 0 denoted no detectable bands, whereas the integer 10 equaled the highest band intensity. The results were calculated by using delta ct delta delta ct analysis. GAPDH values were used for normalization.
Statistical analysis
The aim of our statistical analysis was to evaluate the ratios of expression of the genes according to “0” value. All the values were calculated as mean ± standard deviation for all the evaluated growth factors using SPSS version 10.0 (SPSS, Chicago, IL, USA). The obtained data were analyzed by Student’s t test (one sample t test) for evaluation of significance of expression of TRPM genes according to “0” value (α = 0.05).
Results
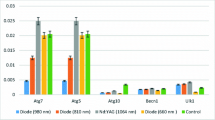
The mean values of the results of this study are shown in Table 1. This table was established according to the significance levels of the outcomes of mRNA expression tests of TRPM2–4 and TRPM6–8. Each group was evaluated in accordance with the control group.
After detailed statistical evaluation of mRNA expression in the 980-nm group in comparison with control group, TRPM4 (p < 0.005) expression was significantly higher. TRPM6 and TRPM7 expression was also significantly higher than in the control group (p < 0.05). No mRNA expression was observed for TRPM2, TRPM3, and TRPM8 in the 980-nm group. Analysis of mRNA expression in the 810-nm group revealed that only expression of TRPM6 was significantly higher than that of the other genes (p < 0.05). Expression of the TRPM4 and TRPM7 genes in this group did not differ significantly from the control group (p > 0.05). No mRNA expression was observed for TRPM2, TRPM3, and TRPM8 in the 810-nm group. In the 660-nm group, again only TRPM6 gene expression (p < 0.01) was significantly higher after laser application compared with the other genes. TRPM7 gene was not significantly departed from the control group (p > 0.05). No mRNA expression was observed for TRPM2–4 and TRPM8 in the 660-nm group. In the 1064-nm group, TRPM4 gene expression was found to have a borderline significant increase (p > 0.05), while TRPM6 expression was significantly higher (p < 0.005). Expression of other genes as for TRPM2 and TRPM6–8 did not differ significantly from the control group (p > 0.05) while no mRNA expression was observed for TRPM3 in this group.
Gene expression in the sham and control groups revealed that there were no significant changes in expression of TRPM2–4 and TRPM6–8 (p > 0.05). No mRNA expression was observed during the evaluation of these groups.
Discussion
Wound damage usually triggers a stable and successful healing response, including rectifying tissue defects, following an associated inflammatory response against the incidental wound infection. Xu and Chisholm [11] have shown that an increase in intracellular Ca2+ level mediated by a TRP channel is one of the initial wound signaling phenomena, and is an important step in maintenance of the WHM. They have also reported that this calcium flash is dependent on epidermal expression of one of the TRP channels, TRPM (GTL-2 in worms). One possible mechanism might include TRPM being engaged as a detector of changes in membrane tension within the lead edge cells [10]. Cell behavior could be affected dramatically by tension changes within epithelial cells and during wound closure. A lot is known about the response of the cells to growth factor signals, but almost nothing is known about how cells respond to the considerable changes in tissue tensions that take place at a wound site [10].
A large number of TRP channels are expressed by mammalian epidermal cells, and some of them are related to epidermal wound healing [21]. TRPM agonists can stimulate barrier generation after wounding [22], and this might be due to the wound healing effect of TRPM. According to the literature, Ca2+ responses require epidermal TRPM channels [11]. Xu and Chisholm [11] have discovered that wound closure is triggered by Ca2+ signals in the adult Caenorhabditis elegans epidermis. However, they have added that mutant organisms that are unable to produce Ca2+ are remarkably capable of generating an immune response to the injured site. Parallel to these findings, mutant worms that are unable to initiate this immune response have demonstrated a natural calcium flash into the wounded area, demonstrating that, after tissue injury, this primitive calcium signal behaves similarly to the immune response. The importance of H2O2 in the WHM has recently been described. Studies have shown that this chemical mediator is one of the initial damage signals that first attract immune cells to the wound area, necessitating the activation of NADPH oxidase [23, 24]. Wood has speculated that stimulation of H2O2 production by DUOX activation might be due to activation of a wound-influenced calcium wave [10]. He concluded that if this is the case, then calcium could be one of the essential mediators of wound healing, which includes both the control mechanism of re-epithelialization and the associated inflammatory response.
In our study, although none of the TRPM channels were expressed in our control (group 1) and sham (group 6) groups, expression of at least one TRPM channel was activated in every laser-treated group (groups 2–5). The expression of TRPM was not expected in the sham group in which no oral mucositis was induced or any treatment was performed. In contrast, because of the tissue damage, expression was expected in the control group. However, owing to the injection of 5-fluorouracil, this mechanism might have been suppressed.
According to Medline, TRPM6 gene is predominantly expressed in the kidney and colon, and encodes a protein including an ion channel domain and a protein kinase domain. It is essential for magnesium homeostasis, and plays a critical role in epithelial magnesium transport and in active magnesium absorption in the gut and kidney. Hypomagnesemia and secondary hypocalcemia may be associated with the mutant version of this gene [25]. TRPM6 is primarily used in cell growth as well as in intestinal absorption and renal reabsorption of magnesium [26]. There are some studies in the literature about interaction of magnesium and wound healing. André et al. have reported that viability of random skin flaps of rats could not be increased by magnesium sulfate applied by iontophoresis [27]. However, Alizadeh et al. have suggested that skin wound healing and tissue strength may be accelerated by magnesium hydroxide by means of activating collagen generation in rats [28]. In our study, the TRPM6 gene was activated in all groups. This activation may have triggered stimulation of magnesium channels and contributed to wound healing. Ikari et al. have suggested that epithelial growth factor (EGF) increases human TRPM6 mRNA expression in renal epithelial cells [26]. Gobbo et al. have reported that high-level laser therapy is effective in the healing of acneiform rash associated with epidermal growth factor receptor inhibitors, with no adverse effects [29]. Although the laser dose in the current study was not as high as that of Gobbo et al., epidermal growth factor receptors might still have been activated. In addition, because TRPM6 gene seemed to be activated in all the LLLT groups, it could be thought that LLLT might increase EGF, indirectly activating TRPM6 for starting the WHM.
TRPM6 and TRPM7 can construct hetero-oligomeric channel complexes. Among the TRPM family members, only TRPM7 seems to be obligatory for TRPM6 passage to the plasma membrane [30–33]. Studies of various single-channel conductance and biophysical characteristics have proposed that TRPM6, TRPM7, and their combination are obvious ion channels that demonstrate divalent cation permeability, pH sensitivity, and unique single-channel transmittance [34]. A question remains about the exact role of the TRPM6/TRPM7 complex in Mg2+ homeostasis. In our study, TRPM7 was activated only in the 980-nm laser group, together with both TRPM6 and TRPM4. This could have been due to the parallel function of TRPM6 and TRPM7 in epithelial magnesium transport and human cell activation, meaning that co-stimulation of these genes by 980-nm LLLT is more efficient in terms of cellular regeneration.
Novoselova et al. have added that LLLT may stimulate the immunomodulatory system, which could be possible when it is applied to anatomically projecting areas of the thymus [35]. They have also asserted that low-power laser radiation could be a useful noninvasive tool in immunotherapy, whereas it may be a damaging factor, suppressing immune cell activity with prolonged application [35]. In our study, TRPM4 was found to be activated in the 980-nm laser group. A previous study has demonstrated that nonprolonged LLLT enhances the immune system [35]. In the current study, LLLT was applied for only 4 days; therefore, it can be inferred that LT activates TRPM4 channels and causes stimulation of the immune system.
The limitations of our study were that only one dosage of laser (8 J/cm2) in only one certain period (4 days) was used; only TRPM channels genes were evaluated; this study was based on animal model of wound healing but not human.
Conclusion
Within the limitations of our study, the following conclusions can be drawn. Our findings suggest that TRPM6 gene was highly expressed during LLLT with different wavelengths, which may lead to accelerated wound healing and tissue repair. There was some evidence that the 980-nm diode laser caused increased expression of TRPM4, TRPM6, and TRPM7 genes, which are responsible for stimulation of Ca2+ and Mg2+ metabolism, as well as apoptotic pathways of controlled cell death. Further studies are required for full understanding of the relationship between laser irradiation and TRPM gene activity.
References
Tuncdemir AR, Yildirim C, Guller F, Ozcan E, Usumez A (2013) The effect of post surface treatments on the bond strength of fiber posts to root surfaces. Lasers Med Sci 28(1):13–18. doi:10.1007/s10103-012-1053-z
Secilmis A, Bulbul M, Sari T, Usumez A (2013) Effects of different dentin thicknesses and air cooling on pulpal temperature rise during laser welding. Lasers Med Sci 28(1):167–170. doi:10.1007/s10103-012-1108-1
Karaarslan ES, Usumez A, Ozturk B, Cebe MA (2012) Effect of cavity preparation techniques and different preheating procedures on microleakage of class V resin restorations. Eur J Dent 6(1):87–94
Usumez A, Cengiz B, Oztuzcu S, Demir T, Aras MH, Gutknecht N (2014) Effects of laser irradiation at different wavelengths (660, 810, 980, and 1,064 nm) on mucositis in an animal model of wound healing. Lasers Med Sci 29(6):1807–1813. doi:10.1007/s10103-013-1336-z
Sezer U, Aras MH, Aktan AM, Cengiz B, Ozkul N, Ay S (2012) Cytomorphological changes in buccal mucosa of patients treated with low-level 1,064-nm laser radiation. Lasers Med Sci 27(1):219–222. doi:10.1007/s10103-011-0947-5
Piva JA, Abreu EM, Silva Vdos S, Nicolau RA (2011) Effect of low-level laser therapy on the initial stages of tissue repair: basic principles. An Bras Dermatol 86(5):947–954
Balbino CA, Pereira LM, Curi R (2005) Mecanismos envolvidos na cicatrização: uma revisão. Braz J Pharm Sci 41:27–51
Migliorati C, Hewson I, Lalla RV, Antunes HS, Estilo CL, Hodgson B, Lopes NN, Schubert MM, Bowen J, Elad S (2013) Systematic review of laser and other light therapy for the management of oral mucositis in cancer patients. Support Care Cancer: Off J Multinatl Assoc Support Care Cancer 21(1):333–341. doi:10.1007/s00520-012-1605-6
McNulty S, Fonfria E (2005) The role of TRPM channels in cell death. Pflugers Arch 451(1):235–242. doi:10.1007/s00424-005-1440-4
Wood W (2012) Wound healing: calcium flashes illuminate early events. Curr Biol: CB 22(1):R14–R16. doi:10.1016/j.cub.2011.11.019
Xu S, Chisholm AD (2011) A Galphaq-Ca(2)(+) signaling pathway promotes actin-mediated epidermal wound closure in C. elegans. Curr Biol: CB 21(23):1960–1967. doi:10.1016/j.cub.2011.10.050
Timofeyev VT, Poryadin GV, Goloviznin MV (2001) Laser irradiation as a potential pathogenetic method for immunocorrection in rheumatoid arthritis. Pathophysiol: Off J Int Soc Pathophysiol / ISP 8(1):35–40
Arora H, Pai KM, Maiya A, Vidyasagar MS, Rajeev A (2008) Efficacy of He-Ne Laser in the prevention and treatment of radiotherapy-induced oral mucositis in oral cancer patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 105(2):180–186. doi:10.1016/j.tripleo.2007.07.043, 186 e181
Sonis ST, Oster G, Fuchs H, Bellm L, Bradford WZ, Edelsberg J, Hayden V, Eilers J, Epstein JB, LeVeque FG, Miller C, Peterson DE, Schubert MM, Spijkervet FK, Horowitz M (2001) Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. J Clin Oncol: Off J Am Soc Clin Oncol 19(8):2201–2205
Sonis ST (2004) Oral mucositis in cancer therapy. J Support Oncol 2(6 Suppl 3):3–8
Saadeh CE (2005) Chemotherapy- and radiotherapy-induced oral mucositis: review of preventive strategies and treatment. Pharmacotherapy 25(4):540–554
Mizutani K, Musya Y, Wakae K, Kobayashi T, Tobe M, Taira K, Harada T (2004) A clinical study on serum prostaglandin E2 with low-level laser therapy. Photomed Laser Surg 22(6):537–539. doi:10.1089/pho.2004.22.537
Viegas VN, Abreu ME, Viezzer C, Machado DC, Filho MS, Silva DN, Pagnoncelli RM (2007) Effect of low-level laser therapy on inflammatory reactions during wound healing: comparison with meloxicam. Photomed Laser Surg 25(6):467–473. doi:10.1089/pho.2007.1098
Gavish L, Perez LS, Reissman P, Gertz SD (2008) Irradiation with 780 nm diode laser attenuates inflammatory cytokines but upregulates nitric oxide in lipopolysaccharide-stimulated macrophages: implications for the prevention of aneurysm progression. Lasers Surg Med 40(5):371–378. doi:10.1002/lsm.20635
Albertini R, Aimbire FS, Correa FI, Ribeiro W, Cogo JC, Antunes E, Teixeira SA, De Nucci G, Castro-Faria-Neto HC, Zangaro RA, Lopes-Martins RA (2004) Effects of different protocol doses of low power gallium-aluminum-arsenate (Ga-Al-As) laser radiation (650 nm) on carrageenan induced rat paw ooedema. J Photochem Photobiol, B 74(2-3):101–107. doi:10.1016/j.jphotobiol.2004.03.002
Yamada T, Ueda T, Ugawa S, Ishida Y, Imayasu M, Koyama S, Shimada S (2010) Functional expression of transient receptor potential vanilloid 3 (TRPV3) in corneal epithelial cells: involvement in thermosensation and wound healing. Exp Eye Res 90(1):121–129. doi:10.1016/j.exer.2009.09.020
Denda M, Tsutsumi M, Denda S (2010) Topical application of TRPM8 agonists accelerates skin permeability barrier recovery and reduces epidermal proliferation induced by barrier insult: role of cold-sensitive TRP receptors in epidermal permeability barrier homoeostasis. Exp Dermatol 19(9):791–795. doi:10.1111/j.1600-0625.2010.01154.x
Moreira S, Stramer B, Evans I, Wood W, Martin P (2010) Prioritization of competing damage and developmental signals by migrating macrophages in the Drosophila embryo. Curr Biol: CB 20(5):464–470. doi:10.1016/j.cub.2010.01.047
Niethammer P, Grabher C, Look AT, Mitchison TJ (2009) A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 459(7249):996–999. doi:10.1038/nature08119
TRPM6 transient receptor potential cation channel, subfamily M, member 6 [Homo sapiens (human)] Gene ID: 140803. http://www.ncbi.nlm.nih.gov/gene/140803. Accessed 14 Apr 2013
Ikari A, Okude C, Sawada H, Yamazaki Y, Sugatani J, Miwa M (2008) TRPM6 expression and cell proliferation are up-regulated by phosphorylation of ERK1/2 in renal epithelial cells. Biochem Biophys Res Commun 369(4):1129–1133. doi:10.1016/j.bbrc.2008.03.002
Andre Yu R, Brumini C, Esteves Junior I, Masako Ferreira L, Eloin Liebano R (2009) Magnesium sulphate given topically by iontophoresis for viability of random skin flaps in rats. Scand J Plast Reconstr Surg Hand Surg / Nord Plastikkirurgisk Foren Nord Klubb Handkirurgi 43(4):197–200. doi:10.1080/02844310902840122
Alızadeh A, Mohagheghi M, Khaneki M, Saeedpour K (2001) A study of the effect of magnesium hydroxide on the wound healing process in rats. Med J Islam World Acad Sci 16(4):165–170
Gobbo M, Ottaviani G, Mustacchi G, Di Lenarda R, Biasotto M (2012) Acneiform rash due to epidermal growth factor receptor inhibitors: high-level laser therapy as an innovative approach. Lasers Med Sci 27(5):1085–1090. doi:10.1007/s10103-011-1029-4
Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM (2003) Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 114(2):191–200
Schmitz C, Dorovkov MV, Zhao X, Davenport BJ, Ryazanov AG, Perraud AL (2005) The channel kinases TRPM6 and TRPM7 are functionally nonredundant. J Biol Chem 280(45):37763–37771. doi:10.1074/jbc.M509175200
Schlingmann KP, Waldegger S, Konrad M, Chubanov V, Gudermann T (2007) TRPM6 and TRPM7–Gatekeepers of human magnesium metabolism. Biochim Biophys Acta 1772(8):813–821. doi:10.1016/j.bbadis.2007.03.009
Chubanov V, Schlingmann KP, Waring J, Heinzinger J, Kaske S, Waldegger S, Mederos Y, Schnitzler M, Gudermann T (2007) Hypomagnesemia with secondary hypocalcemia due to a missense mutation in the putative pore-forming region of TRPM6. J Biol Chem 282(10):7656–7667. doi:10.1074/jbc.M611117200
Li M, Jiang J, Yue L (2006) Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol 127(5):525–537. doi:10.1085/jgp.200609502
Novoselova EG, Glushkova OV, Cherenkov DA, Chudnovsky VM, Fesenko EE (2006) Effects of low-power laser radiation on mice immunity. Photodermatol Photoimmunol Photomed 22(1):33–38. doi:10.1111/j.1600-0781.2006.00191.x
Ethical approval
All studies were performed in accordance with the National Institutes of Health Guidelines on Animal Care and with the approval of the Ethics Committee of the Gaziantep University Medical Faculty, TURKEY; app# 12.2010-01. All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Conflict of interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Isman, E., Aras, M.H., Cengiz, B. et al. Effects of laser irradiation at different wavelengths (660, 810, 980, and 1064 nm) on transient receptor potential melastatin channels in an animal model of wound healing. Lasers Med Sci 30, 1489–1495 (2015). https://doi.org/10.1007/s10103-015-1750-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-015-1750-5