Abstract
A biopsy is a surgical procedure performed to establish a clear diagnosis of a lesion in order to clarify a clinical diagnostic suspicion. During a biopsy procedure it is fundamental to maintain safe and readable cut margins in order to permit histological visualization of possible marginal infiltrations or malignant transformation of a lesion. The aim of this study was to evaluate the histological peripheral damage caused by application of a KTP (potassium titanium phosphate) laser during oral soft tissue biopsy procedures. A KTP laser (λ 532 nm) at different power settings and fluences was used to obtain 45 samples from pig cadaver tongues. The samples were then subdivided into five groups of nine samples each. A final specimen was taken by scalpel as a control. All samples were put into test tubes containing 10% buffered formalin solution, and were examined separately under an optical microscope by two pathologists to evaluate the peripheral thermal damage induced by the laser. In all specimens the cut edges of the incision were free from histological artefacts, especially when lower settings were applied. Statistical analysis showed no differences among the groups. The KTP laser demonstrated surgical effectiveness and caused little peripheral damage to the cut edges, and therefore would always allow a safe histological diagnosis to be obtained.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A biopsy is a surgical procedure performed to establish a clear diagnosis of a lesion in order to clarify a clinical diagnostic suspicion. A biopsy may be incisional or excisional. An incisional biopsy removes one or more pathological samples so that adequate therapy can be started after histopathological evaluation. By contrast, an excisional biopsy involves complete removal of the lesion, so it is a diagnostic and therapeutic procedure. During a biopsy procedure it is fundamental to maintain safe and readable cut margins in order to permit histological visualization of possible marginal infiltrations or malignant transformation of a lesion.
Due to the considerable controversy concerning the reliability of laser biopsies [1] and the effects of thermal damage to the edge of laser wounds, we recently evaluated in vitro the effects of the use of different lasers (diode 808 nm and 980 nm, Nd:YAG 1064 nm and Er,Cr:YSGG 2780 nm) on histological diagnosis. We found that, in almost all cases, it was possible to perform histological diagnosis even when cut edges showed some thermal damage. In some cases the damage was very large and deep (Nd:YAG and diode 980 nm); in other cases it was only a few microns (diode 808 nm and Er,Cr:YSGG) [2].
In recent years the KTP laser (potassium titanium phosphate, also called the doubled frequency Nd:YAG laser) has been introduced in dentistry. The first medical use of the KTP laser was in 1986 in dermatology. Although there are more than 200 scientific reports about the use of the laser in urology [3], vascular surgery [4] and dermatology [5], there are very few reports about its use in the field of dentistry. The KTP laser has a wavelength of 532 nm and emits green visible radiation. Light is produced, as in the Nd:YAG laser, via a solid active material (yttrium, aluminium, garnet crystal doped with neodymium). The resulting radiation is filtered by a system of mirrors of potassium, titanium and phosphate, that halves the initial wavelength.
In dentistry, the KTP laser has been tested in oral surgery [6], endodontics [7], dental bleaching [8] and periodontology [9]. The output can be continuous or pulsed, depending on the application. The wavelength of the KTP laser is more strongly absorbed by oxyhaemoglobin than any other wavelength, so lower levels of energy and fluence can be used to cut vascularized tissues.
The aim of this study was to evaluate in vitro the peripheral effects induced by the KTP laser in oral soft tissues with increasing settings of power, fluence and irradiance.
Materials and methods
The study was performed in vitro on pig cadaver tongue, chosen because of its similar histological and physiological structure to human tongue, in cooperation with the Department of Cytology and Cellular Diagnosis of Regina Elena Institute of Rome. Five pig tongues were used in the experiments. A KTP laser (532 nm; SmartLite, Deka, Florence, Italy) with a 300-μm optical fibre was used to obtain 45 1-cm2 mucosal samples. The samples were taken by the same operator, an oral surgeon with expertise in the use of lasers, to prevent errors resulting from interindividual differences in ability. The tissue samples were subdivided into five groups, called G1 to G5, comprising nine samples each. The samples of the various groups were obtained with the following laser settings:
-
G1
Power 2 W, T on 50 ms, T off 50 ms, irradiance 2,800 W/cm2, fluence 141 J/cm2.
-
G2
Power 2.3 W, T on 50 ms, T off 50 ms, irradiance 3,200 W/cm2, fluence 162 J/cm2.
-
G3
Power 2.5 W, T on 50 ms, T off 50 ms, irradiance 3,500 W/cm2, fluence 176 J/cm2.
-
G4
Power 2.7 W, T on 50 ms, T off 50 ms, irradiance 3,800 W/cm2, fluence 191 J/cm2.
-
G5
Power 3 W, T on 50 ms, T off 50 ms, irradiance 4,200 W/cm2, fluence 212 J/cm2.
A final specimen, also of area 1 cm2, was taken using a Bard-Parker 15 scalpel (sample S) to serve as a control sample.
Following excision, a second operator placed the specimens in sterile test tubes marked with alphanumeric codes and containing a 10% buffered formalin solution. When the laser was used, both the operators wore protective glasses. The specimens were embedded in paraffin, cut into 3-μm samples and stained with haematoxylin-eosin for histological evaluation that was performed separately under an optical microscope (Primo Star, Zeiss, Oberkochen, Germany) at a magnification of ×10 with a micrometric lens and applying the conversion factor relative to the magnification (0.035 mm/70 pins, corresponding to 5 μm). Histological analysis was performed by two blinded pathologists to avoid any personal bias. They assigned a score from 0 to 3 to the peripheral thermal damage according to the following scale: 0 no damage, 1 little damage, 2 moderate damage, and 3 severe damage.
Finally, a statistical evaluation was conducted using GraphPad Prism 5.0 software. All groups were compared with each other using Dunn’s test to assess whether there were statistically significant differences among them.
Results
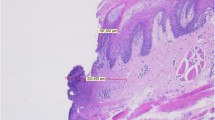
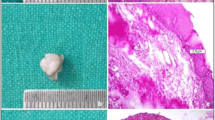
Samples of every group showed clear and readable cut margins. G1 samples generally showed clean and safe cut edges and thermal damage was limited to two or three lines of cells (Fig. 1). G2 samples generally showed signs of poor tissue coagulation both in the epithelium and corium (Fig. 2). Thermal damage in both G1 and G2 samples was always limited to less than 50 μm. By contrast, G3 samples generally showed the highest amount of thermal damage over the epithelium, but the subepithelial corium was absolutely free from thermal alteration, as shown in Fig. 3. G4 samples showed generally few signs of cellular hyperthermia near the cut edges, but they were always readable and showed clear detail (Fig. 4). G5 samples showed almost intact epithelium and signs of cellular damage were limited to 200 μm (Fig. 5). Obviously, control samples showed no thermal damage to the cut edges (Fig. 6).
Figure 7 shows the thermal damage scores for samples of all groups.
Dunn’s test showed no statistically significant differences among the groups (p>0.05).
Discussion
A biopsy is a surgical diagnostic procedure performed to establish a clear diagnosis of a lesion in order to clarify the clinical hypothesis. In performing a biopsy it is fundamental to maintain cut margins safe and readable in order to permit the pathologist to clearly evaluate possible marginal infiltration and malignant transformation of dysplastic or neoplastic lesions. In oral pathology, it is essential for a clear histological diagnosis that the whole biopsy specimen was intact and clearly readable, especially in suspected lesions. The peripheral cellular layers of the specimen are extremely important for the evaluation of the infiltrative potential of the lesion. So it is not safe to use any surgical device that creates thermal or mechanical damage to surrounding tissues for biopsy procedures. Moreover, it is well known that the partial excision of a dysplastic lesion enhances the degree of dysplasia [10, 11].
Since the ruby laser was developed by Maiman in 1960, many researchers have investigated laser applications in dentistry. A laser is a device that transforms light of various frequencies into chromatic radiation in the visible, infrared, and ultraviolet regions with all the waves in phase such that the emitted radiation is able to mobilize immense heat and power when focused at close range [12]. After initial experiments with the ruby laser [13], clinicians began using other lasers, such as argon [14], carbon dioxide [15], neodymium:yttrium-aluminium-garnet (Nd:YAG) [16], diode [17] and erbium (Er:YAG and Er,Cr:YSGG) lasers [18, 19].
Every type of laser creates thermal damage to the target tissues by a photothermal effect. The laser beam transmits thermal energy that increases the temperature at the point of incidence by more than 100°C. This vaporization is the basis of the laser effect, so lasers cut by heating the tissue. A side effect of this thermal reaction is an increase in the temperature of the surrounding tissues, and this creates permanent or reversible damage. The extent of this damage is related to both the wavelength and the settings of the laser.
The KTP laser has a visible green emission (532 nm wavelength) that is strongly absorbed by oxyhaemoglobin, so it works on all vascularized tissues, mainly with a thermal effect. As a diode laser, this device can be used in continuous or pulsed mode, with the possibility of changing power, frequency and energy settings. Optical fibres of different diameters, from large size to small (about 200 µm), provide light transmission. Because of its properties, the KTP laser can be successfully applied in many operative fields of dentistry, such as oral surgery for the management of soft tissue lesions [6], endodontics for root canal disinfection [7], dental bleaching to provide a chemical whitening process [8], and periodontology for nonsurgical periodontal treatment [9]. The advantages of the employment of the KTP laser in oral soft tissue surgery are its high cutting ability, the bloodless operative field, its relative ease and rapidity of use, and the reduced use of infiltrative anaesthesia.
In our in vitro experience, the KTP laser gave excellent results with all the tested parameters. In fact, specimens of all the groups were free from thermal artefacts at very short distances from the cut margin, even at higher fluence and irradiance settings. Obviously, we obtained the best results at the lowest fluence settings (G1 and G2 141 and 162 J/cm2, respectively); however even at higher settings thermal injury was always slight and limited to a few microns in both the epithelium and corium, and, as the statistical analysis showed, there were no differences among the groups. According to our in vitro and clinical experience we may say that the KTP laser seems to be the least harmful device amongst the lasers of different wavelength we have studied [2]. Our results show the high performance of this wavelength and dispel the doubts concerning the use of this laser in oral pathology.
However, we should not forget two important considerations. First, our study was performed in vitro irradiating bloodless tissues. The presence in vivo of normal or pathological amounts of blood (e.g. in inflammatory or autoimmune diseases) could lead to an improvement in cutting ability that could permit the parameters applied to be reduced with less damage to cut margins, but on the other hand to higher local heat build-up with larger thermal artefacts. Second, there is always thermal damage to the cut edges. So, according to our experience, we believe that it is still necessary to separate oral soft tissue lesions into two groups, according their dysplastic potential:
-
1.
Clinically nonsuspicious lesions (e.g. fibroma, haemangioma, gingival hyperplasia, mucocele, etc), where laser treatment is fundamental in the surgical resolution of the pathology.
-
2.
Suspected dysplastic or neoplastic lesions (e.g. leukoplakia, lichen planus, carcinoma, melanoma, etc), where the peripheral thermal damage may lead to the risk of misunderstanding the real extent of the lesion, with great problems both in treatment and prognosis. So it would be prudent to enlarge the surgical incision for these lesions to at least about 0.5 mm, with the aim of making the histological diagnosis totally free from uncertainty.
In conclusion, the KTP laser is an effective instrument for performing oral soft tissue excision, because of its excellent surgical properties and the almost total lack of thermal damage. However, these indisputable advantages cannot replace the ability and knowledge of the oral surgeon.
References
Rizoiu IM, Eversole LR, Kimmel AI (1996) Effects of an erbium, chromium: yttrium, scandium, gallium, garnet laser on mucocutaneous soft tissues. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 82:386–395
Romeo U, Del Vecchio A, Ripari F, Palaia G, Chiappafreddo C, Tenore G, Visca P (2007) Effects of different laser devices on oral soft tissues: in vitro experience. J Oral Laser Appl 7(3):155–159
Hernández Fernández C, Subirá Rios D, Bueno Chomón G, Tabares Jiménez J (2008) Prostate and KTP laser. Arch Esp Urol 61(9):1023–1027
Kishimoto Y, Hirano S, Kato N, Suehiro A, Kanemaru S, Ito J (2008) Endoscopic KTP laser photocoagulation therapy for pharyngolaryngeal venous malformations in adults. Ann Otol Rhinol Laryngol 117(12):881–885
Dabis R, Rosbotham J, Jones L, Knowles S, Harland CC (2006) Potassium titanyl phosphate (KTP) laser treatment for molluscum contagiosum. J Dermatol Treat 17(1):45–47
Miyazaki H, Kato J, Watanabe H, Harada H, Kakizaki H, Tetsumura A, Sato A, Omura K (2009) Intralesional laser treatment of voluminous vascular lesions in the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 107(2):164–172
Schoop U, Kluger W, Dervisbegovic S, Goharkhay K, Wernisch J, Georgopoulos A, Sperr W, Moritz A (2006) Innovative wavelengths in endodontic treatment. Lasers Surg Med 38(6):624–630
Zhang C, Wang X, Kinoshita J, Zhao B, Toko T, Kimura Y, Matsumoto K (2007) Effects of KTP laser irradiation, diode laser, and LED on tooth bleaching: a comparative study. Photomed Laser Surg 25(2):91–95
Romeo U, Palaia G, Botti R, Leone V, Rocca JP, Polimeni A (2009) Non-surgical periodontal therapy assisted by potassium-titanyl-phosphate laser: a pilot study. Laser Med Sci. doi:10.1007/s10103-009-0738-4
Zhang L, Poh CF, Lam WL, Epstein JB, Cheng X, Zhang X, Priddy R, Lovas J, Le ND, Rosin MP (2001) Impact of localized treatment in reducing risk of progression of low-grade oral dysplasia: molecular evidence of incomplete resection. Oral Oncol 37(6):505–512
Ma G, Sano K, Ikeda H, Inokuchi T (1999) Promotional effect of CO2 laser and scalpel incision on 4-NQO-induced premalignant lesions of mouse tongue. Laser Surg Med 25(3):207–212
Kimura Y, Wilder-Smith P, Matsumoto K (2000) Lasers in endodontics: a review. Int Endod J 33:173–185
Stern RH, Sognnaes RF (1964) Laser beam effect on dental hard tissues. J Dent Res 43:873
Conrado LA, Munin E, Frois IM, Zingaro RA (2004) Root apex sealing with different filling materials photopolymerized with fiber optic-delivered argon laser light. Lasers Med Sci 19:95–99
Matsumoto K, Suzuki H, Usami Y, Hattori M, Komoro T (2008) Histological evaluation of artifacts in tongue tissue produced by the CO2 laser and the electrotome. Photomed Laser Surg 26(6):573–577
Vivek V, Jayasree RS, Balan A, Sreelatha KT, Gupta AK (2008) Three-year follow-up of oral leukoplakia after neodymium:yttrium aluminum garnet (Nd:YAG) laser surgery. Lasers Med Sci 23(4):375–379
Ergun S, Mete O, Yeşil S, Tanyeri H (2009) Solitary angiokeratoma of the tongue treated with diode laser. Lasers Med Sci 24(1):123–125
Rosa DS, Aranha AC, Eduardo Cde P, Aoki A (2007) Esthetic treatment of gingival melanin hyperpigmentation with Er:YAG laser: short-term clinical observations and patient follow-up. J Periodontol 78(10):2018–2025
Zola M, Rosenberg D, Anakwa K (2006) Treatment of a ranula using an Er,Cr:YSGG laser. J Oral Maxillofac Surg 64(5):823–827
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romeo, U., Palaia, G., Del Vecchio, A. et al. Effects of KTP laser on oral soft tissues. An in vitro study. Lasers Med Sci 25, 539–543 (2010). https://doi.org/10.1007/s10103-010-0756-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-010-0756-2