Abstract
Aim
To investigate the effects of low-level laser therapy (LLLT) as an adjunct to non-surgical periodontal treatment (NSPT) on the plasminogen-activating system.
Materials and Methods
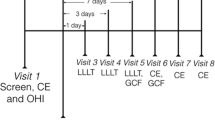
Stage 3–4 Grade C periodontitis and age-gender-matched healthy individuals participated in the split-mouth study (ClinicalTrials.gov identifier, NCT05233501). The study groups were Periodontitis/NSPT (Sham); Periodontitis/NSPT + LLLT (LLLT); Healthy (Control). Following NSPT, LLLT was applied on Days 0, 2 and 7. Clinical parameters were recorded at baseline and on Day 30. Gingival crevicular fluid (GCF) was collected at baseline, on days 7, 14, and 30; tissue-type plasminogen activator (tPA) and plasminogen activator inhibitor-1 (PAI-1) levels were measured with ELISA.
Results
Clinical parameters, total GCF tPA (tPAt) and PAI-1 (PAI-1t) levels significantly reduced in LLLT and Sham groups (< 0.001). GCF tPAt levels in LLLT were significantly lower (< 0.05) than Sham on Day 7. GCF tPAt levels in periodontitis groups were significantly higher than the Control at baseline, on Days 7 and 14 (< 0.01). By Day 30, both groups decreased to control levels (> 0.05). GCF PAI-1t levels were significantly lower in LLLT than the Sham on day 30 (< 0.01), comparable to healthy controls (> 0.05).
Conclusion
Adjunctive LLLT modulates the plasminogen activating system in severe periodontitis by altering GCF tPA and PAI-1 levels.
Clinical relevance
LLLT as an adjunct to non-surgical periodontal treatment in patients with Stage 3–4 Grade C leads to reduced plasminogen activation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontitis is one of the most prevalent chronic diseases, affecting more than 65% of the population worldwide. The new 2018 Classification of Periodontal Diseases has provided a multidimensional characterization of periodontitis comprised of stage and grade to capture disease severity, extent, and progression. Untreated periodontitis results in the progressive development of pocket formation, loss of attachment, alveolar bone atrophy, and tooth loss. Non-surgical periodontal treatment (NSPT) and individualized supportive periodontal therapy (SPT) have been shown to reduce tooth loss and increase long-term tooth retention [1]. Mechanical debridement to eradicate pathological bacteria within infected pockets is the gold standard in periodontal treatment [2]. This consists primarily of scaling and root planing (SRP), which has demonstrated clinical efficacy [3], particularly for 4–6 mm pockets. However, as pocket depth increases, controlling plaque and inflammation becomes more complicated because the complex anatomy of the furcation area, pocket depth, and penetration of microorganisms into tissue makes it difficult to gain access for mechanical debridement. Studies have shown that Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis are capable of aggressive tissue invasion and are not entirely eradicated using mechanical debridement alone, which is unable to provide complete relief to patients suffering from generalized aggressive periodontitis [4,5,6,7]. Considering the contradictory data and the multifactorial biological interactions that affect the post-treatment healing process, it is difficult to form a solid conclusion regarding the effectiveness and long-term stability of NSPT in severe and progressive patients [8, 9]. Various strategies, including supplementary anti-infective therapies [10,11,12] and laser treatment [13,14,15] have been suggested to improve the success of traditional treatment methods in such severe and progressive periodontitis cases.
Also known as soft laser therapy or photobiomodulation, low-level laser therapy (LLLT) entails exposure to low-level laser light to achieve tissue ablation and hemostasis, eliminate periodontal pathogens, enhance tissue growth and regeneration, resolve inflammation, reduce pain, and promote wound healing [16]. LLLT has been shown to possess biostimulatory action on various cell and tissue types through its ability to affect the mitochondrial respiratory chain, thereby increasing adenosine triphosphate production, facilitating fibroblast proliferation, angiogenesis, growth factor release, and collagen synthesis [17]. Limited clinical trials have investigated the benefits of LLLT as an adjunct to NSPT with conflicting results [13, 18,19,20,21].
One of the potential targets of LLLT in tissues is the plasminogen-activating (PA) system. This pathway involves various physiological and pathological processes, including tissue repair and remodeling, wound healing, angiogenesis, and local inflammatory reactions [22]. The PA system remains in balance through the activities of plasminogen activators – such as the urokinase-type plasminogen activator (u-PA) and the tissue/blood vessel-type plasminogen activator (t-PA) – and plasminogen activator inhibitors – such as PAI-1 produced primarily by endothelial and malignant cells, and PAI-2 produced mainly by monocytes, macrophages, epithelial cells, and fibroblasts [23, 24]. The PA system has been implicated in the degradation of periodontal tissue due to periodontal inflammatory processes as well as the entire process of periodontal wound healing [23, 25,26,27]. Limited data is available regarding the effects of various periodontal treatment protocols on GCF t-PA and PAI-1 levels and the extent to which LLLT in conjunction with NSPT affects these levels in patients with Stage 3–4, Grade C periodontitis. Therefore, this study was undertaken to evaluate the clinical outcomes and GCF t-PA and PAI-1 levels in patients with Stage 3–4 Grade C periodontitis to assess the use of LLLT as an adjunct to standard NSPT.
Materials and methods
Study design and participants
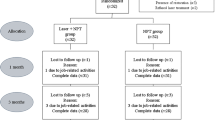
This study was designed as a split-mouth randomized controlled clinical trial. The study protocol was approved by the Local Ethics Committee of Istanbul University (No: 2013/1225), and written informed consent was obtained from all study participants following the Helsinki Declaration (1975; revised, 2002). Participants were recruited from those individuals applying consecutively to the Istanbul Aydin University Dental Faculty's Department of Periodontology between January and September 2017. (ClinicalTrials.gov identifier, NCT05233501).
Stage 3–4, Grade C periodontitis was diagnosed according to the clinical criteria of the World Workshop in Periodontitis [28, 29]. Criteria for inclusion in the study group were as follows: (i) Stage 3–4, Grade C periodontitis (at least one site with probing depth (PD) and clinical attachment level (CAL) ≥ 5 mm in their incisors and first molars and at least 6 other teeth with similar PD and CAL measurements, with alveolar bone loss confirmed by radiography; familial aggregation; the (ii) presence of ≥ 16 teeth; (iii) no periodontal treatment in the 6 months before data collection; (iv) non-smoker. Exclusion criteria were as follows: (i) systemic problems, including a medical history of cancer, rheumatoid arthritis, diabetes mellitus, or cardiovascular disease; (ii) compromised immune system; (iii) pregnancy, menopause, or lactation; (iv) ongoing drug therapy that might affect the clinical characteristics of periodontitis; (v) use of systemic antimicrobials during the 6 weeks before data collection [30]. Of 254 individuals screened, 15 were included in the split-mouth-designed study. Patients fulfilling these criteria were randomly assigned to one of two groups based on the treatment protocol as follows: NSPT + Sham Group (n = 15): Stage 3–4, Grade C periodontitis receiving NSPT only and NSPT + LLLT (n = 15): Stage 3–4, Grade C periodontitis receiving NSPT and LLLT. In addition, a control group (C) (n = 15) comprised periodontally healthy individuals who fulfilled the other criteria described above.
NSPT
Following clinical measurements and GCF sampling, NSPT was performed on periodontitis patients. Treatment consisted of oral-hygiene procedures, full-mouth scaling, and root planing (SRP) performed under local anesthesia using scalers, curettes, and ultrasonic devices. NSPT was completed in a single visit. Supragingival polishing was also performed; however, no subgingival irrigation or mouth rinsing/tongue brushing with chlorhexidine was performed, and patients were instructed not to use any antimicrobial mouth-rinsing solutions for the length of the study.
LLLT Application
In the LLLT group, LLLT was performed following SRP in either the left or right maxillary quadrant selected randomly by a coin toss. LLLT was applied to the selected group of teeth 3 times (Days 0, 2, and 7) [31]. Treatment was performed using a 940 nm indium Gallium Arsenide Phosphorous (lnGaAsP) diode laser (Epic Biolase, Irvine, CA, USA) applied perpendicularly to the periodontal pocket for 20 s at a constant distance of 15 mm and with a continuous wavelength (3.76 J/cm2 delivery with a 1.76 cm2 spot and average output of 0.3 W). In the Sham group, laser application was simulated without pushing the start button.
GCF sampling and processing
The same clinician (CZK) blinded to the study groups performed all clinical procedures and GCF sampling. All treatments were performed by another clinician (FP). For each patient, the 5 teeth with the highest PPD were identified, and GCF sampling was performed on these teeth and the contralateral teeth (total: 10 teeth). The following clinical parameters were recorded: Silness & Löe plaque index (PI) [32]; Löe & Silness gingival index (GI) [33]; probing pocket depth (PPD); clinical attachment level (CAL); bleeding on probing (BOP). Measurements were performed on 6 sites per tooth (mesio-buccal, mid-buccal, disto-buccal, mesio-lingual, mid-lingual, disto-lingual locations) using a Williams periodontal probe (Nordent Manufacturing Inc., Elk Grove Village, IL, USA) calibrated in mm for both treatment and control groups. Clinical measurements were repeated on the same teeth during follow-up visits on Day 30.
The sites selected for sampling showed the most clinical signs of inflammation (i.e., redness, bleeding on probing, and edema) and greatest PPD, with radiographic confirmation of alveolar bone loss, before periodontal therapy. GCF samples were collected on the initial visit after clinical measurements were obtained and again on follow-up visits on Days 7, 14, and 30. Before sampling, sites were gently air-dried and isolated with cotton rolls to prevent saliva contamination, and supragingival plaque, if present, was removed using a sterile curette. GCF was sampled by placing strips of filter paper (Periopaper, ProFlow, Inc, Amityville, NewYork, USA) into the crevice until mild resistance was felt and then leaving the strips in this position for 30 s. Any strips with visible signs of saliva or blood contamination were discarded. The GCF volume of each strip was determined by electronic impedance (Periotron 8000, ProFlow Inc, New York, USA). Samples were placed in sterile polypropylene tubes and stored at -70 °C until analysis.
GCF elution was performed according to Curtis et al. [34] with a slight modification. A total of 150 μL 2% bovine serum albumin (0.01 M, pH 7.2) in phosphate-buffered saline (PBS) was added to each tube, and the samples were incubated at 4 °C for 60 min. Following incubation, a sterile drill was used to bore a hole in the bottom of each tube, which was then placed inside a 1.5 mL tube, and the nested tubes were centrifuged at 10,000 g for 10 min at 4 °C.
ELISA
The amounts of t-PA (eBioscience catalog no: BMS258/2/BMS258/2TEN; Bender MedSystems GmbH Campus Vienna Biocenter 2, 1030 Vienna, Austria) and PAI-1(eBioscience catalog no: BMS2033/BMSTEN; Bender MedSystems GmbH Campus Vienna Biocenter 2, 1030 Vienna, Austria) in each GCF sample were determined using a standard enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions. Reactions were terminated by adding an acid solution, and color change was measured spectrophotometrically at a wavelength of 450 nm. Concentrations of t-PA and PAI-1 and total amounts (pg/30 s) of t-PA and PAI-1 collected from each sample in 30 s were calculated using standard curves and recorded for analysis.
Statistical analysis and sample size calculation
Statistical analysis was performed using the statistical software program SPSS (SPSS v.21.0 Inc., Chicago, IL, USA). A Shapiro–Wilk test was performed to evaluate data normality. Data showing normal distribution were evaluated with One-way ANOVA followed by post-hoc Tukey and Student-t tests, with the results presented as mean ± standard error of the mean (SEM). Data not showing normal distribution were evaluated with non-parametric Mann Whitney U and Wilcoxon Signed rank tests with Bonferroni correction, with the results presented as median (min–max). A p-value of < 0.05 was considered statistically significant. The sample size required to ensure adequate power for this study was calculated based on changes in clinical attachment levels (CAL) [35], with a minimum requirement of 15 patients per group identified to ensure accuracy of α = 0.05 at a confidence level of 82%.
Results
All study participants completed the 1-month evaluation and treatment period without missing appointments. Healing was uneventful in all cases, with no reports of adverse effects (e.g., burning, pain) related to laser irradiation. Pre- and post-treatment clinical measurements and the change in the clinical parameters (PPD reduction and CAL gain) before and after treatment are given in Tables 1 and 2. All clinical parameters showed statistically significant reductions between baseline and Day 30 in the LLLT and Sham groups, with no significant differences in the reductions observed between groups. Significant reductions in GCF volumes were observed at all time points in both treatment groups (Table 3, p < 0.001). Steady decreases in GCF volumes were observed in both the LLLT and Sham groups throughout the treatment period. GCF volumes did not vary significantly between the LLLT and Sham groups at any time tested (> 0.05). Whereas GCF volumes had been reduced to levels similar to those of the healthy Control group by Day 14 in the LLLT group (> 0.05), similar reductions were not observed in the Sham group until Day 30 (> 0.05) (Tables 1 and 2).
GCF tPA
No significant decreases (> 0.05) over time were observed in the GCF concentrations of tPA levels of either the LLLT or Sham group at any time. Total GCF tPA decreased significantly (< 0.001) in both groups at each time point examined (Table 3, Fig. 1). When LLLT and Sham groups were compared, no differences were observed between the groups in either GCF concentrations of tPA or total GCF tPA levels at any time point, except the total GCF tPA levels measured on Day 7, which were significantly (< 0.05) lower in the LLLT group as compared to the Sham group. Moreover, the LLLT group generally tended to have lower total GCF tPA levels than the Sham group (Table 3). When compared to the Control group, both periodontitis treatment groups had significantly lower GCF concentrations of tPA levels at baseline (< 0.05); however, these differences were no longer observed after Day 7 (> 0.05). Compared to the Control group, both periodontitis treatment groups had significantly higher total GCF tPA levels at baseline, and these differences continued to be observed on both Days 7 and 14 (< 0.01). It was not until Day 30 that total GCF tPA levels in both treatment groups decreased to a level similar to that of the healthy Control group (> 0.05; Table 3, Fig. 1).
A GCF concentrations of tPA levels (tPAc) of the control and the study groups at baseline and during follow-up visits on days 7, 14, and 30. B Total GCF tPA levels (tPAt) of the control and the study groups at baseline and during follow-up visits on days 7, 14, and 30. Box plots show the median, first and third quartiles, and minimum and maximum values (whiskers). (*Significantly different compared to baseline in NSPT + Sham, post-hoc Tukey test, P < 0,001; †Significantly different compared to baseline in NSPT + LLLT, post-hoc Tukey test, P < 0,001; ‡Significantly different compared to healthy control, Student's t-test, P < 0,05)
GCF PAI-1
As with GCF concentrations of tPA levels, no significant decreases (> 0.05) over time were observed in the GCF concentrations of PAI-1 levels of either the LLLT or Sham group at any time. By contrast, steady reductions in total GCF PAI-1 levels of both treatment groups were observed over time, with significant differences detected at each examination time point (< 0.001; Table 3, Fig. 2). When LLLT and Sham groups were compared, no differences were observed between either the GCF concentrations of PAI-1 or total GCF PAI-1 levels of the groups at any time point except total GCF PAI-1 levels on Day 30, which were significantly (< 0.01) lower in the LLLT group (Table 3). Compared to the Control group, baseline GCF concentrations of PAI-1 levels were significantly lower in both periodontitis treatment groups (< 0.05). Levels in both treatment groups increased to a level similar to that of the Control group by Day 7 and remained similar throughout the study (> 0.05). By contrast, total GCF PAI-1 levels were significantly higher in both periodontitis treatment groups compared to the Control group at baseline (< 0.001). While the levels in the LLLT group had decreased to those of the Control group on Day 30 (> 0.05), levels in the Sham group continued to remain significantly (< 0.001) higher than the levels of the Control group throughout the study, and, increased significantly at each time point measured (Table 3, Fig. 2).
A GCF concentrations of PAI-1 levels (PAI-1c) of the control and the study groups at baseline and during follow-up visits on days 7, 14, and 30. B Total GCF PAI-1 levels (PAI-1t)of the control and the study groups at baseline and during follow-up visits on days 7, 14, and 30. Box plots show the median, first and third quartiles, and minimum and maximum values (whiskers). (*Significantly different compared to baseline in NSPT + Sham, post-hoc Tukey test, P < 0, 001; † Significantly different compared to baseline in NSPT + LLLT, post-hoc Tukey test, P < 0, 001; ‡ Significantly different compared to healthy control, Student's t-test, P < 0, 05)
Discussion
The use of diode lasers or any low-level laser irradiation as either monotherapy or as an adjunct to SRP in treating any form of periodontitis is still controversial [36,37,38]. While Annaji et al. [39] and Kamma et al. [40] found clinical and immunological outcomes in Stage 3–4, Grade C patients to be significantly better with LLLT compared to SRP, Ertugrul et al. [41] reported comparable improvements in the clinical outcomes with both LLLT and SRP. Given the current lack of clarity on the subject, this study examined the effects of LLLT as an adjunct to NSPT on clinical parameters and inflammatory markers in non-smoking Stage 3–4, Grade C periodontitis patients. In all cases, postoperative healing was uneventful, and no complications were observed. While using LLLT did not significantly improve clinical periodontal parameters, decreases in total tPA and PAI-1 GCF levels were observed, suggesting that LLLT, in conjunction with NSPT, may have beneficial effects on the healing of periodontal tissue.
NSPT combined with plaque control improve clinical conditions, reduce clinical inflammation, decrease pocket depth, and increase clinical attachment levels in patients with Stage 3–4, Grade C periodontitis [4]. In the present study, the clinical parameters of patients receiving NSPT improved significantly throughout the 1 month following initial treatment, regardless of whether or not LLLT was applied as an adjunct therapy. These findings align with a split-mouth study [41], in which SRP was only performed in the right maxillary and mandibular quadrants. In contrast, SRP plus Er, Cr: YSGG laser treatment was applied in the left maxillary quadrant, and SRP plus diode laser treatment was applied in the left mandibular quadrant. The authors concluded that diode lasers did not provide additional clinical benefits over conventional treatment in patients with Stage 3–4, Grade C periodontitis. Several previous studies [39, 40, 42, 43] have reported significantly better outcomes when laser treatment is used as an adjunct to non-surgical treatment for Stage 3–4, Grade C periodontitis. The differences in study findings may be attributed to differences in examination periods of studies, some of which conducted short-term follow-up periods of the month. In our study, for example, the 1-month follow-up period may not have been long enough to detect appropriate re-epithelization and the regeneration of epithelial attachment by healing connective tissue [44]. Besides differences in follow-up periods, differences in laser energy fluence, power output, application procedures, and duration could explain the conflicting findings among studies [31].
The initiation and progression of periodontal disease, wound healing, and tissue remodeling are affected by the plasminogen activating system [45], which must be kept under strict control of tissue integrity [24]. Components of the plasminogen system have been found in periodontal tissue [26, 27] suggesting that plasminogen activators and inhibitors are produced locally in both gingival tissue and GCF [27, 46, 47]. Gingivitis and periodontitis patients have higher GCF tPA levels than healthy individuals [23, 46, 48], and gingival biopsies have shown PAI-1 levels to be higher in patients with the periodontal disease when compared to healthy individuals [49]. The present study found that total GCF tPA and PAI-1 decreased significantly in Stage 3–4, Grade C periodontitis patients following NSPT treatment with or without LLLT; however, no changes were observed in GCF concentrations of tPA and PAI-1 levels. This may be related to the decrease in the volume of GCF found in periodontal tissue once the inflammation has been resolved through periodontal treatment and the concurrent increase in the concentrations of the component biological molecules [50].
Studies evaluating changes in t-PA about periodontal treatment in Stage 3–4, Grade C periodontitis patients are rare. Tuter et al. [48] reported reductions in GCF t-PA levels of both aggressive and chronic periodontitis patients following periodontal treatment; however, neither the levels nor the reductions in the amounts of GCF t-PA were affected by periodontitis type. The GCF levels of several cytokines and t-PA in patients with different types of periodontal disease were also evaluated by Toyman et al. [23], who found that regardless of periodontitis type, total amounts of t-PA were higher in patients with the periodontal disease when compared to normal controls. Similarly, our study found higher t-PA levels among periodontal patients and reductions in t-PA following periodontal treatment.
The present study is the first to evaluate GCF PAI-1 levels before and after periodontal treatment in Stage 3–4, Grade C periodontitis. Previously, we investigated the effects of LLLT as an adjunct to SRP on smoking and non-smoking patients with chronic periodontitis [51]. We chose to study up to 30 days mainly based on the biological actions of the PAI-I. PAI-1 plays an important role in the early/initial response of periodontal tissue to treatment. Therefore, we evaluated the short-term inflammation period up to 30 days. We found that GCF PAI levels showed steady reductions over time in both the LLLT and Sham groups of smokers as well as non-smokers, with the differences in reductions achieved with LLLT as compared to sham treatment significant only for smokers (P < 0.05). Moreover, the changes in GCF PAI-1 levels did not vary significantly between the LLLT and Sham groups. Compared to the control group, the gingival crevicular fluid PAI-1 levels in chronic periodontitis remained significantly higher. Deppe et al. [49] which looked at biopsied periodontal tissue, but not GCF, found the amounts of urokinase-type plasminogen activator (uPA) and its PAI-1 to be higher in inflamed periodontal tissue when compared to the healthy oral mucosa [49] consistent with our study. Behle et al. [52] examined serum PAI-1 levels in periodontitis patients receiving comprehensive periodontal treatment and found significant reductions within a 6-week period, which is consistent with the findings of the present study. Similarly, Taylor et al. [53] reported declines in serum PAI-1 levels within 3 months of the initiation of periodontal treatment. Both Bizzaro et al. [54] and Akman et al. [55] documented the importance of serum PAI-1 as a marker in periodontal disease, with PAI-1 levels increasing with increases in inflammation and disease progression. The reduction of total GCF PAI-1 in our study reflects the reductions in tissue inflammation achieved through periodontal treatment. Ozawa et al. [56] found that low-energy diode-laser irradiation applied to human periodontal ligament cells in a culture medium significantly inhibited the production of stress-induced tPA. Takema et al. [57] reported that low-level laser irradiation reduced tPA mRNA levels, significantly decreasing lipopolysaccharide-induced PA activity in human gingival fibroblasts. Another study in which changes in GCF tPA and PAI-1 were evaluated about laser irradiation [51] concluded that by modulating GCF tPA and PAI-1 levels, LLLT administered as an adjunct to non-surgical periodontal treatment could help resolve inflammation and support periodontal tissue-healing, particularly in patients with chronic periodontitis who smoke. A recent review study by Mokeem et al. [38] stated that SRP combined with LLLT in the treatment of aggressive periodontitis appears to promote positive outcomes in terms of periodontal parameters but that the lack of available data makes it impossible to reach a definitive conclusion as to whether SRP plus LLLT adjunct therapy is superior to SRP alone. In the present study, changes in GCF concentrations of tPA and PAI-1 levels did not vary significantly between the LLLT and Sham groups. The concentrations of these markers in both treatment groups were similar to those of healthy controls after Day 7. Although total GCF tPA levels in the LLLT group tended to be lower than those in the Sham group at all time points, it was only on Day 7 that any statistically significant differences between the two groups were observed, with the LLLT group exhibiting significantly lower total GCF tPA levels as well as lower total amounts of GCF tPA and PAI-1 at this time. The LLLT group also showed lower total GCF PAI-1 levels than the Sham group at all times, but the difference was only statistically significant on Day 30. Moreover, while the whole GCF PAI-1 level of the LLLT group had decreased to a level similar to that of the healthy Control group on Day 30, a similar decrease was not observed in the total GCF PAI-1 levels of the Sham group on Day 30. This differs from the outcomes for total GCF tPA, for which levels in the LLLT and Sham groups dropped to levels similar to those of the healthy controls on Day 30.
LLLT may play a role in the observed fluctuations in plasminogen inhibitors and activators. The ability of specific periodontal pathogens to invade periodontal tissue is well known. For example, A.actinomycetemcomitans has been found in the connective tissue of both active and inactive sites [58], and P.gingivalis has been shown to adhere to and enter oral epithelial cells [59]. Both these organisms can invade tissue in Stage 3–4, Grade C periodontitis patients and are thus highly pathogenic. Due to their bactericidal and detoxifying properties, diode lasers have previously been proposed as an adjunctive treatment to facilitate non-surgical periodontal treatment [60]. Laser-assisted treatment has shown strong antibacterial effects in terms of periodontopathogen counts [40]. A significant dose-dependent drop in bacterial LPS-induced elevated plasminogen activity in gingival fibroblasts has also been demonstrated following laser irradiation [57] Furthermore, the ability of bacteria to, directly and indirectly, modulate plasminogen activation [61] implies that a well-balanced equilibrium must be maintained [62]. Through its effects on the levels of plasminogen activator system components, periodontal treatment may modulate local proteolytic activity.
The present study has several methodological limitations. The study's small sample size may affect the reproducibility of the results, which must, therefore, be interpreted with caution. Standardized criteria for periodontal laser therapy that address energy levels, application time, irradiation modes, power settings, and laser types are needed. In summary, the application of LLLT as an adjunct to non-surgical periodontal treatment of Stage 3–4, Grade C periodontitis patients can have a considerable positive impact on the resolution of inflammation and periodontal tissue-healing by modulating GCF tPA and PAI-1 levels.
Data Availability
N/A.
References
Manresa C, Sanz-Miralles EC, Twigg J, Bravo M (2018) Supportive periodontal therapy (SPT) for maintaining the dentition in adults treated for periodontitis. Cochrane Database Syst Rev 1:CD009376. https://doi.org/10.1002/14651858.CD009376.pub2
Sanz I, Alonso B, Carasol M, Herrera D, Sanz M (2012) Nonsurgical treatment of periodontitis. J Evid Based Dent Pract 12:76–86. https://doi.org/10.1016/S1532-3382(12)70019-2
Umeda M, Takeuchi Y, Noguchi K, Huang Y, Koshy G, Ishikawa I (2004) Effects of nonsurgical periodontal therapy on the microbiota. Periodontol 2000 36:98–120. https://doi.org/10.1111/j.1600-0757.2004.03675.x
Keestra JA, Grosjean I, Coucke W, Quirynen M, Teughels W (2015) Non-surgical periodontal therapy with systemic antibiotics in patients with untreated aggressive periodontitis: a systematic review and meta-analysis. J Periodontal Res 50:689–706. https://doi.org/10.1111/jre.12252
Belibasakis GN, Belstrom D, Eick S, Gursoy UK, Johansson A, Kononen E (2023) Periodontal microbiology and microbial etiology of periodontal diseases: Historical concepts and contemporary perspectives. Periodontol 2000. https://doi.org/10.1111/prd.12473
Sedghi L, DiMassa V, Harrington A, Lynch SV, Kapila YL (2021) The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontol 2000 87:107–131. https://doi.org/10.1111/prd.12393
Joseph S, Curtis MA (2021) Microbial transitions from health to disease. Periodontol 2000 86:201–209. https://doi.org/10.1111/prd.12377
Teughels W, Dhondt R, Dekeyser C, Quirynen M (2014) Treatment of aggressive periodontitis. Periodontol 2000 65:107–133. https://doi.org/10.1111/prd.12020
Darveau RP, Curtis MA (2021) Oral biofilms revisited: A novel host tissue of bacteriological origin. Periodontol 2000 86:8–13. https://doi.org/10.1111/prd.12374
Sgolastra F, Petrucci A, Gatto R, Monaco A (2012) Effectiveness of systemic amoxicillin/metronidazole as an adjunctive therapy to full-mouth scaling and root planing in the treatment of aggressive periodontitis: a systematic review and meta-analysis. J Periodontol 83:731–743. https://doi.org/10.1902/jop.2011.110432
Rabelo CC, Feres M, Goncalves C, Figueiredo LC, Faveri M, Tu YK, Chambrone L (2015) Systemic antibiotics in the treatment of aggressive periodontitis. A systematic review and a Bayesian Network meta-analysis. J Clin Periodontol 42:647–657. https://doi.org/10.1111/jcpe.12427
Herrera D, van Winkelhoff AJ, Matesanz P, Lauwens K, Teughels W (2023) Europe's contribution to the evaluation of the use of systemic antimicrobials in the treatment of periodontitis. Periodontol 2000. https://doi.org/10.1111/prd.12492
Calderin S, Garcia-Nunez JA, Gomez C (2013) Short-term clinical and osteoimmunological effects of scaling and root planing complemented by simple or repeated laser phototherapy in chronic periodontitis. Lasers Med Sci 28:157–166. https://doi.org/10.1007/s10103-012-1104-5
Meimandi M, TalebiArdakani MR, EsmaeilNejad A, Yousefnejad P, Saebi K, Tayeed MH (2017) The Effect of Photodynamic Therapy in the Treatment of Chronic Periodontitis: A Review of Literature. J Lasers Med Sci 8:S7–S11. https://doi.org/10.15171/jlms.2017.s2
Grzech-Lesniak K, Matys J, Dominiak M (2018) Comparison of the clinical and microbiological effects of antibiotic therapy in periodontal pockets following laser treatment: An in vivo study. Adv Clin Exp Med 27:1263–1270. https://doi.org/10.17219/acem/70413
Schwarz F, Aoki A, Becker J, Sculean A (2008) Laser application in non-surgical periodontal therapy: a systematic review. J Clin Periodontol 35:29–44. https://doi.org/10.1111/j.1600-051X.2008.01259.x
Ren C, McGrath C, Jin L, Zhang C, Yang Y (2017) The effectiveness of low-level laser therapy as an adjunct to non-surgical periodontal treatment: a meta-analysis. J Periodontal Res 52:8–20. https://doi.org/10.1111/jre.12361
Qadri T, Miranda L, Tuner J, Gustafsson A (2005) The short-term effects of low-level lasers as adjunct therapy in the treatment of periodontal inflammation. J Clin Periodontol 32:714–719. https://doi.org/10.1111/j.1600-051X.2005.00749.x
Aykol G, Baser U, Maden I, Kazak Z, Onan U, Tanrikulu-Kucuk S, Ademoglu E, Issever H, Yalcin F (2011) The effect of low-level laser therapy as an adjunct to non-surgical periodontal treatment. J Periodontol 82:481–488. https://doi.org/10.1902/jop.2010.100195
Lai SM, Zee KY, Lai MK, Corbet EF (2009) Clinical and radiographic investigation of the adjunctive effects of a low-power He-Ne laser in the treatment of moderate to advanced periodontal disease: a pilot study. Photomed Laser Surg 27:287–293. https://doi.org/10.1089/pho.2007.2206
Takasaki AA, Aoki A, Mizutani K, Schwarz F, Sculean A, Wang CY, Koshy G, Romanos G, Ishikawa I, Izumi Y (2009) Application of antimicrobial photodynamic therapy in periodontal and peri-implant diseases. Periodontol 2000 51:109–40. https://doi.org/10.1111/j.1600-0757.2009.00302.x
Kurgan S, Onder C, Balci N, Fentoglu O, Eser F, Balseven M, Serdar MA, Tatakis DN, Gunhan M (2017) Gingival crevicular fluid tissue/blood vessel-type plasminogen activator and plasminogen activator inhibitor-2 levels in patients with rheumatoid arthritis: effects of nonsurgical periodontal therapy. J Periodontal Res 52:574–581. https://doi.org/10.1111/jre.12425
Toyman U, Tuter G, Kurtis B, Kivrak E, Bozkurt S, Yucel AA, Serdar M (2015) Evaluation of gingival crevicular fluid levels of tissue plasminogen activator, plasminogen activator inhibitor 2, matrix metalloproteinase-3 and interleukin 1-beta in patients with different periodontal diseases. J Periodontal Res 50:44–51. https://doi.org/10.1111/jre.12179
Sarajlic J, Agis H, Kandler B, Watzek G, Gruber R (2007) Plasminogen activation by fibroblasts from periodontal ligament and gingiva is not directly affected by chemokines in vitro. Arch Oral Biol 52:663–668. https://doi.org/10.1016/j.archoralbio.2006.12.020
Schmid J, Cohen RL, Chambers DA (1991) Plasminogen activator in human periodontal health and disease. Arch Oral Biol 36:245–250. https://doi.org/10.1016/0003-9969(91)90093-a
Wyganowska-Swiatkowska M, Surdacka A, Skrzypczak-Jankun E, Jankun J (2014) The plasminogen activation system in periodontal tissue (Review). Int J Mol Med 33:763–768. https://doi.org/10.3892/ijmm.2014.1653
Kinnby B (2002) The plasminogen activating system in periodontal health and disease. Biol Chem 383:85–92. https://doi.org/10.1515/BC.2002.008
Armitage GC (1999) Development of a classification system for periodontal diseases and conditions. Ann Periodontol 4:1–6. https://doi.org/10.1902/annals.1999.4.1.1
Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, Flemmig TF, Garcia R, Giannobile WV, Graziani F, Greenwell H, Herrera D, Kao RT, Kebschull M, Kinane DF, Kirkwood KL, Kocher T, Kornman KS, Kumar PS, Loos BG, Machtei E, Meng H, Mombelli A, Needleman I, Offenbacher S, Seymour GJ, Teles R, Tonetti MS (2018) Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol 89(Suppl 1):S173–S182. https://doi.org/10.1002/JPER.17-0721
Kanmaz B, Lappin DF, Nile CJ, Buduneli N (2020) Effects of smoking on non-surgical periodontal therapy in patients with periodontitis Stage III or IV, and Grade C. J Periodontol 91:442–453. https://doi.org/10.1002/JPER.19-0141
Aoki A, Mizutani K, Schwarz F, Sculean A, Yukna RA, Takasaki AA, Romanos GE, Taniguchi Y, Sasaki KM, Zeredo JL, Koshy G, Coluzzi DJ, White JM, Abiko Y, Ishikawa I, Izumi Y (2015) Periodontal and peri-implant wound healing following laser therapy. Periodontol 2000 68:217–69. https://doi.org/10.1111/prd.12080
Silness J, Loe H (1964) Periodontal disease in pregnancy. Ii. correlation between oral hygiene and periodontal condtion. Acta Odontol Scand 22:121–135. https://doi.org/10.3109/00016356408993968
Loe H, Silness J (1963) Periodontal disease in pregnancy. I prevalence and severity. Acta Odontol Scand 21:533–551. https://doi.org/10.3109/00016356309011240
Curtis MA, Griffiths GS, Price SJ, Coulthurst SK, Johnson NW (1988) The total protein concentration of gingival crevicular fluid. Variation with sampling time and gingival inflammation. J Clin Periodontol 15:628–632. https://doi.org/10.1111/j.1600-051x.1988.tb02263.x
Saglam M, Kantarci A, Dundar N, Hakki SS (2014) Clinical and biochemical effects of diode laser as an adjunct to nonsurgical treatment of chronic periodontitis: a randomized, controlled clinical trial. Lasers Med Sci 29:37–46. https://doi.org/10.1007/s10103-012-1230-0
Slot DE, Jorritsma KH, Cobb CM, Van der Weijden FA (2014) The effect of the thermal diode laser (wavelength 808–980 nm) in non-surgical periodontal therapy: a systematic review and meta-analysis. J Clin Periodontol 41:681–692. https://doi.org/10.1111/jcpe.12233
Dederich DN (2015) Little evidence for the use of diode lasers as an adjunct to non-surgical periodontal therapy. Evid Based Dent 16:16. https://doi.org/10.1038/sj.ebd.6401078
Mokeem S (2018) Efficacy of adjunctive low-level laser therapy in the treatment of aggressive periodontitis: A systematic review. J Investig Clin Dent 9:e12361. https://doi.org/10.1111/jicd.12361
Annaji S, Sarkar I, Rajan P, Pai J, Malagi S, Bharmappa R, Kamath V (2016) Efficacy of photodynamic therapy and lasers as an adjunct to scaling and root planing in the treatment of aggressive periodontitis - a clinical and microbiologic short term study. J Clin Diagn Res 10:ZC08-12. https://doi.org/10.7860/JCDR/2016/13844.7165
Kamma JJ, Vasdekis VG, Romanos GE (2009) The effect of diode laser (980 nm) treatment on aggressive periodontitis: evaluation of microbial and clinical parameters. Photomed Laser Surg 27:11–19. https://doi.org/10.1089/pho.2007.2233
Ertugrul AS, Tekin Y, Talmac AC (2017) Comparing the efficiency of Er, Cr:YSGG laser and diode laser on human beta-defensin-1 and IL-1beta levels during the treatment of generalized aggressive periodontitis and chronic periodontitis. J Cosmet Laser Ther 19:409–417. https://doi.org/10.1080/14764172.2017.1334923
Matarese G, Ramaglia L, Cicciu M, Cordasco G, Isola G (2017) The effects of diode laser therapy as an adjunct to scaling and root planing in the treatment of aggressive periodontitis: a 1-year randomized controlled clinical trial. Photomed Laser Surg 35:702–709. https://doi.org/10.1089/pho.2017.4288
Moreira AL, Novaes AB Jr, Grisi MF, Taba M Jr, Souza SL, Palioto DB, de Oliveira PG, Casati MZ, Casarin RC, Messora MR (2015) Antimicrobial photodynamic therapy as an adjunct to non-surgical treatment of aggressive periodontitis: a split-mouth randomized controlled trial. J Periodontol 86:376–386. https://doi.org/10.1902/jop.2014.140392
Borrajo JL, Varela LG, Castro GL, Rodriguez-Nunez I, Torreira MG (2004) Diode laser (980 nm) as adjunct to scaling and root planing. Photomed Laser Surg 22:509–512. https://doi.org/10.1089/pho.2004.22.509
Uitto VJ, Overall CM, McCulloch C (2003) Proteolytic host cell enzymes in gingival crevice fluid. Periodontol 2000 31:77–104. https://doi.org/10.1034/j.1600-0757.2003.03106.x
Olofsson A, Lindberg P, Lanke J, Matsson L, Kinnby B (2003) Relationship between fibrinolytic activity and gingival inflammatory reaction in young individuals. J Periodontal Res 38:104–108. https://doi.org/10.1034/j.1600-0765.2003.01370.x
Buduneli N, Buduneli E, Kardesler L, Lappin D, Kinane DF (2005) Plasminogen activator system in smokers and non-smokers with and without periodontal disease. J Clin Periodontol 32:417–424. https://doi.org/10.1111/j.1600-051X.2005.00694.x
Tuter G, Ozdemir B, Kurtis B, Serdar M, Yucel AA, Ayhan E (2013) Short term effects of non-surgical periodontal treatment on gingival crevicular fluid levels of tissue plasminogen activator (t-PA) and plasminogen activator inhibitor 2 (PAI-2) in patients with chronic and aggressive periodontitis. Arch Oral Biol 58:391–396. https://doi.org/10.1016/j.archoralbio.2012.08.008
Deppe H, Hohlweg-Majert B, Holzle F, Kesting MR, Wagenpfeil S, Wolff KD, Schmitt M (2010) Content of urokinase-type plasminogen activator (uPA) and its inhibitor PAI-1 in oral mucosa and inflamed periodontal tissue. Quintessence Int 41:165–171
Buduneli N, Buduneli E, Kutukculer N (2009) Interleukin-17, RANKL, and osteoprotegerin levels in gingival crevicular fluid from smoking and non-smoking patients with chronic periodontitis during initial periodontal treatment. J Periodontol 80:1274–1280. https://doi.org/10.1902/jop.2009.090106
Pamuk F, Lutfioglu M, Aydogdu A, Koyuncuoglu CZ, Cifcibasi E, Badur OS (2017) The effect of low-level laser therapy as an adjunct to non-surgical periodontal treatment on gingival crevicular fluid levels of transforming growth factor-beta 1, tissue plasminogen activator and plasminogen activator inhibitor 1 in smoking and non-smoking chronic periodontitis patients: A split-mouth, randomized control study. J Periodontal Res 52:872–882. https://doi.org/10.1111/jre.12457
Behle JH, Sedaghatfar MH, Demmer RT, Wolf DL, Celenti R, Kebschull M, Belusko PB, Herrera-Abreu M, Lalla E, Papapanou PN (2009) Heterogeneity of systemic inflammatory responses to periodontal therapy. J Clin Periodontol 36:287–294. https://doi.org/10.1111/j.1600-051X.2009.01382.x
Taylor B, Tofler G, Morel-Kopp MC, Carey H, Carter T, Elliott M, Dailey C, Villata L, Ward C, Woodward M, Schenck K (2010) The effect of initial treatment of periodontitis on systemic markers of inflammation and cardiovascular risk: a randomized controlled trial. Eur J Oral Sci 118:350–356. https://doi.org/10.1111/j.1600-0722.2010.00748.x
Bizzarro S, van der Velden U, ten Heggeler JM, Leivadaros E, Hoek FJ, Gerdes VE, Bakker SJ, Gans RO, Ten Cate H, Loos BG (2007) Periodontitis is characterized by elevated PAI-1 activity. J Clin Periodontol 34:574–580. https://doi.org/10.1111/j.1600-051X.2007.01095.x
Akman PT, Fentoglu O, Yilmaz G, Arpak N (2012) Serum plasminogen activator inhibitor-1 and tumor necrosis factor-alpha levels in obesity and periodontal disease. J Periodontol 83:1057–1062. https://doi.org/10.1902/jop.2011.110548
Ozawa Y, Shimizu N, Abiko Y (1997) Low-energy diode laser irradiation reduced plasminogen activator activity in human periodontal ligament cells. Lasers Surg Med 21:456–463. https://doi.org/10.1002/(sici)1096-9101(1997)21:5%3c456::aid-lsm7%3e3.0.co;2-p
Takema T, Yamaguchi M, Abiko Y (2000) Reduction of plasminogen activator activity stimulated by lipopolysaccharide from periodontal pathogen in human gingival fibroblasts by low-energy laser irradiation. Lasers Med Sci 15:35–42. https://doi.org/10.1007/s101030050045
Saglie FR, Marfany A, Camargo P (1988) Intragingival occurrence of Actinobacillus actinomycetemcomitans and Bacteroides gingivalis in active destructive periodontal lesions. J Periodontol 59:259–265. https://doi.org/10.1902/jop.1988.59.4.259
Sandros J, Papapanou PN, Nannmark U, Dahlen G (1994) Porphyromonas gingivalis invades human pocket epithelium in vitro. J Periodontal Res 29:62–69. https://doi.org/10.1111/j.1600-0765.1994.tb01092.x
Moritz A, Schoop U, Goharkhay K, Schauer P, Doertbudak O, Wernisch J, Sperr W (1998) Treatment of periodontal pockets with a diode laser. Lasers Surg Med 22:302–311. https://doi.org/10.1002/(sici)1096-9101(1998)22:5%3c302::aid-lsm7%3e3.0.co;2-t
Fleetwood AJ, O’Brien-Simpson NM, Veith PD, Lam RS, Achuthan A, Cook AD, Singleton W, Lund IK, Reynolds EC, Hamilton JA (2015) Porphyromonas gingivalis-derived RgpA-Kgp complex activates the macrophage urokinase plasminogen activator system: implications for periodontıtıs. J Biol Chem 290:16031–16042. https://doi.org/10.1074/jbc.M115.645572
Andreasen PA, Egelund R, Petersen HH (2000) The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol Life Sci 57:25–40. https://doi.org/10.1007/s000180050497
Funding
This study was supported by the Scientific Research Fund of Beykent University (Grant/Award Number: 2018–19-BAP-05).
Author information
Authors and Affiliations
Contributions
Pamuk F prepared the manuscript and applied all treatment protocols. Lütfioğlu M prepared the manuscript and applied the statistical analysis and sample size calculation. Paksoy T prepared the manuscript and organised tables. Koyuncuoglu CZ performed all clinical measurements and GCF sampling. Polat NG performed ELISA. Cifcibasi E contributed to all ethical procedures and patient screening. Yildirim S organized the figures and organized data and the results. Kantarci A edited the manuscript and guided the study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The informed consent was obtained from study participants by written.
Competing interests
The authors declare no competing interests.
Conflict of interests
The authors report no conflict of interest related to this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pamuk, F., Lütfioğlu, M., Paksoy, T. et al. Impact of low-level laser therapy as an adjunct to non-surgical periodontal treatment on the levels of tissue plasminogen activator and plasminogen activator inhibitor 1 in Stage 3–4, Grade C periodontitis patients: a split-mouth, randomized control study. Clin Oral Invest 27, 6439–6449 (2023). https://doi.org/10.1007/s00784-023-05248-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-023-05248-z