Abstract
The aim of this study was to examine the effect of low-level laser therapy (LLLT) on the cell viability and the expression of hypoxia-inducible factor-1s (HIF-1s), bone morphogenic protein-2 (BMP-2), osteocalcin, type I collagen, transforming growth factor-β1 (TGF-β1), and Akt in hypoxic-cultured human osteoblasts. Human fetal osteoblast cells (cell line 1.19) were cultured under 1 % oxygen tension for 72 h. Cell cultures were divided into two groups. At the experimental side, low-level laser (808 nm, GaAlAs diode) was applied at 0, 24, and 48 h. After irradiation, each cell culture was incubated 24 h more under hypoxia. Total energy was 1.2, 2.4, and 3.6 J/cm2, respectively. Non-irradiated cultures served as controls. Comparisons between the two groups were analyzed by t test; a p value <0.05 was considered statistically significant. Hypoxia resulted in a decrease in the expression of type I collagen, osteocalcin, and TGF-β1 (p < 0.001, p < 0.001, and p < 0.01, respectively). Cell viability and BMP-2 expression were not decreased by hypoxic condition. On the other hand, LLLT on hypoxic-cultured osteoblast promoted the expression of BMP-2, osteocalcin, and TGF-β1 (p < 0.05, p < 0.01, and p < 0.001, respectively). Cell proliferation was also increased time-dependently. However, hypoxia decreased in type I collagen expression (p < 0.001), and LLLT did not affect type I collagen expression in hypoxic-cultured osteoblasts. Furthermore, LLLT inhibited HIF-1 and Akt expression in hypoxic conditioned osteoblasts. We concluded that LLLT induces the expression of BMP-2, osteocalcin, and TGF- β1 in 1 % hypoxic-cultured human osteoblasts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
O2 tension in the environment surrounding bone cells can vary under certain circumstances. Normally, O2 tension is around 13 % (100 mmHg) in arterial blood, approximately 6 % (45 mmHg) in mixed venous blood, and considerably lower than 6 % in most tissues including bone tissues [1, 2]. Measurements of bone marrow aspirates from normal human volunteer donors yielded mean O2 tension values of 6.6 % [3].
Lack of oxygen can result in a failure to generate sufficient ATP to maintain essential cellular functions, whereas excess oxygen (hyperoxia) results in the generation of damaging reactive oxygen intermediates [4]. Therefore, regulating constant O2 tension constantly is an important factor for tissue homeostasis including bone remodeling. However, in the context of a lack of oxygen, there are many circumstances causing hypoxic condition on bone. Hypoxia occurs when the blood supply to tissues is reduced or disrupted and can occur in situations such as: aging [5], inflammation [6], fractures [7], diabetes [8], and possibly also at bone graft site. Bones are not static but are rather dynamic, and therefore, bone remodeling continuously occurs in these conditions.
Although the data are inconclusive, hypoxia has been shown to have significant effects on bone cell physiology. Some studies have shown that hypoxia increases osteoblast vascular endothelial growth factor (VEGF) [9, 10]. In addition, hypoxia enhances bone morphogenic protein-2 (BMP-2) expression in osteoblasts by hypoxia-inducible factor-1α (HIF-1α) [11], while others have demonstrated decreased osteogenic differentiation [12], cell proliferation [4, 13], and osteocalcin [14].
Hypoxia activates a distinct transcription factor called hypoxia-inducible factor-1 (HIF-1) [15]. Under normoxic condition, HIF-1α undergoes rapid ubiquidation and proteosomal degradation. Under hypoxic conditions, this degradation is inhibited, HIF-1α translocates to the nucleus, dimerizes with HIF-1β, and binds to its consensus sequence within the hypoxia-responsive element, and regulates transcription of VEGF, erythropoietin, and glycolytic enzymes that enhance cellular adaptation to hypoxia [16–18].
Some studies have investigated the effects of low-level laser therapy (LLLT) on osteoblasts or osteoblastic cells. These studies have demonstrated that LLLT promotes proliferation and maturation of human osteoblasts [19], acts as a proliferative stimulus on osteoblast [20], stimulates mineralization, and increases BMP expression [21]. However, these studies that show the effects of LLLT on osteoblasts and osteoblastic cells have been conducted under normoxic condition. In fact, hypoxic condition is readily encountered in clinical practice as previously described. Therefore, our study focuses on the effect of LLLT on osteoblasts under hypoxic condition.
There are many cytokines that are involved in osteoblast differentiation and proliferation. BMP-2 plays an important role in the process of bone formation and remodeling as well as bone development and osteoblast differentiation [22, 23]. Osteocalcin is a marker of mature osteoblasts. Type I collagen is the major organic component of bone matrix and a primary product of the differentiated osteoblast. Additionally, transforming growth factor-βs (TGF-βs) are potent growth regulatory molecules that stimulate osteoblast proliferation and induce the synthesis of collagen, osteocalcin, and other extracellular matrix proteins [24–28]. It has been reported that Akt, a serine–threonine protein kinase, related signaling pathways are involved in osteogenic process [29–31]. We postulated that LLLT affects these cytokines and genes of osteoblast of hypoxic condition.
To test this hypothesis, we investigated the effect of LLLT on human osteoblast cell line in which human fetal osteoblast (hFOB) cells were exposed to 1 % oxygen tension at different time intervals. We first examined whether hypoxia would affect cell viability. Additionally, using real-time PCR and Western blot analysis, we analyzed the effect of hypoxia and LLLT on the expression of BMP-2, TGF-β1, type I collagen, osteocalcin, HIF-1s, and Akt.
Materials and methods
Cell culture
A hFOB 1.19 human osteoblast cell line was purchased from the ATCC (Rockville, MD, USA). This cell line was maintained at 34°C with 5 % CO2 in air atmosphere in D-MEM/F-12 medium with 4 mM l-glutamine, 1.5 μg/L sodium bicarbonate, 4.5 g/L glucose, and 1.0 mM sodium pyruvate supplemented with 10 % fetal bovine serum.
Hypoxia of cultured osteoblasts
The cells were cultured under 1 % oxygen tension. Cells were seeded on six-well plate (1 × 105 cells) before exposure to hypoxia. Cells were gassed with 95 % N2 and 5 % CO2 (Anaerobic System PROOX model 110; BioSpherix) and incubated at 34°C within the chamber for different time intervals (0, 24, 48 and 72 h).
Low-level laser irradiation
Cultures were irradiated with a low-level GaAlAs laser (λ = 808 ± 3 nm, 1,000 mW, 80 mA, NDLux, Seoul, Korea). Laser energy was delivered to the culture in continuous mode, with the laser positioned vertically above each well. Irradiation time for each well was 15 s. Cell cultures were divided into two groups. Laser irradiation was applied at 0, 24, and 48 h. After irradiation, each cell culture was incubated 24 h more under hypoxic condition. For example, 72-h cultured samples were irradiated three times (0, 24, and 48 h). Total energy was 1.2, 2.4, and 3.6 J/cm2, respectively. Control non-irradiated cells were incubated at 0, 24, 48, and 72 h under hypoxia without laser irradiation.
MTT assay
Cells were cultured in a 96-well plate and incubated for 24 h. The cells were exposed to low-level laser irradiation and hypoxic condition for different time intervals. After treatment (laser irradiation), 100 μl of MTT (0.5 mg/ml final concentration) was added and incubated in the dark for an additional 4 h to induce the production of formazan crystals at 37°C, and the supernatants were discarded. The medium was aspirated, and formed formazan crystals were dissolved in DMSO. Cell viability was monitored on an ELISA reader (Sunrise Remote Control, Tecan, Austria) at 570 nm excitatory emission wavelength.
Quantitative reverse transcriptional PCR
Total RNA was extracted from the hFOB cells using Trizol reagent (Invitrogen, Life Technologies, Carlsbad, NM, USA) according to manufacturer’s instructions. Total RNA (2 μg) was reverse transcribed using a RevertAid™ First Strand cDNA synthesis kit (Thermo, Fremont, CA, USA) according to the manufacturer’s protocols. Real-time PCR was performed on ABI 7500 Fast Real-Time PCR System (Applied Biosystems 7500 System Sequence Detection System (SDS) software version 2.0.1) using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA). The primers for BMP-2 (forward: 5′-ACC AGG TTG GTG AAT CAG AA-3′ and reverse: 5′-TTT GGC TTG ACG TTT TTC TC-3′), type I collagen (forward: 5′-CGT GGT GTA ACT GGT CCT TC-3′ and reverse: 5′-ACC GGG CTC TCC CTT ATC -3′), osteocalcin (forward: 5′- ATG AGA GCC CTC ACA CTC CT-3′ and reverse: 5′-GGA TTG AGC TCA CAC ACC TC-3′), and TGF- β1 (forward: 5′-TCT TTT GAT GTC ACC GGA GT-3′ and reverse: 5′-CGT GGA GCT GAA GCA ATA GT-3′) were used. GAPDH (forward: 5′-GGA AGG ACT CAT GAC CAC AG-3′ and reverse: 5′-TTG GCA GGT TTT TCT AGA CG-3′) was used as an internal control. The conditions for the PCR were as follows: 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s, 60°C for 1 min, and 72°C for 30 s. Real-time PCR data were analyzed by the SDS 2.0.1 software package (Applied Biosystems, Foster, CA, USA).
Western blot assay
Cells were plated at a density of 1 × 105 cells in six-well plates. Cells were washed twice with ice-cold PBS and centrifuged at 2,000 rpm for 10 min. Total cell proteins were lysed with a RIPA buffer [300 mM NaCl, 50 mM Tris–HCl (pH 7.6), 0.5 % Triton X-100, 2 mM PMSF, 2 μg/ml aprotinin, and 2 μg/ml leupeptin] and incubated at 4°C for 1 h. The lysates were centrifuged at 14,000 revolutions per min for 15 min at 4°C, and sodium dodecyl sulfate (SDS) and sodium deoxycholic acid (0.2 % final concentration) were added. Protein concentrations of cell lysates were determined with Bradford protein assay (Bio-Rad, Richmond, CA, USA), and BSA was used as a protein standard. A sample of 50 μg protein from each well was separated and loaded onto 7.5–10 % SDS/PAGE. The gels were transferred to a nitrocellulose membrane (Amersham Pharmacia Biotech, Piscataway, UK) and reacted with each antibody. Immunostaining with antibodies was performed using SuperSignal West Pico enhanced chemiluminescence substrate and detected with Alpha Imager HP (Alpha Innotech, San Leandro, USA). Equivalent protein loading was confirmed by Ponceau S staining.
Statistical analysis
Experiments were repeated four to six times. Comparisons between the two groups were analyzed by t test. The data were expressed as the mean ± standard deviation. Values of p < 0.05 were considered significant.
Results
Hypoxia does not alter cell viability and LLLT stimulate cell proliferation
The proliferation assay was performed at 0 (immediately), 24, 48, and 72 h; the results are presented in Table 1. Control non-irradiated group has shown that there are increases in cell proliferation time-dependently (48 h, p = 0.0002 and 72 h, p = 0.0003). Therefore, hypoxia does not seem to reduce cell viability. In order to assess the effect of LLLT on hypoxic-cultured osteoblast, cells were irradiated at different time intervals (0, 24, and 48 h). At 24 and 72 h, there are significant increases in cell proliferation compared to control non-irradiated group (p < 0.05 and p < 0.01, respectively).
BMP-2 expression is increased by LLLT in hypoxic-cultured osteoblasts
To examine the effects of hypoxia and laser irradiation on BMP-2 expression, hFOB cells were exposed to hypoxia and laser irradiation at different time intervals. The results are presented in Table 2 and Fig. 1. Hypoxic condition did not (p > 0.05) alter BMP-2 expression time-dependently in the non-irradiated group except at the 72-h exposure that showed a slight increase (1.05-fold, p = 0.0440). However, BMP-2 expression was significantly increased throughout the experiment in the irradiated group compared to the non-irradiated group (0 h, p < 0.05; 24 h, p < 0.05; 48 h, p < 0.001; and 72 h, p < 0.001, and peak BMP-2 expression was noted at 72 h (2.8-fold).
TGF- β1 expression is increased by LLLT in hypoxic-cultured osteoblasts
In order to assess the effect of hypoxia and laser irradiation on TGF-β1 expression in hFOB cells, cells were exposed to hypoxia at 0, 24, 48, and 72 h. The results are presented in Table 3 and Fig. 1. Hypoxia resulted in a marked decrease in TGF-β1 expression at 24, 48, and 72 h compared to 0 h in non-irradiated group (p = 0.0003, p = 0.0010, and p = 0.0028, respectively). TGF-β1 expression remained below baseline levels at 24, 48, and 72 h (46, 54, and 67 %, respectively). In the irradiated group, however, TGF-β1 expression was markedly increased at 24, 48, and 72 h (2.9-, 2.6-, 2.5-fold, respectively) compared to the hypoxic-cultured group at the same time intervals (p < 0.001, p < 0.001, and p < 0.001, respectively).
Hypoxia decreases type I collagen expression, and laser irradiation does not affect on type I collagen expression in hypoxic-cultured osteoblasts
To determine the effects of hypoxia and laser irradiation on type I collagen, hFOB cells were cultured under hypoxic condition. In the irradiated group, laser irradiation was done as scheduled. The results are presented in Table 4 and Fig. 1. Hypoxia resulted in a marked decrease in type I collagen expression in both groups throughout the experimental period (p < 0.001). Type I collagen expression remained below baseline levels at 24, 48, and 72 h (32, 32, and 29 %, respectively).
In addition, laser irradiation had little or negative effect on hypoxic-cultured osteoblasts. Although the expression of type I collagen was higher at 24 h in the irradiated group compared to the non-irradiated group (p < 0.01), type I collagen expression remained lower than in the non-irradiated group for the remainder of the experimental period, at 48 (p < 0.001) and 72 h (p < 0.001).
Effect of hypoxia and laser irradiation on osteocalcin expression in osteoblasts
The expressions of osteocalcin under hypoxia and laser irradiation are summarized in Table 5 and Fig. 1. In hypoxia-only condition, osteocalcin expression had decreased time-dependently (p = 0.0000, respectively). The expression of osteocalcin at 24, 48, and 72 h was 53, 47, and 26 %, respectively, compared to 0 h. However, laser irradiation induced an increase in osteocalcin expression immediately after irradiation (0 h, 1.09-fold, p < 0.05), and this result remained as irradiation frequency was increased (24 h, 2.4-fold, p < 0.001; 48 h, 1.5-fold, p < 0.001; and 72 h, 2.7-fold, p < 0.001, respectively). Peak expression was noted at 24 h (1.3-fold compared to normoxic condition). Although the expression of osteocalcin was higher in the irradiated group than in the non-irradiated group at 48 and 72 h, absolute expression levels at these intervals were lower than in normoxic control (71 %, respectively).
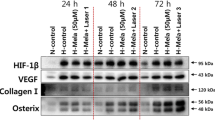
LLLT inhibits HIF-1 expression in hypoxic conditioned osteoblasts
To determine the effect of hypoxia on HIF-1 activation, hFOB cells were cultured under hypoxia for 0, 24, 48, and 72 h. Furthermore, to assess the effect of LLLT on protein levels of HIF-1 in hFOB cells under hypoxic condition, low-level laser irradiated at different time intervals. Our results on the effect of LLLT on HIF-1 expression in osteoblasts that are cultured under hypoxia are shown in Fig. 2. Western blot analysis indicated that hypoxia induced a marked increase in protein levels of HIF-1α time-dependently. In addition, HIF-1β protein levels at 24 h were slightly increased, and markedly increased at 48 and 72 h in the hypoxia-only cultured group. However, in the irradiated group, HIF-1α was not expressed throughout the entire experimental period. Furthermore, like HIF-1α, HIF-1β expression was not increased in the irradiated group.
LLLT inhibits phosphorylation of Akt in hypoxic conditioned osteoblasts
Our results on the effect of LLLT on Akt activation in osteoblasts that are cultured under hypoxia are shown in Fig. 2. Both groups were cultured under hypoxia for 0, 24, 48, and 72 h; one group was irradiated with low-level laser (LLL) at different time intervals. In non-irradiated group, expression of phosphorylated Akt (pAkt) was increased at 48 and 72 h. However, in the irradiated group, pAkt protein levels were not changed time-dependently.
Discussion
Several studies have investigated the effect of hypoxia on osteoblasts and osteoblastic cells [9–14]. However, to our knowledge, the effect of LLLT on osteoblasts under hypoxia has not been described until this paper.
The present study showed that hypoxia reduced the expression of type I collagen, osteocalcin, and TGF-β1 in human osteoblast cells. There are some studies on the contradictory effects of hypoxia on osteoblasts and osteoblastic cells. Warren et al. [32] studied the effect of hypoxia on the gene expression in rat osteoblasts. They demonstrated that hypoxia induced TGF-β1 mRNA and type I collagen mRNA expression, in contrast to our findings. Meanwhile, Park et al. [14] showed that hypoxia reduced the expression of type I collagen and osteocalcin in human osteoblastic cells. In addition, hypoxia reduced the expression of osteocalcin, TGF-β, collagen in rat calvarial osteoblasts in a time-dependent manner [4]. Taking together our results with other investigations and considering the meaning and function of type I collagen, osteocalcin, and TGF-β, we conclude that hypoxia inhibits osteoblast differentiation, maturation, and bone-forming capacity. Interestingly, however, cell viability was not affected by hypoxia in the present study. Previous studies demonstrated that hypoxia inhibits osteoblast proliferation [4] and osteoblastic differentiation [12] and reduces the viability of osteoblastic cells [14]. On the other hand, Salim et al. [33] demonstrated that hypoxia had little effect on osteoblast differentiation. Our results showed that hypoxia does not affect osteoblast survival. Rather, although under hypoxic condition, cell counts increased time-dependently. In addition, our preliminary studies comparing the osteoblast viability between under normoxic and hypoxic condition demonstrated that there were no significant differences in cell viability between the two groups (data not shown). From these results, we conclude that 1 % oxygen tension has no cytotoxicity on human osteoblast cell line.
In contrast to other cytokines and proteins, hypoxia did not alter BMP-2 expression in osteoblasts in the present study. BMP-2 plays an important role in the process of bone formation and remodeling as well as bone development and osteoblast differentiation [22, 23]. In addition, some studies have demonstrated that Akt-related signaling pathways are involved osteoblast differentiation [29–31]. Furthermore, Akt suppresses osteoblast apoptosis [31]. Tseng et al. [11] have investigated the relationship between hypoxia and BMP-2 expression. They demonstrated that hypoxia induced BMP-2 expression via ILK/Akt/mTOR and HIF-1α pathways in osteoblasts time-dependently. However, our study showed contradictory results. Except at 72 h, hypoxia did not alter BMP-2 expression in the present study. There are differences in culture time intervals between their experiment and our investigation. Therefore, we should consider that altered expression would have been revealed at different time intervals.
However, interestingly, phosphorylation of Akt had occurred throughout the entire experimental period in hypoxic-cultured osteoblasts in our experiment. Therefore, BMP-2 and Akt-related signaling pathways may function normally in osteoblasts exposed to 1 % hypoxia. Aditi and Peter [29] demonstrated that activation of Akt promotes BMP-2-mediated osteoblast differentiation. Taken together, our results suggest that osteoblast could differentiate via BMP-2 expression and Akt-related signaling pathways, even under hypoxia. This hypothesis may explain how osteoblasts can survive and differentiate rather than result in apoptosis in hypoxic condition. However, we also show evidence of delayed osteoblast differentiation in hypoxia through the inhibition of type I collagen, osteocalcin, and TGF-β1 expression. Thus, it seems to suggest that osteoblast can survive and proliferate although in a quiescence state and differentiated slowly in moderate ischemic condition until vascularization occurs or oxygen tension is increased.
It is proposed that biostimulation by LLLT may enhance the osteogenic potential of osteoblasts [34]. In addition, our previous studies have demonstrated that LLLT promote metabolic bone activity and bone remodeling [35, 36]. As the application of hypothesis to hypoxic-cultured osteoblast was unknown, we investigated the effect of LLLT on osteoblasts exposed to 1 % oxygen tension.
In our investigation, in the irradiated group, osteoblasts proliferated more than in the non-irradiated group, especially at 24 and 72 h. Likewise, LLL induced BMP-2 expression in hypoxic-cultured osteoblast time-dependently, and peak expression was noted at 72 h. These results were consistent with previous investigations that LLLT promotes osteoblast proliferation and increases BMP expression [19–21], even under normoxic conditions. Thus, we conclude that LLLT promotes osteoblast proliferation in hypoxic condition. However, the connection between low-level laser and increased BMP-2 expression remains unknown necessitating further study on their exact relationship.
In addition to osteoblast proliferation and BMP-2 expression, TGF-β1 expression in the irradiated group increased over time compared to the non-irradiated group, and expression levels were higher than normoxic 0-h control throughout the experiment. As previously described, BMP-2 induces osteoblast differentiation. Furthermore, TGF-β1 stimulates osteoblast proliferation, production of type I collagen and osteocalcin, a marker of mature osteoblast. From this, we can hypothesize that LLLT on hypoxic-cultured osteoblasts overcomes the inhibitory effect on osteoblasts. However, surprisingly, the peak expression level of type I collagen was noted at 24 h in the irradiated group, and the expression level decreased over time even though the frequency of laser irradiation increased. The expression of osteocalcin also showed similar results. Although expression levels were higher than the non-irradiated group and peak expression was noted at 24 h (1.3-fold) in the irradiated group, absolute expression levels at 48 and 72 h were below baseline. These observations suggest that LLLT on hypoxic-cultured osteoblasts promotes cell differentiation and proliferation via BMP-2 and TGF-β1 expressions. Furthermore, osteoblast maturation would be increased by LLLT at an early stage, and these results could be maintained to some extent compared to the hypoxia-only cultured group.
As shown in our results, hypoxia decreased significantly in type I collagen expression, and LLLT had no effect on its expression. It has been reported that hypoxia decreases collagen synthesis and type I collagen expression in osteoblastic cells and osteoblasts exposed to 1 % oxygen tension [4, 14], while Warren et al. [32] demonstrated that type I collagen expression was increased in short-term cultures. Therefore, hypoxia may have a dual effect in type I collagen expression. Nevertheless, it is interesting that type I collagen expressions were largely unaffected by LLLT in our systems. Hirata et al. [37] have demonstrated that LLLT increases type I collagen expression in osteoblastic cells. In addition, low-level laser stimulates mineralization via increased BMPs in osteoblastic cells [38]. However, in our study, although cell maturation and differentiation rate increased and increased TGF-β1 expression possibly stimulated productions of type I collagen, amounts of collagen synthesis did not increase despite the addition of laser irradiation. Thus, it is reasonable to assume that proper oxygen tension is essential to synthesize type I collagen. Further investigations are needed on hypoxia-related type I collagen synthesis and effect of low-level laser irradiation.
It is well known that hypoxia signaling is mainly affected via HIF-1 activation [15]. As previously described, under hypoxic condition, HIF-1α translocates to the nucleus, dimerizes with HIF-1β, and binds to its consensus sequence. Our data also showed that HIF-1α and 1β were expressed immediately 24 h after hypoxic incubation, and expression levels were increased as culture time was extended. In the irradiated group, however, HIF-1s were only slightly expressed at 48 h; we did not find strong HIF-1 activation during the entire experimental period. In addition to HIF-1s, phosphorylated Akt (pAkt) was not expressed in the irradiated group. It has been reported that low-level laser has a biostimulative effect. Furthermore, some laboratories have observed the formation of reactive oxygen species (ROS) in cells in vitro after LLLT [39, 40]. In studies on the relationship between Akt and HIF-1, Tseng et al. [11] demonstrated that Akt pathway regulates HIF-1 expression and activity in osteoblasts. Likewise, Kanichai et al. [41] stated that hypoxia induces a rapid increase in Akt phosphorylation and a subsequent stabilization of HIF-1α in rat mesenchymal stromal cells.
Taken together, we hypothesize that LLLT can generate ROS even though in hypoxic-cultured osteoblast and hypoxic condition were camouflaged by generated ROS. However, how LLLT affects the interaction between HIF-1 and Akt in hypoxic-cultured osteoblast remains unknown. Thus, further investigation is needed.
In conclusion, hypoxia did not affect osteoblast viability in vitro, although it resulted in a decrease in the expression of type I collagen, osteocalcin, and TGF-β1. These findings provide evidence that hypoxia has a negative effect on differentiation and bone-forming capacity of human osteoblast. In contrast, LLLT on hypoxic-cultured osteoblast stimulates osteoblast differentiation and proliferation through increased expression of BMP-2, osteocalcin, and TGF-β1. However, the effect of LLLT on extracelluar matrix synthesis and HIF-related signaling in hypoxic osteoblast remains unclear. Further investigations are required to evaluate the possible mechanisms that may explain the response of hypoxic-cultured osteoblast to LLLT.
References
Vanderkooi JM, Erecinska M, Silver IA (1991) Oxygen in mammalian tissue: methods of measurement and affinities of various reactions. Am J Physiol 260:C1131–1150
Vaupel P, Schlenger K, Knoop C, Hockel M (1991) Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res 51:3316–3322
Harrison JS, Rameshwar P, Chang V, Bandari P (2002) Oxygen saturation in the bone marrow of healthy volunteers. Blood 99:394
Utting JC, Robins SP, Brandao-Burch A, Orriss IR, Behar J, Arnett TR (2006) Hypoxia inhibits the growth, differentiation and bone-forming capacity of rat osteoblasts. Exp Cell Res 312:1693–1702
Dinenno FA, Seals DR, DeSouza CA, Tanaka H (2001) Age-related decreases in basal limb blood flow in humans: time course, determinants and habitual exercise effects. J Physiol 531:573–579
Lewis JS, Lee JA, Underwood JC, Harris AL, Lewis CE (1999) Macrophage responses to hypoxia: relevance to disease mechanisms. J Leukoc Biol 66:889–900
Spector JA, Mehrara BJ, Greenwald JA, Saadeh PB, Steinbrech DS, Bouletreau PJ, Smith LP, Longaker MT (2001) Osteoblast expression of vascular endothelial growth factor is modulated by the extracellular microenvironment. Am J Physiol Cell Physiol 280:C72–80
Oikawa A, Siragusa M, Quaini F, Mangialardi G, Katare RG, Caporali A, van Buul JD, van Alphen FP, Graiani G, Spinetti G, Kraenkel N, Prezioso L, Emanueli C, Madeddu P (2010) Diabetes mellitus induces bone marrow microangiopathy. Arterioscler Thromb Vasc Biol 30:498–508
Steinbrech DS, Mehrara BJ, Saadeh PB, Chin G, Dudziak ME, Gerrets RP, Gittes GK, Longaker MT (1999) Hypoxia regulates VEGF expression and cellular proliferation by osteoblasts in vitro. Plast Reconstr Surg 104:738–747
Steinbrech DS, Mehrara BJ, Saadeh PB, Greenwald JA, Spector JA, Gittes GK, Longaker MT (2000) VEGF expression in an osteoblast-like cell line is regulated by a hypoxia response mechanism. Am J Physiol Cell Physiol 278:C853–860
Tseng WP, Yang SN, Lai CH, Tang CH (2010) Hypoxia induces BMP-2 expression via ILK, Akt, mTOR, and HIF-1 pathways in osteoblasts. J Cell Physiol 223:810–818
D'Ippolito G, Diabira S, Howard GA, Roos BA, Schiller PC (2006) Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone 39:513–522
Lee CM, Genetos DC, You Z, Yellowley CE (2007) Hypoxia regulates PGE(2) release and EP1 receptor expression in osteoblastic cells. J Cell Physiol 212:182–188
Park JH, Park BH, Kim HK, Park TS, Baek HS (2002) Hypoxia decreases Runx2/Cbfa1 expression in human osteoblast-like cells. Mol Cell Endocrinol 192:197–203
Semenza GL (1998) Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev 8:588–594
D'Angio CT, Finkelstein JN (2000) Oxygen regulation of gene expression: a study in opposites. Mol Genet Metab 71:371–380
Hwang JM, Weng YJ, Lin JA, Bau DT, Ko FY, Tsai FJ, Tsai CH, Wu CH, Lin PC, Huang CY, Kuo WW (2008) Hypoxia-induced compensatory effect as related to Shh and HIF-1alpha in ischemia embryo rat heart. Mol Cell Biochem 311:179–187
Sharp FR, Bernaudin M (2004) HIF1 and oxygen sensing in the brain. Nat Rev Neurosci 5:437–448
Stein A, Benayahu D, Maltz L, Oron U (2005) Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in vitro. Photomed Laser Surg 23:161–166
Renno AC, McDonnell PA, Parizotto NA, Laakso EL (2007) The effects of laser irradiation on osteoblast and osteosarcoma cell proliferation and differentiation in vitro. Photomed Laser Surg 25:275–280
Kiyosaki T, Mitsui N, Suzuki N, Shimizu N (2010) Low-level laser therapy stimulates mineralization via increased Runx2 expression and ERK phosphorylation in osteoblasts. Photomed Laser Surg 28(Suppl 1):S167–172
Rodan GA, Martin TJ (2000) Therapeutic approaches to bone diseases. Science 289:1508–1514
Li X, Cao X (2006) BMP signaling and skeletogenesis. Ann N Y Acad Sci 1068:26–40
Centrella M, McCarthy TL, Canalis E (1987) Transforming growth factor beta is a bifunctional regulator of replication and collagen synthesis in osteoblast-enriched cell cultures from fetal rat bone. J Biol Chem 262:2869–2874
Centrella M, Massague J, Canalis E (1986) Human platelet-derived transforming growth factor-beta stimulates parameters of bone growth in fetal rat calvariae. Endocrinology 119:2306–2312
Lundy MW, Hendrix T, Wergedal JE, Baylink DJ (1991) Growth factor-induced proliferation of osteoblasts measured by bromodeoxyuridine immunocytochemistry. Growth Factors 4:257–264
Robey PG, Young MF, Flanders KC, Roche NS, Kondaiah P, Reddi AH, Termine JD, Sporn MB, Roberts AB (1987) Osteoblasts synthesize and respond to transforming growth factor-type beta (TGF-beta) in vitro. J Cell Biol 105:457–463
Wrana JL, Maeno M, Hawrylyshyn B, Yao KL, Domenicucci C, Sodek J (1988) Differential effects of transforming growth factor-beta on the synthesis of extracellular matrix proteins by normal fetal rat calvarial bone cell populations. J Cell Biol 106:915–924
Mukherjee A, Rotwein P (2009) Akt promotes BMP2-mediated osteoblast differentiation and bone development. J Cell Sci 122:716–726
Choi YH, Jeong HM, Jin YH, Li H, Yeo CY, Lee KY (2011) Akt phosphorylates and regulates the osteogenic activity of Osterix. Biochem Biophys Res Commun 411:637–641
Kawamura N, Kugimiya F, Oshima Y, Ohba S, Ikeda T, Saito T, Shinoda Y, Kawasaki Y, Ogata N, Hoshi K, Akiyama T, Chen WS, Hay N, Tobe K, Kadowaki T, Azuma Y, Tanaka S, Nakamura K, Chung UI, Kawaguchi H (2007) Akt1 in osteoblasts and osteoclasts controls bone remodeling. PLoS One 2:e1058
Warren SM, Steinbrech DS, Mehrara BJ, Saadeh PB, Greenwald JA, Spector JA, Bouletreau PJ, Longaker MT (2001) Hypoxia regulates osteoblast gene expression. J Surg Res 99:147–155
Salim A, Nacamuli RP, Morgan EF, Giaccia AJ, Longaker MT (2004) Transient changes in oxygen tension inhibit osteogenic differentiation and Runx2 expression in osteoblasts. J Biol Chem 279:40007–40016
Khadra M, Lyngstadaas SP, Haanaes HR, Mustafa K (2005) Effect of laser therapy on attachment, proliferation and differentiation of human osteoblast-like cells cultured on titanium implant material. Biomaterials 26:3503–3509
Kim YD, Kim SS, Hwang DS, Kim SG, Kwon YH, Shin SH, Kim UK, Kim JR, Chung IK (2007) Effect of low-level laser treatment after installation of dental titanium implant-immunohistochemical study of RANKL, RANK, OPG: an experimental study in rats. Lasers Surg Med 39:441–450
Kim YD, Song WW, Kim SS, Kim GC, Hwang DS, Shin SH, Kim UK, Kim JR, Chung IK (2009) Expression of receptor activator of nuclear factor-kappaB ligand, receptor activator of nuclear factor-kappaB, and osteoprotegerin, following low-level laser treatment on deproteinized bovine bone graft in rats. Lasers Med Sci 24:577–584
Hirata S, Kitamura C, Fukushima H, Nakamichi I, Abiko Y, Terashita M, Jimi E (2010) Low-level laser irradiation enhances BMP-induced osteoblast differentiation by stimulating the BMP/Smad signaling pathway. J Cell Biochem 111:1445–1452
Fujimoto K, Kiyosaki T, Mitsui N, Mayahara K, Omasa S, Suzuki N, Shimizu N (2010) Low-intensity laser irradiation stimulates mineralization via increased BMPs in MC3T3-E1 cells. Lasers Surg Med 42:519–526
Houreld NN, Sekhejane PR, Abrahamse H (2010) Irradiation at 830 nm stimulates nitric oxide production and inhibits pro-inflammatory cytokines in diabetic wounded fibroblast cells. Lasers Surg Med 42:494–502
Zhang J, Xing D, Gao X (2008) Low-power laser irradiation activates Src tyrosine kinase through reactive oxygen species-mediated signaling pathway. J Cell Physiol 217:518–528
Kanichai M, Ferguson D, Prendergast PJ, Campbell VA (2008) Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: a role for AKT and hypoxia-inducible factor (HIF)-1alpha. J Cell Physiol 216:708–715
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011–0026921).
Author information
Authors and Affiliations
Corresponding author
Additional information
Dr. Song WW and Dr. Pyo SJ equally contributed to this study as the first authors.
Rights and permissions
About this article
Cite this article
Pyo, SJ., Song, WW., Kim, IR. et al. Low-level laser therapy induces the expressions of BMP-2, osteocalcin, and TGF-β1 in hypoxic-cultured human osteoblasts. Lasers Med Sci 28, 543–550 (2013). https://doi.org/10.1007/s10103-012-1109-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-012-1109-0