Abstract
In 30 patients with periodontitis, a total of 278 teeth exhibiting bleeding on probing, subgingival calculus, and a probing depth between 3–6 mm were examined. For each participant, two treatment types were alternatively applied on the contralateral quadrants: scaling and root planing (SRP) as control, and SRP followed by Er,Cr:YSGG laser application (SRP+laser), as a test method. Five clinical parameters: plaque level, bleeding on probing, probing depth, gingival recession and clinical attachment level were examined at baseline and at 2, 3, 6, 12 months after treatment. Of the total of 1,668 sites examined in all patients, 1,088 sites were found with a probing depth of 3–6 mm. In these sites, differences in clinical parameters between SRP and SRP+laser-treated quadrants were analyzed, assuming the level of p < 0.05 as significant. After 2 months from baseline, the mean probing depth reduction and the clinical attachment level gain were significantly greater in SRP+laser than in SRP quadrants, and remained so throughout the study (p < 0.001). A marked reduction of the bleeding scores occurred in all examined sites, irrespective of the treatment method. However, after 12 months, significantly less teeth exhibited bleeding on probing in SRP+laser quadrants than in SRP quadrants (p < 0.001). The mean plaque and gingival recession levels did not differ between the SRP and SRP+laser quadrants neither before nor after the treatment. The periodontal procedures either using Er,Cr:YSGG laser after SRP or SRP alone, lead to significant improvements in all clinical parameters investigated. However, laser application, as an adjunct to SRP, appeared to be more advantageous.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic periodontitis is an infectious disease related to inflammation of the supporting tissues of teeth and to the following progressive loss of attachment and of the bone. The primary goal in treatment of periodontitis is the removal of subgingival deposits, bacterial biofilm, and smear layer, in order to prevent progression of the disease [1, 2]. This treatment strategy aims to restore the periodontal attachment level so that the periodontal fibers could connect into newly formed cementum [3].
Conventional periodontal procedures, otherwise called scaling and root planing, consist of debridement of the contaminated root surfaces as well as of elimination of bacteria and their endotoxins from the cementum and from the adjacent periodontal tissues [4]. However, removal of calculus by means of manual instruments and ultrasonic scalers is often incomplete, due to the root topography, painful and rather time-consuming [5]. In cases where gingival recession exists, opened root surfaces may remain sensitive after the procedure. Furthermore, such mechanical treatment usually produces a smear layer and deep grooves on the root surfaces that may adversely affect healing of the periodontal tissue [6]. Considering these disadvantages, in recent years lasers were suggested as an alternative for treatment of periodontitis. Lasers are designed to ablate or to vaporize only the diseased tissue from the inner epithelial lining of a periodontal pocket, resulting in a better, more predictable end result to treatment by cauterizing blood vessels, nerve endings, and lymph glands, providing hemostasis, post-operative pain control, and rapid healing [7].
However, due to the complex structure of periodontium (combination of gingiva, periodontal ligament, cementum, and alveolar bone) the use of lasers for periodontal treatment can be rather problematic. As concluded by several researchers, neither CO2 nor Nd:YAG or diode lasers are effective in removing calculus from the root surfaces [7]. When these lasers were used directly on the cementum or on the alveolar bone, thermal side-effects such as carbonization and melting were reported [8].
Recently, Er:YAG (erbium-doped:yttrium, aluminium and garnet, wavelength 2,940 nm) and the Er,Cr:YSGG (erbium-chromium-yttrium-scallium-gallium-garnet, wavelength 2,790 nm) lasers were introduced. Several studies suggested that erbium lasers could effectively remove calculus [9, 10] as well as superficial layers of the contaminated cementum and exhibit high bactericidal effects [11, 12] without any thermal damage to the root surface or to the adjacent tissue [13, 14].
The most common mechanism of periodontal wound healing is characterized by the epithelization of the internal surface of the flap in contact with the root cementum, forming so-called long epithelial attachment [15, 16]. The epithelial tissue possesses migrating cells that are able to invade the periodontal wound area faster than other cells, and to facilitate periodontal re-attachment. Therefore, it was proposed to increase the distance for the epithelial cells to travel in order to allow the periodontal ligament cells to reach the radicular surface first. By means of a laser, it is possible to de-epithelize the flap and thus delay epithelial migration. Consecutively, formation of the connective tissue and new alveolar bone is facilitated [17, 18]. These processes are expected to improve clinical parameters in periodontal treatment.
The purpose of this study was to compare the clinical results of conventional scaling and root planing with a therapy using the Er,Cr:YSGG laser in adjunct to scaling and root planing during 1 year of application in patients with early to moderate periodontitis.
Materials and methods
The study was conducted at the Clinic of Dental and Oral Pathology, Kaunas University of Medicine, during the period from March 2006 to March 2008. The Ethical Committee of Kaunas University of Medicine, Lithuania, approved the study protocol.
Selection of subjects
The study subjects were selected from the patients applying for periodontal treatment at the university clinic. Patient selection was based on signed informed consent forms and that the patients met the following criteria:
-
No periodontal treatment received within the last 12 months
-
No systemic diseases that could potentially influence the outcome of the therapy (diabetes, immune deficiencies, cancer, hemorrhagic disorders, epilepsy, etc.)
-
No use of systemic antibiotics at least 6 months prior to, during, and 12 months after the treatment
-
Non-smokers
-
No pregnancy
Thus, 30 patients with a diagnosis of early or moderate periodontitis between 26 and 58 years of age (16 men, 14 women) were included in the study.
Study design
The study was performed according to a split-mouth design on single-rooted teeth (lower or upper incisors and canines, upper second premolars, lower first and second premolars). A total of 278 teeth (123 in the maxilla, and 155 in the mandible) exhibiting gingival inflammation with positive bleeding on probing (BOP), subgingival calculus, and a PD (probing depth) of 3–6 mm on at least one site of the tooth were selected for examination. Of the 1,668 sites examined, 1,088 exhibited a probing depth of more than 3 mm (range: 3–6 mm). A detailed description of the distribution of treatment sites is presented in Fig. 1.
Two types of treatment were performed for the study participants:
-
1.
Conventional scaling and root planing (SRP)-‘control’ method
-
2.
Conventional scaling and root planing followed by immediate Er,Cr:YSGG application-(SRP+laser)-‘test’ method.
Each participant received both types of treatment using different mouth quadrants for at least two quadrants. On one side, the teeth were treated by scaling and root planing only (control method, SRP), whereas the teeth of the contra-lateral side were treated by Er,Cr:YSGG laser immediately after SRP (test method, SRP+laser). Selection of the mouth quadrants to be treated with either test, or control method, was performed randomly (for details, see Kelbauskiene, Maciulskiene, 2007) [19]. The selected treatment protocols were coded in the patients’ case descriptions in order to prevent biased measurements of the treatment outcomes. Two weeks prior to treatment, all patients were scheduled for oral hygiene instructions as well as for professional supra-gingival tooth cleaning according to individual needs. The same oral hygiene procedures were performed after 3 and 6 months.
The baseline recordings of the clinical parameters and the following treatment procedures were performed by a periodontist (SK). The clinical parameters of periodontal status at 2, 3, 6, and 12 months after the treatment were measured by another blinded and calibrated examiner who did not know which quadrants were treated by SRP alone or by SRP+laser. The probing measurements were taken after the recording of plaque and of the bleeding scores.
For all patients, in the control quadrants, scaling and root planing was performed immediately after the baseline examination and subsequently supra-gingival scaling and polishing were performed 3 and 6 months after the initial treatment. In the test quadrants, SRP+laser was performed immediately after the baseline examination. Supra-gingival scaling and polishing were performed 3 and 6 months after the initial treatment.
Scaling and root planing (SRP)
Subgingival instrumentation of the root surfaces was performed using an ultrasonic scaler (Satelec, Acteon, Switzerland) with a sharp-pointed tip. The ultrasonic scaler was conducted by contacting the probe obliquely to the root surface at an angle of approximately 15° and moving the tip in a sweeping motion. The instrumentation was accomplished using Gracey curettes (American Eagle, USA). The procedure was continued until the operator felt that the root surfaces were adequately scaled and planed.
Er,Cr:YSGG laser application after SRP (SRP+laser)
An Er,Cr:YSGG laser device (Waterlase, Biolase, USA) was used in this study. This laser system emits photons at a wavelength of 2.78 µm and has a pulse duration of 140 to 200 µs with a repetition rate of 20 Hz. The average power output can vary from 0 to 6 W. A Z-6 series tip of 600 µm in diameter and 9 mm in length was used to remove the inner epithelial lining (the epithelium inside the periodontal pocket) to the depth of the pocket, and 5 mm of the outer epithelium (oral epithelium near the free gingival margin). This technique allows cells that arise from periodontal ligament and the bone to repopulate the root surface, while excluding epithelial and gingival cells from the initial wound healing [20, 21]. A 9-mm Z-6 tip marked to the depth of the pocket was used at a setting of 1 W, 10% air and 15% water. The treatment was performed from coronal to apical paths parallel to the long axis to the root surfaces. To condition the root, the laser tip was angled 5–15° toward the root and moved up and down until the root surface was left with an acid-etched appearance. The same procedure was performed once a week for each millimeter of pocket reduction desired to obtain a normal probing depth of 3 mm or less. This required an average of three appointments. At subsequent visits, inner epithelium to the depth of the pocket (1 mm less than at the previous appointment) and 5 mm of the outer epithelium was removed.
Clinical measurements and data collection
The following parameters were recorded before the treatment, and 2, 3, 6, 12 months after the treatment: plaque index (PI), bleeding on probing (BOP), probing depth (PD), gingival recession (GR), and clinical attachment level (CAL).
Plaque index was assessed for every tooth examined using the following scale modified from Silness and Löe [22]: 0–no plaque; 1–plaque detected by probe only; 2–visible, average amount of plaque; 3–a lot of visible plaque near the gingival margin and into the pocket.
Bleeding on probing and pocket measurements were assessed simultaneously and the absence or presence of bleeding up to 30 s after probing was recorded. Probing depth (PD) was measured from the gingival margin to the depth of the pocket. Gingival recession (GR) was measured from the cementum-enamel junction (CEJ) to the gingival margin. Clinical attachment level (CAL) was measured from the CEJ to the bottom of the probable sulcus. The measurements were made at six sites per tooth: mesio-vestibular (mv), mid-vestibular (v), disto-vestibular (dv), mesio-lingual (ml), mid-lingual (l), and disto-lingual (dl) using a manual periodontal probe (PCP 12, Hu-Friedy, USA).
Statistical analysis
The data were analyzed using SPSS 13.0 (SPSS inc., Chicago, III) statistical package. Statistical significance of difference in proportion was tested by Chi-square test. The mean values and standard deviations of the clinical parameters were calculated. Statistical testing between the groups (SRP and SRP+laser) for differences of GR, PD, as well as of CAL were assessed by comparing the mean change values estimated for all tooth aspects, in the sites with baseline PD 3–6 mm, at different time points. The evaluation of mean values was performed using Student's t test. For the data that were not normally distributed, as shown by a Kolmogorov-Smirnov test, Mann–Whitney U test was applied. For testing the measurements of dependent variables, the paired t test for the continuous data, and Wilcoxon’s test for the ordinal data were used. The difference with significance level below 0.05 was evaluated as significant.
The power of the study, given PD of 0.56 (1.09) mm as a significant difference between the groups, was calculated to be 0.99, which justified the sample size of 30 patients, presenting 1,088 examined sites.
Plaque distribution was evaluated at the tooth level and was determined by percentages of teeth with different scores recorded. Score 0 and score 1 were added together and were defined as “no plaque”, score 2 and score 3 were added together and defined as “visible plaque”. Plaques levels were estimated in percentages of teeth presenting scores with “visible plaque”.
Changes in BOP were expressed as percentages of presence or absence of bleeding up to 30 s after probing was recorded.
Inter-examiner and intra-examiner reproducibility testing: four patients, each presenting two pairs of contra-lateral teeth with the probing depth ≥4 mm on at least one aspect of the tooth, were examined twice, with an interval of 48 h to calibrate gingival recession and probing depth. Variation between the repeated measurements not exceeding 1 mm was accepted. Reproducibility testing was performed using a manual periodontal probe (PCP 12, Hu-Friedy, USA). The percentages of inter-examiner and intra-examiner agreements were 93.8% and 90%, respectively.
Results
Baseline clinical examination of the patients revealed no statistically significant differences in the investigated parameters between SRP-treated and SRP+laser-treated quadrants. However, in the sites with baseline PD of 3–6 mm, the baseline mean PD and mean CAL values were significantly higher in the SRP+laser quadrants as compared to SRP quadrants (Table 1).
During the entire study period, the status of oral hygiene remained good in all patients. No statistically significant differences in PI values were observed between the SRP-treated and SRP+laser-treated quadrants neither before nor after the instrumentation. The percentage of teeth presenting “visible plaque” increased in all treated quadrants, when estimated 2 and 3 months after the instrumentation (also 6 months after the treatment, in the SRP quadrants), however, they were not different significantly from the baseline plaque levels when estimated 12 months after the instrumentation (Fig. 2).
The changes in bleeding on probing (BOP) levels during the study period are presented in Fig. 3. At baseline, 79.0% of the teeth examined in the SRP+laser-treated quadrants, and 74.1% of the teeth examined in the SRP-treated quadrants, exhibited bleeding on probing. After the instrumentation, a marked reduction of the bleeding scores occurred in all examined sites treated with either SRP or SRP+laser method (Fig. 3). However, at the final examination after 12 months, significantly less teeth exhibited BOP in the SRP+laser-treated quadrants than in the SRP-treated quadrants: 9.8% and 26.7%, respectively (p < 0.001).
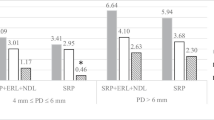
The mean values of gingival recession (GR) for the investigated tooth sites at baseline did not differ between SRP-treated and SRP+laser-treated quadrants. The estimated changes of gingival recession were marginal and remained similar between SRP and SRP+laser quadrants through the entire study period (Table 1). The mean PD changes from baseline values, estimated at all following examination time points (2, 3, 6, and 12 months) after the instrumentation, differed significantly between SRP-treated and SRP+laser-treated quadrants, the mean PD reduction being greater for the SRP+laser-treated sites than for the sites treated by SRP alone (p < 0.001). The same tendency was observed regarding CAL measurements: statistically significant differences of CAL gain (p < 0.001) were found between SRP-treated and SRP+laser-treated quadrants at 2, 3, 6, and 12 months examination points, the CAL gain being greater in SRP+laser-treated sites (Table 1).
When CAL changes were analyzed for the lingual and vestibular surfaces separately, the same tendency was observed as for all tooth aspects: the significantly greater attachment gain was estimated in SRP+laser-treated lingual and vestibular sites as compared to SRP-treated sites, thorough the entire study period (Fig. 4).
Discussion
The explanation of periodontal healing is largely based on Melcher’s hypothesis [23], which suggests that formation of attachment between the tooth and the periodontal tissue depends on the origin of the cells repopulating the wound area, and that the only cells able to achieve true periodontal regeneration are periodontal ligament cells (‘pdl’) [24]. For new attachment to form, the epithelium within the periodontal pocket needs to be removed, otherwise the epithelial cells would inhibit proliferation as well as migration of ‘pdl’ cells from the periodontal space to the wound area. As soon as ‘pdl’ cells reach the root surface, they start to stimulate cementoblasts to form the new cementum, and mediate the attachment of the connective tissue and the bone to this cementum. Thus, the major advantage of laser application as compared to other treatment methods such as conventional scaling and root planing is thought to be the facilitated process of epithelium removal from the periodontal pockets.
The results of the present study have demonstrated that non-surgical periodontal treatment with either combination of a laser and SRP or root scaling and planing alone lead to clinically and statistically significant improvements in all investigated parameters at 2, 3, 6, and 12 months following treatment. The observation that in all cases there were no post-operative complications indicates good tolerance of all conservative treatment procedures received. These results are in agreement with other studies where a significant PD reduction and CAL gain was achieved after the subgingival debridement procedures with an Er:YAG laser [25–28]. Thus, it has been suggested that the Er:YAG laser could be considered as a meaningful alternative to hand instruments in treatment of periodontitis [12].
However, in the present study, the use of the Er,Cr:YSGG laser in addition to scaling and root planing resulted in a statistically significant and consistently greater reduction of the probing depth and gain of the clinical attachment level when compared to the results of SRP alone. According to our results, the most obvious changes of the periodontal depth in SRP-treated and SRP+laser-treated quadrants were achieved in 6 months after the treatment (Table 1). However, after 12 months, the estimated mean PD reduction values decreased significantly in both treatment groups when compared to the mean PD reduction values estimated after 6 months from baseline. It appeared that in the SRP-treated quadrants, the mean PD value after 12 months was still lower than the respective baseline value, but became indicative for early periodontitis (mean PD > 3 mm: 4.07 (0.79) – 0.89 (1.04) = 3.19 (1.03), Table 1). However, such tendency was not observed in SRP+laser-treated quadrants (4.33 (1.08) – 1.71 (1.35) = 2.62 (1.17), Table 1). Considering the fact that at baseline the mean estimated PD values in SRP quadrants were even lower than in the SRP+laser quadrants, the obtained results suggest that a combined SRP and laser therapy provides better longitudinal results than SRP alone. The observation of the decreased PD reduction in SRP-treated quadrants over time can potentially explain the increase of BOP values in these quadrants in 12 months after the treatment.
In the present study, plaque values (PI) increased in both treatment groups 3 months after treatment compared to baseline (p < 0.05), although there were no significant differences between groups through the whole study. The potential explanation for this could be that supragingival professional teeth cleaning was performed just 2 weeks before baseline examination and there was no professional hygiene visit between the baseline appointment and the 3-month follow-up visit. Consequently, the amount of plaque present was dependent completely on the patients' home care compliance.
Blomlof et al. [29] showed that ultrasonic debridement resulted in a smooth surface covered by a smear layer, whereas the Er:YAG laser induced glazed microstructures presenting a relative rough surface topography. A smear layer, which contains bacteria and inflammatory substances, may adversely affect the healing of periodontal tissue [30]. Crespi et al. [31] reported that Er:YAG-treated root surfaces that were roughened promoted fibroblast attachment and contributed to a significant gain of clinical attachment level. Removal of the smear layer and etching of the root surface could explain the increased fibroblast attachment, which might be a partial explanation for the improved probing depth and clinical attachment level.
Findings from several studies reported a high bactericidal potential of the Er:YAG laser [12, 32]. In addition, besides viable bacteria, laser radiation was capable of removing endotoxins from the root surfaces [33]. Mechanical periodontal treatment alone usually improves clinical conditions; however, it is not effective in eliminating all types of bacteria [34, 35]. Furthermore, the literature suggests that laser application helps to remove sulcular epithelium more precisely and effectively than the manual instruments, without causing underlying damage to the connective tissue [36]. Consequently, laser de-epithelization blocks the down-growth of epithelium into the healing periodontal pocket and allows cells to arise from the periodontal ligament, and so enhances periodontal reattachment [18].
Schwarz et al. [26] compared the use of an Er:YAG laser+SRP with the application of laser alone in a split-mouth design for the treatment of 20 patients with moderate to advanced periodontitis. They concluded that a combined Er:YAG laser and SRP treatment did not improve the outcome of the therapy compared to Er:YAG laser alone. The same group of investigators compared laser treatment with scaling and root planing as mono-therapies and again, obtained more favorable results in the laser-treated teeth. In this study, the mean values of gingival recession increased after 3 months from the treatment, and remained at the increased level throughout the whole study.
On the contrary, we found that gingival recession increased slightly in the SRP-treated quadrants, however, remained stable in the SRP+laser-treated quadrants. This explains that the obtained gain of CAL was not related to increased GR but was as a result of decreased PD. This is a very positive finding, indicating a more efficient outcome of the SRP+laser therapy compared to scaling and root planing alone, which is possibly explained by the retarded epithelial migration related with an increased connective tissue formation.
Conclusions
Based on the present study conditions, we conclude that:
-
Non-surgical periodontal therapy using either SRP+Er,Cr:YSGG laser, or scaling and root planing alone, lead to significant improvements in all clinical parameters investigated.
-
The treatment using Er,Cr:YSGG laser as an adjunct to SRP appeared to be more advantageous when compared to SRP alone.
-
Combined therapy, using SRP+Er,Cr:YSGG laser appeared to have a prolonged clinical improvement throughout the study period when compared to SRP alone.
References
O’Leary TJ (1986) The impact of research on scaling and root planing. J Periodontol 57(2):69–75
Kepic TJ, O’Leary TJ, Kafrawy AH (1986) Total calculus removal: an attainable objective? J Periodontol 57:69–75
Nyman S, Lindhe J, Karring T (1992) Reattachment–New attachment. In: Lindhe J (ed) Textbook of clinical periodontology. Munksgaard, Copenhagen
Adrians PA, Edwards CA, De Boever IA et al (1988) Ultrastructural observations on bacterial invasion in cementum and radicular dentin of periodontally diseased human teeth. J Periodontol 59:493–503
Yukna RA, Scott JB, Aichelmann-Reidy ME et al (1997) Clinical evaluation of the speed and effectiveness of subgingival calculus removal on single-rooted teeth with diamond-coated ultrasonic tips. J Periodontol 68(5):436–442
Polson AM, Frederic GT, Ladenheim S et al (1984) The production of a root surface smear layer by instrumentation and its removal by citric acid. J Periodontol 55:443–446
Moritz A, Schoop U, Goharkhay K et al (1998) Treatment of periodontal pockets with a diode laser. Lasers Surg Med 22:302–311
Rossman JA, Cobb M (1995) Lasers in periodontal therapy. Periodontol 2000(9):150–164
Watanabe H, Ishikawa I, Suzuki M (1996) Clinical assessments of the erbium:YAG laser for soft tissue surgery and scaling. J Clin Laser Med Surg 14(2):67–75
Folwaczny M, Mehl A, Haffner C et al (2003) Root substance removal with Er:YAG laser radiation at different parameters using a new delivery system. J Periodontol 74(2):147–155
Ando Y, Aoki A, Watanabe H et al (1996) Bactericidal effect of erbium YAG laser on periodontopathic bacteria. Lasers Surg Med 19:190–200
Folwaczny M, Mehl A, Aggstaller H et al (2002) Antimicrobial effects of 2.94 micron Er:YAG laser radiation on root surfaces: an in vitro study. J Clin Periodontol 29:73–78
Yamaguchi H, Kobayashi K, Osada R et al (1997) Effects of irradiation of an erbium:YAG laser radiation on root surfaces. J Periodontol 68:1151–1155
Theodoro LH, Haaypek P, Bachmann L et al (2003) Effect of Er:YAG and diode laser irradiation on the root surfaces: morphological and thermal analysis. J Periodontol 74:838–843
Caton J, Zander HA (1976) Osseous repair of an intrabony pocket without new attachment of connective tissue. J Clin Periodontol 3:54–58
Listgarten MA, Rosenberg MM (1979) Histological study of repair following new attachment procedures in human periodontal lesions. J Periodontol 50:333–344
Centry IG, Blank LW, Levy BA et al (1997) Carbon dioxide laser for de-epithelization of periodontal flaps. J Periodontol 68(8):763–768
Israel M, Rossmann JA, Froum SJ (1995) Use of the carbon dioxide laser in retarding epithelial migration: a pilot histological study utilizing case reports. J Periodontol 66:197–203
Kelbauskiene S, Maciulskiene V (2007) A pilot study of Er,Cr:YSGG laser therapy used as an adjunct to scaling and root planing in patients with early and moderate periodontitis. Stomatologija 9(1):21–26
Nyman S, Gottlow J, Karring T et al (1982) The regenerative potential of the periodontal ligament: an experimental study in monkeys. J Clin Periodontol 9:257
Nyman S, Lindhe J, Karring T et al (1982) New attachment following surgical treatment of human periodontal disease. J Clin Periodontol 9:290
Loe H, Silness J (1963) Periodontal disease in pregnancy. Prevalence and severity. Acta Odontol Scand 21:553–551
Melcher AH (1976) On the repair potential of periodontal tissues. J Periodontol 47(5):256–260
Melcher AH, Mcculloch CAG, Cheong T et al (1987) Cells from bone synthesize cementum-like and bone-like tissue in vitro and may migrate into periodontal ligament in vivo. J Periodontal Res 22:246–247
Shulean A, Schwarz F, Berakdar M et al (2004) Periodontal treatment with an Er:YAG laser compared to ultrasonic instrumentation: a pilot study. J Periodontol 75:966–973
Schwarrz F, Berakdar M, Georg T et al (2003) Clinical evaluation of an Er:YAG laser combined with scaling and root planing for non-surgical periodontal treatment. J Clin Periodontol 30:26–34
Crespi R, Cappare P, Toscanelli I, Gherlone E et al (2007) Effects of Er:YAG laser compared to ultrasonic scaler in periodontal treatment: a 2-year follow-up split mouth clinical study. J Periodontol 78:1195–1200
Schwarz F, Berakdar M, Georg T, Reich E et al (2003) Clinical evaluation of an Er:YAG laser combined with scaling and root planing for non-surgical periodontal treatment. J Clin Periodontol 30:26–34
Blomlof JP, Blomlof LB, Lindskog SF (1996) Smear removal and collagen exposure after non-surgical root planing followed by etching with an EDTA gel preparation. J Periodontol 67(9):841–845
Polson AM, Frederich GT, Ladenheim S, Hanes PJ (1984) The production of a root surface smear layer by instrumentation and its removal by citric acid. J Periodontol 55:443–446
Crespi R, Romanos GE, Cassinelli C, Gherlone E (2006) Effects of Er:YAG laser and ultrasonic system on fibroblast attachment to root surfaces: in vitro study. J Periodontol 77:1217–1222
Schwarz F, Shulean A, Georg T, Reich E (2001) Periodontal treatment with an Er:YAG laser compared to scaling and root planing. A controlled clinical study. J Periodontol 72:361–368
Folwaczny M, Aggsataller H, Mehl A, Hickel R (2003) Removal of bacterial endotoxin from root surface with Er:YAG laser. Am J Dent 16:3–5
Renvert S, Wikstrom M, Dahlen G, Slots J et al (1990) Effect of root debridement on the elimination of Actinobacillus actinomycetemcomitans. J Clin Periodontol 17:345–350
Takamatsu N, Yano K, He T, Umeda M et al (1999) Effect of initial periodontal therapy on the frequency of detecting Bacteroides forsythus, Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans. J Periodontol 70:574–580
Centty I, Blank L, Levy B et al (1997) Carbon dioxide laser for de-epithelization of periodontal flaps. J Periodontol 763–768
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kelbauskiene, S., Baseviciene, N., Goharkhay, K. et al. One-year clinical results of Er,Cr:YSGG laser application in addition to scaling and root planing in patients with early to moderate periodontitis. Lasers Med Sci 26, 445–452 (2011). https://doi.org/10.1007/s10103-010-0799-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-010-0799-4