Abstract
The treatment of severely atrophied posterior mandibles with standard-diameter root-form implants may present a challenge. Bone reconstructive surgery represents the treatment of choice; however, it may not be accepted by some patients for economic reasons or due to higher morbidity. Computer-aided design/computer-aided manufacturing (CAD/CAM) technologies have recently opened new frontiers in biomedical applications. Selective laser sintering (SLS) is a CAD/CAM technique that allows the fabrication of complex three-dimensional (3D) structures created by computer-generated image-based design techniques. The aim of this study is to present a protocol for the manufacture and clinical use of custom-made SLS titanium blade implants as a non-conventional therapeutic treatment for the prosthetic rehabilitation of extremely atrophied posterior mandibles. Computed tomography datasets of five patients were transferred to a specific reconstruction software, where a 3D projection of the atrophied mandible was obtained, and custom-made endosseous blade implants were designed. The custom-made implants were fabricated with SLS technique, placed in the extremely atrophied posterior (<4 mm width) mandible, and immediately restored with fixed partial restorations. After 2 years of loading, all implants were in function, showing a good esthetic integration. Blade implants can be fabricated on an individual basis as a custom-designed device. This non-conventional approach may represent an option for restoring the atrophied posterior mandible of elderly patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prosthetic rehabilitation of partially and totally edentulous patients with endosseous dental implants has become common practice in the last few decades, with reliable long-term results [1, 2]. In the severely resorbed posterior mandible, however, the use of standard-diameter root-form implants may present a challenge [2]. Some residual ridges, in fact, are very thin and cannot accept standard-diameter root-form implants within the confines of the available bone with 1- to 2-mm circumferential bone thickness [2].
The ideal approach would be to augment the bone horizontally in a predictably successful way, and the first therapeutic option to provide better functional and esthetic results is alveolar ridge augmentation that simultaneously provides better implant support and decreased inter-arch length [2, 3]. Bone reconstructive surgery represents the treatment of choice to recreate the correct bone volume and morphology; however, reconstructive procedures may not be accepted by the patients for economic reasons or due to higher morbidity [2, 3]. Most of these patients, in fact, are elderly who may have general health problems or may simply not want to pursue this option due to the complexity of the treatment and financial concerns.
In most indications, the use of narrow-diameter implants would enable the dentist to rehabilitate the patient without grafting [2, 4]; however, in case of residual alveolar ridge less than 4-mm wide, even narrow-diameter implants cannot be used. Endosseous dental implants have been traditionally manufactured in two basic designs: root-form (cylinders and screws) and plate-form (blades) [5, 6].
The blade implants are thin, flat, and usually rectangular-shaped pieces of metal with either one or two prongs on the long side of the blade [5–11]; the prongs stick out into the mouth where they support fixed partial restorations. This transgingival design is featured for one-stage surgical procedure [5–11]. The plate-form cross-sectional dimension of blade implants is such that they can be placed in as little as 3 mm of bone; whether the cross section of the bone is narrow or wide, in either the maxillary or the mandibular arch, these implants can be used [5–11].
In modern oral implantology, the history of blade implants was started by Professor Linkow in the USA [5]. The blade implant is a modality that has been used for several decades, although its popularity has declined in favor of the root-form dental implants. Blade implants, in fact, had a high incidence of failure due to the difficulty of achieving primary stability at placement, loading protocol, and postoperative complications [10–14]. However, some plate-form implants placed 20–30 years ago still survive; there are many papers describing the blade implants [9–11], and these implants can be successfully used in the case of thin posterior mandibular ridges [10, 11].
Computer-aided design/computer-aided manufacturing (CAD/CAM) technologies have recently opened new frontiers in biomedical applications [15]. In particular, technologies for producing CAD models based on computed tomography (CT) techniques have been developed, such as rapid prototyping (RP) [15–19]. RP comprises a group of techniques that can generate a physical model directly from CAD data or data provided by computer-based medical imaging technologies in a layer-by-layer manner, where each layer is the shape of the cross section of the model at a specific level [15–19].
Selective laser sintering (SLS) is a laser-based RP technique in which an object is built layer-by-layer using powdered materials, radiant heaters, and a computer-controlled laser [17–22]. The SLS models are generated directly from three-dimensional (3D) computer data converted to stereolithographic (STL) files, which are then sliced into thin layers using the associated software. The laser sintering machine produces the models on a moveable platform by applying incremental layers of the pattern material. For each layer, the machine lays down a film of powdered material with an accurate thickness (0.1 mm/0.004 in.). The laser then melts selected areas so that they conform to the previous layer. The platform moves down the preprogrammed layer thickness, a fresh film of powder is laid down, and the next layer is melted with exposure to the laser source. This process continues, layer by layer, until the object is fabricated [17–22].
With SLS, patient-specific medical devices and implants may be prepared using CAD models derived from patient CT data [18, 19, 21, 22]. In fact, techniques have been developed for fabricating custom-made titanium implants based on models of the jaws made via CT scan and CAD/CAM technology [18, 19, 21, 22]. These patient-specific implants may possess suitable features, including geometry, size, and weight for a given patient and a given medical condition [18, 19, 21, 22]. The aim of this study is to present a new protocol for the fabrication and clinical use of custom-made SLS titanium blade implants as a non-conventional therapeutic treatment for the prosthetic rehabilitation of extremely atrophied posterior mandibles.
Materials and methods
Patient selection
Between January 2009 and January 2010, all patients referred to the dental clinic of the University of Varese, Italy, and to a private clinic (Brescia, Italy) for treatment with oral implants were considered for inclusion in this study. Only elderly patients (65–75 years old) presenting with partial edentulism associated with horizontal defects of the lateral posterior alveolar ridges of the mandible (from the first premolar to the second molar), which render the placement of standard-diameter implant (4 mm) difficult or impossible, were selected for this study. Exclusion criteria consisted of poor oral hygiene, active periodontal infections, uncontrolled diabetes, bruxism, and smoking habit. The study protocol was explained to each subject, and a signed informed consent was obtained. The study was performed according to the principles outlined in the World's Medical Association's Declaration of Helsinki on experimentation involving human subjects, as revised in 2008, and it was approved by the Local Ethics Committee for Human Studies at the University of Varese, Italy.
Preoperative work-up
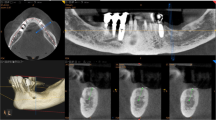
A complete examination of the oral hard and soft tissues was carried out for each patient (Fig. 1). Preoperative work-ups included an assessment of the partially edentulous posterior ridge using casts and diagnostic wax-up. Panoramic X-ray and mandibular posterior cone beam CT scan were taken for each patient, which revealed that the width of the residual posterior mandibular ridge was minimal (<4 mm). CT datasets were transferred in the DICOM (Digital Imaging and Communications in Medicine) format to a specific implant navigation software (MimicsR, Materialise, Leuven, Belgium) to perform a 3D reconstruction of the mandible. Through this navigation software, it was possible to correctly assess the width of the jawbone, the thickness and the density of the cortical plates and the cancellous bone, and the ridge angulation. Bone density was also assessed to obtain a reliable and valid description of preoperative jawbone condition.
Implant design and fabrication
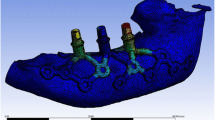
Within the confines of the 3D projection of the atrophied posterior mandibular ridge, a custom-made endosseous blade implant was drawn. This “virtual” implant was isolated as a STL file and transferred to a proprietary reverse-engineering software (Leader-NovaxaR, Milan, Italy). The implant was smoothed in order to obtain a regular surface. The STL file was returned to the 3D reconstruction software again (MimicsR, Materialise, Leuven, Belgio) to test the congruence between the implant and the implant site. Then, the file was transferred to Pro/Engineering CAD 3D software (PTC GroupR, Needham, MA, USA) where two prosthetic conical abutments were designed and added to the implant structure (Fig. 2). Finally, with the aid of another 3D reconstruction software (MagicsR, Materialise, Leuven, Belgium), copies of the final STL file (virtual blade implant plus abutments) were prepared, with sequential percentage dimensional increments in order to provide the surgeon with at least three different STL files representing different size increments (0, 5, and 10 %) of the same object (to avoid potential distortions or errors related to the 3D projection steps). All three STL copies were then used to manufacture the implants using the SLS technique (TixOsR, Leader-Novaxa, Milan, Italy), as previously reported [2, 18, 19, 23–26]. The SLS implants were made of master alloy powder (Ti-6Al-4 V), with a particle size of 25 to 45 μm, as the basic material. Processing was carried out in an argon atmosphere using a powerful Yb (ytterbium) fiber laser system with the capacity to build a volume up to 250 × 250 × 215 mm using a wavelength of 1,054 nm with a continuous power of 200 W at a scanning rate of 7 m/s. The size of the laser spot was 0.1 mm. To remove residual particles from the manufacturing process, the sample was sonicated for 5 min in distilled water at 25 °C, immersed in sodium hydroxide (20 g/L) and hydrogen peroxide (20 g/L) at 80 °C for 30 min, and then further sonicated for 5 min in distilled water. Acid etching was carried out by immersion of the samples in a mixture of 50 % oxalic acid and 50 % maleic acid at 80 °C for 45 min, followed by washing for 5 min in distilled water in a sonic bath. The surface topography of the SLS implants had no clear orientation. The direct laser preparation provided an implant surface with a roughness with the mean ± SD of the absolute values of all profile points, the root-mean-square of the values of all points, and the average value of the absolute heights of the five highest peaks and the depths of the five deepest valleys of 66.8 ± 6.6 μm, 77.6 ±11.1 μm, and 358.3 ±101.9 μm, respectively [2, 18, 19, 23–26]. The SLS implant was a blade-type device design consisting of one-piece construction having a body and two posts. The axis of the implant posts were adjusted to align with the adjacent teeth, and the posts were designed to receive the fabricated fixed partial prosthesis. The standard implant body dimensions were 2-mm thick, 8- or 10-mm high, and 10-, 12-, or 15-mm long. Hemi-holes through the inferior implant body serve as added retention by providing physical space for bone to grow through the device body.
Surgical and prosthetic procedure
Local anesthesia was obtained by infiltrating with articaine 4 % containing 1:100.000 adrenaline (UbistesinR, 3 M Espe, St. Paul, MN, USA). A ridge crest incision was made in the edentulous posterior mandible. Minor reflection of the soft tissues was made exposing only the labial and lingual crestal bone. This provided direct assessment of the bone width while retaining the periosteum and blood supply. The residual alveolar ridge was less than 4-mm wide and concave in the anterior segment. Osteotomies to a depth of 8–10 mm were prepared under copious water irrigation. The entire procedure was performed atraumatically using a surgical technique called piezosurgery [27]. The custom-made implants were transferred to the site, inserted, and seated solidly with the aid of a surgical mallet. The custom-made implants fitted perfectly into the osteotomy sites, which were prepared according to the implant dimensions (Fig. 3). The implant body was placed approximately 1 mm below the alveolar ridge peak, with the abutments protruding into the mouth. The flaps were repositioned, and suturing (SupramidR, Novaxa, Milan, Italy) was performed on either side of the two protruding implant abutments. Immediately after implant placement, provisional restorations, fabricated in the dental laboratory prior to surgery using either a technique where a diagnostic waxing was duplicated to create a gypsum cast or by using cast-based guided surgery with prefabricated provisional restorations, were placed in position, relined intraorally (Unifast LCR; GC Corp, Tokyo, Japan), and cemented with temporary cement (Temp-BondR, Kerr, Orange, CA, USA). Care was taken to prevent the cement from penetrating subgingivally or to remove it thoroughly if this occurred. The occlusion was carefully checked. The provisional restorations were placed in light maximum intercuspation contact without working and nonworking contacts. Finally, the patients received postoperative instructions. All patients received oral antibiotics, 2 g each day for 6 days (AugmentinR, Glaxo-Smithkline Beecham, Brentford, UK). Postoperative pain was controlled by administering 100 mg nimesulide (AulinR, Roche Pharmaceutical, Basel, Switzerland) every 12 h for 2 days, and detailed instructions about oral hygiene were given, with mouth rinses with 0.12 % chlorhexidine (ChlorexidineR, OralB, Boston, MA, USA) administered for 7 days. The patients were seen on a weekly basis during the first 4 weeks. At the first control visit, 7 days after the surgery, a clinically healthy marginal area was present, and no postoperative pain or swelling was reported. There was no bleeding or wound infection. Sutures were removed. After 2 weeks, the peri-implant tissues showed a good marginal adaptation. After 3 weeks, the peri-implant tissues were stable and in optimal conditions. Provisional restorations were used to monitor implant stability under a progressive load and to obtain good soft tissue healing around the implant before fabrication of the definitive restorations. The placement of the definitive prosthetic restorations was performed on an individual basis after soft tissue maturation, at least 3 months after implant placement. All definitive restorations were ceramo-metallic, cemented with temporary cement (Temp-BondR, Kerr, Orange, CA, USA). These restorations were carefully evaluated for proper occlusion, and protrusion and laterotrusion were assessed on the articulator and intraorally.
Results
A total of five patients (three males and two females, aged between 57 and 74 years, mean age 66.7 years) were enrolled in this prospective clinical study. In total, five blade implants were positioned in the extremely atrophied posterior mandible by two experienced surgeons. The prosthetic restorations comprised five fixed partial prostheses. Two years after placement, the blade implants have been shown to be functional and esthetic (Fig. 4). In fact, at the end of the study, all implants were in function. None of the implants caused pain or clinical mobility, suppuration, or exudation, and none had any prosthetic complication. No implant fractures or prosthetic complications related to the suprastructure (fixed partial prostheses) were observed. The good conditions of the peri-implant tissues were confirmed by radiographic examination, with unchanged peri-implant marginal bone level and no peri-implant radiolucency (Fig. 5).
Discussion
In the treatment of severely atrophied posterior mandibles using osseointegrated root-form implants, extensive bone-grafting procedures are often required prior to placing the implants. Such procedures are time consuming as they can take over a year before the prosthesis can be fabricated, and the surgical expense can be high [3]. Multiple surgeries are required with a team of doctors from different surgical disciplines, and morbidity of the donor site may occur, simply to increase masticatory function [3]; elderly patients shouldn't be subjected to extensive and multiple surgeries unless absolutely necessary. For these reasons, the use of conventional root-form implants may be rejected by patients.
In modern implant dentistry, priority should be given to those interventions that look simpler, are less invasive, involve less risk of complications, and reach their goal within the shortest timeframe. In most indications, a stable dental prosthesis is the primary objective; therefore, from the standpoint of longevity, load-bearing capabilities, functional efficiency, and ease of prosthetic maintenance, blade implants could be considered for the rehabilitation of severely atrophied posterior mandibles.
Blade implants require less bone width for placement than that required for the placement of standard root-form implants [5–11]. For this reason, they may offer potential advantages of minimizing ridge augmentation treatment needs, reducing the length of treatment time required to restore lost prosthetic function, and possibly providing a lower financial burden for some patients, in comparison to conventional treatment plans involving root-form dental implant systems [5–11].
Originating from the USA in the early 1960s [5, 6], blade implants appear to be rarely utilized today in oral implantology practices. The popularity of these implants, in fact, greatly diminished after the introduction of the root-form endosseous implants in the early 1980s [10, 11]. In fact, a lack of osseointegration often resulted due to the nature of the surgical techniques, the difficulty to achieve primary stability, and the loading protocol traditionally used for blade implants [10–14]. The placement of blade implants required surgical skills and adequate training. Primary stability of root-form implants can be easily achieved biomechanically by creating an osteotomy that has a diameter less than the diameter of the implant. In the past, it was more difficult to achieve primary stability using blade implants; implant failure could result from insufficient primary stability or by an inadequately stabilized early loading of the implant [10–14].
Recent histologic studies, however, have supported bone integration to titanium independent of whether the implant is in the physical form of a plate or a cylinder [12–14], and showed that osseointegration can be obtained in immediately loaded blade implant inserted into the mandible and that mineralized tissues were maintained at the interface over a long period (20 years) [12–14]. Blade implants, in fact, can present a direct bone contact even if they are loaded immediately after insertion [12–14]. Trisi et al., Proussaefs and Lozada, and Di Stefano et al. reported that blade implants retrieved respectively after 7, 13, and 20 years of function exhibited mature bone in tight contact with the implant and that it was present around most of the implant surface [12–14]. In addition, the peri-implant bone formation did not appear to be disturbed by the stresses and strains at the interface, and mineralized tissues were maintained at the bone–implant interface [28].
Nowadays, with the aim of computer-aided design/computer-aided manufacturing (CAD/CAM) and rapid prototyping (RP) techniques, the previously unpredictable blade implant may become predictable, less invasive, and not as technique sensitive as it was in the past; those patients who have narrow ridges or thin maxillary bone may be treated successfully with this procedure. RP is used to describe the customized production of solid models using 3D computer data and has resulted in fabrication technologies such as stereolithography, fused deposition modeling, and more recently, selective laser sintering [17–22]. SLS is a RP method that creates patterns using thermal fusing (sintering) of powdered materials. In SLS, in fact, the digital representation of an object is mathematically sliced into a number of thin layers. The object is then created by scanning a laser beam and selectively fusing (melting or sintering) patterns into sequentially deposited layers of a powder. Each patterned layer of powder is also fused to its underlying layer and corresponds to a cross section of the object as determined from the mathematical slicing operation [17–22]. This layered manufacturing method allows the fabrication of geometries with a high degree of complexity: it can be used to fabricate 3D structures with complex features such as overhangs and undercuts. The manufacture time is reduced and its cost is less, compared to conventional fabrication techniques. In addition, SLS enables the direct conversion of a scaffold's computer model into its physical realization, allowing patient-specific and tissue-specific reconstruction strategies to be easily developed [17–22].
The main advantage of SLS techniques over other manufacturing techniques is that custom-made devices with complex geometries can be fabricated in order to precisely meet the needs of a given patient or application [17–22]. In fact, with the ability to accurately fabricate structures based on CT data, SLS can be used to produce patient-specific prostheses and other medical devices. The geometry of a given structure, in fact, can be determined by an input STL file, which is manipulated using CAD software [17–22]; in addition, modifications to the geometry of the structure can be readily performed.
The precision of the SLS process has been reported to be within 10 μm [29, 30]. Dimensional differences between SLS models fabricated from CT data and master structures were recently shown to be 1.79 % [29]. Kaim et al. recently compared CT data, the resulting SLS model, and CT data of the SLS model. The differences between these three data sets were shown to be statistically insignificant (the likelihood of this finding by chance alone is less than 5 %), indicating that the limiting factor in CT-based SLS is the resolution of the scanning technique and not the resolution of the prototyping technique [30]. Precisely controlling the macro- and microarchitecture of the scaffold and hence fulfilling a custom design with complex anatomic shapes are of significant importance for clinical applications of the scaffold. Recent studies have demonstrated that nasal and maxillary prosthetics and dental implants fabricated using SLS can be successfully implanted into the human body [18, 19, 21, 22].
In the present study, custom-made SLS blade implants were used for the prosthetic rehabilitation of the atrophied posterior mandible of five patients. The blade implants used in the present study are really simple as they include two parts: the implant and the prosthetic rehabilitation that are joined by cement. There were no other parts such as copings or screws. The denser cortex and the cancellous structure of the atrophied posterior mandible were manipulated by the use of piezosurgery inserts. With piezosurgery, the implant site was prepared rapidly and precisely, and the direction of the osteotomy was controlled [27]. The precise fit of the implant in the bone socket, which is related to the implant design, is relevant. The success rate of healed blade implants depends very much on atraumatic work with bone, together with primary stability; the use of piezosurgery is mandatory in order to guarantee good long-term results. The implants were placed in position and immediately loaded with fixed partial restorations. After 2 years of functional loading, the implants were still in function, showing a perfect esthetic integration. This procedure can be considered as a nonstandard alternative for the rehabilitation of atrophied toothless mandible in elderly patients and in patients who choose this alternative for financial reasons.
Moreover, the fabrication of custom-made blade implants with SLS has two distinct advantages. In fact, SLS implants were shown to have impressive mechanical properties within the reported lower range of properties for human trabecular bone [18, 19, 22–25]. Moreover, SLS allows the fabrication of a porous structure with controlled porosity, pore interconnection, size, shape, and distribution, which are requirements for rapid bone ingrowth [23–26]. Multiple pore size distributions, interconnected pore structure, and high porosity of the implant can facilitate vascularization and diffusion of nutrients and gases [23–26]. Pore interconnectivity, as well as pore size, plays a critical role in bone ingrowth, regulating cell growth and function, manipulating tissue differentiation, and optimizing mechanical function [23–26].
Conclusions
This article describes the use of SLS to produce custom-made blade implants for the rehabilitation of extremely atrophied posterior mandibles. This implant can be indicated in patients with advanced atrophy of the mandible, and it may be an alternative to more conventional techniques, such as bone regeneration and root-form narrow-diameter implants, in patients who either have anatomical problems with their alveolar ridge or do not have enough money to pay for complex regenerative procedures. With the satisfying effect of a fixed implant-supported restoration, patients may be effectively rehabilitated and derive emotional and physical benefit from the treatment provided. Further studies on a larger sample of patients are needed to evaluate the effects and benefits from this treatment; however, this technique could represent suitable therapeutic options for the rehabilitation of extremely atrophied posterior mandible in elderly patients.
References
Chiapasco M, Casentini P, Zaniboni M, Corsi E, Anello T (2011) Titanium–zirconium alloy narrow-diameter implants (Straumann RoxolidR) for the rehabilitation of horizontally deficient edentulous ridges: prospective study on 18 consecutive patients. Clin Oral Implants Res. doi:10.1111/j.1600-0501.2011.02296.x
Mangano C, Mangano F, Shibli JA, Luongo G, De Franco M, Briguglio F, Figliuzzi M, Eccellente T, Rapani C, Piombino M, Macchi A (2012) Prospective clinical evaluation of 201 direct laser metal forming implants: results from a 1-year multicenter study on 62 patients. Lasers Med Sci 27:181–189
Chiapasco M, Casentini P, Zaniboni M (2009) Bone augmentation procedures in implant dentistry. Int J Oral Maxillofac Implants 24(suppl):237–259
Sohn DS, Bae MS, Heo JU, Park JS, Yea SH, Romanos GE (2011) Retrospective multicenter analysis of immediate provisonalization using one-piece narrow-diameter (3.0 mm) implants. Int J Oral Maxillofac Implants 26:163–168
Linkow LI (1968) The blade vent—a new dimension in endosseous implantology. Dent Concepts 11:3–12
Linkow LI, Donath K, Lemons JE (1992) Retrieval analyses of a blade implant after 231 months of clinical function. Implant Dent 1:37–43
Commissionat Y, Poulmaire F (1996) Blade implants: new ideas. Rev Stomatol Chir Maxillofac 97:283–287
Roberts RA (1996) Types, uses, and evaluation of the plate-form implant. J Oral Implantol 22:111–118
Veron C, Chanavaz M (1997) Implant rehabilitation of distal mandibular atrophy using a blade implant. Rev Stomatol Chir Maxillofac 98(Suppl 1):17–22
Roberts R (2002) Placement of plate-form implants using osteotomes. J Oral Implantol 28:283–289
Strecha J, Jurkovic R, Siebert T, Prachar P, Bartakova S (2010) Fixed bicortical screw and blade implants as a non-standard solution to an edentulous (toothless) mandible. Int J Oral Sci 2:105–110
Trisi P, Emanuelli M, Quaranta M, Piattelli A (1993) A light microscopy, scanning electron microscopy and laser scanning microscopy analysis of retrieved blade implants after 7 to 20 years of clinical function. J Periodontol 64:374–378
Proussaefs P, Lozada J (2002) Evaluation of two Vitallium blade-form implants retrieved after 13 and 21 years of function: a clinical report. J Prosthet Dent 87:412–415
Di Stefano D, Iezzi G, Scarano A, Perrotti V, Piattelli A (2006) Immediately loaded blade implant retrieved from a man after a 20-year loading period: a histologic and histomorphometric case report. J Oral Implantol 32:171–176
van Noort R (2012) The future of dental devices is digital. Dent Mater 28:3–12
Feng Z, Dong Y, Zhao Y, Bai S, Zhou B, Bi Y, Wu G (2010) Computer-assisted technique for the design and manufacture of realistic facial prostheses. Br J Oral Maxillofac Surg 48:105–109
Smith MH, Flanagan CL, Kemppainen JM, Sack JA, Chung H, Das S, Hollister SJ (2007) Feinberg SE (2007) Computed tomography-based tissue-engineered scaffolds in craniomaxillofacial surgery. Int J Med Robotics Comput Assist Surg 3:207–216
Mangano F, Cirotti B, Sammons R, Mangano C (2012) Custom-made, root-analogue direct laser metal forming implant: a case report. Lasers Med Sci. doi:10.1007/s10103-012-1134-z
Figliuzzi M, Mangano F, Mangano C (2012) Case report on a novel root analogue dental implant using CT scan and CAD/CAM—selective laser melting technology. Int J Oral Maxillofac Surg 41:858–862
Gittard SD, Narayan RJ (2010) Laser direct writing of micro- and nano-scale medical devices. Expert Rev Med Devices 7:343–356
Williams JV, Revington PJ (2010) Novel use of an aerospace selective laser sintering machine for rapid prototyping of an orbital blowout fracture. Int J Oral Maxillofac Surg 39:182–184
Wu G, Zhou B, Bi Y, Zhao Y (2008) Selective laser sintering technology for customized fabrication of facial prostheses. J Prosthet Dent 100:56–60
Traini T, Mangano C, Sammons RL (2008) Direct laser metal sintering as a new approach to fabrication of an isoelastic functionally graded material for manufacture of porous titanium dental implants. Dent Mater 24:1525–1533
Shibli JA, Mangano C, d'Avila S, Piattelli A, Pecora G, Mangano F, Onuma T, Ferrari D, Aguilar K, Iezzi G (2010) Influence of direct laser fabrication (DLF) implant topography on type IV bone: a histomorphometric study in humans. J Biomed Mater Res (part A) 93:607–614
Mangano C, Piattelli A, Iezzi G, Mangano F, Raspanti M, Shibli JA, Cassoni A (2011) Scanning electron microscopy (SEM) and X-ray dispersive spectrometry evaluation of direct laser metal sintering surface and human bone interface: a case series. Lasers Med Sci 26:133–138
Mangano C, Mangano F, Shibli JA, Ricci M, Perrotti V, d'Avila S, Piattelli A (2012) Immediate loading of mandibular overdentures supported by unsplinted direct laser metal forming (DLMF) implants. Results from a 1-year prospective study. J Periodontol 83:70–78
Pavlíková G, Foltán R, Horká M, Hanzelka T, Borunská H, Sedý J (2011) Piezosurgery in oral and maxillofacial surgery. Int J Oral Maxillofac Surg 40:451–7
Steflik DE, Sisk AL, Parr GR, Hanes PJ, Lake FT, Brewer P, Horner J, McKinney RV (1992) Correlative transmission electron microscopic and scanning electron microscopic observations of the tissues supporting endosteal blade implants. J Oral Implantol 27:110–120
Silva DN, Gerhardt de Oliveira M, Meurer E, Meurer MI, Lopes da Silva JV, Santa-Bárbara A (2008) Dimensional error in selective laser sintering and 3D-printing of models for craniomaxillary anatomy reconstruction. J Craniomaxillofac Surg 36:443–449
Kaim AH, Kirsch EC, Alder P, Bucher P, Hammer B (2009) Preoperative accuracy of selective laser sintering (SLS) in craniofacial 3D modeling: comparison with patient CT data. Rofo 181:644–651
Acknowledgments
The authors are grateful to Federico Rizzi, CAD engineer, for his help in writing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mangano, F., Bazzoli, M., Tettamanti, L. et al. Custom-made, selective laser sintering (SLS) blade implants as a non-conventional solution for the prosthetic rehabilitation of extremely atrophied posterior mandible. Lasers Med Sci 28, 1241–1247 (2013). https://doi.org/10.1007/s10103-012-1205-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-012-1205-1