Abstract
The aim of this study was to investigate the effect of low-level laser therapy (LLLT) on plantar fasciitis documented by the ultrasonographic appearance of the aponeurosis and by patients’ pain scores. Thirty individuals with diagnosis of unilateral plantar fasciitis were enrolled in a randomized, double-blind, placebo-controlled trial, but 25 participants completed the therapeutic protocol. The contralateral asymptomatic fascia was used as control. After enrolment, symptomatic individuals were randomly assigned to receive LLLT, or identical placebo, for 6 weeks. Ultrasonography was performed at baseline and after completion of therapy. The subjective subcalcaneal pain was recorded at baseline and after treatment on a visual analogue scale (VAS). After LLLT, plantar fascia thickness in both groups showed significant change over the experimental period and there was a difference (before treatment and after treatment) in plantar fascia thickness between the two groups. However, plantar fascia thickness was insignificant (mean 3.627 ± 0.977 mm) when compared with that in the placebo group (mean 4.380 ± 1.0042 mm). Pain estimation on the visual analogue scale had improved significantly in all test situations (after night rest, daily activities) after LLLT when compared with that of the placebo group. (P = 0.006 and P = 0.01, respectively). Additionally, when the difference in pain scores was compared between the two groups, the change was statistically significant (after night rest P = 0.000; daily activities P = 0.001). In summary, while ultrasound imaging is able to depict the morphologic changes related to plantar fasciitis, 904 nm gallium–arsenide (GaAs) infrared laser may contribute to healing and pain reduction in plantar fasciitis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plantar fascia or plantar aponeurosis is a thin fibrous band on the plantar surface of the foot and forms a strong mechanical linkage between the calcaneous and the toes [1]. Synonymous with the deep fascia [2], plantar aponeurosis arises from the medial process of the calcaneal tubercle and attaches distally to the plantar aspect of the forefoot as well as the medial and lateral intermuscular septa [3].
Plantar fasciitis may be idiopathic or associated with rheumatoid arthritis, seronegative spondyloarthropathies, or traumatic [4]. Idiopathic plantar fasciitis is the most frequent cause of heel pain. The development of plantar fasciitis is thought to have a mechanical origin. Mechanical overload has been cited as the principal factor involved in the development of plantar fasciitis [5]. In addition, the incidence of plantar fasciitis is high in obese people and sports players, especially athletes [6, 7].
The diagnosis of plantar fasciitis is typically clinical. It is based on the clinical history: pain during weight bearing, especially in the morning, which worsens throughout the day with increased activity. A pathognomonic feature is tenderness at the insertion site of the plantar fascia on the medial calcaneal tubercle [5, 8].
Ultrasound and magnetic resonance imaging have been useful in the differential diagnosis of plantar heel pain and confirmation of diagnosis of plantar fasciitis [5, 8]. Several sonographic studies have been performed to assess plantar fascia thickness and echogenicity [4, 9–12]. In plantar fasciitis the average thickness varies from 2.9 mm to 6.2 mm in symptomatic patients, and the aponeurosis is usually hypoechoic. As a general rule, a plantar fascia thickness of more than 4 mm would be consistent with plantar fasciitis in the proper clinical setting [1]. Plantar fascia thickness in control subjects and in the asymptomatic feet of unilateral cases varies from 2.2 mm to 3.9 mm for control subjects and from 2.3 mm to 3.8 mm for asymptomatic feet [13].
Therapy for plantar fasciitis is primarily conservative [14]. Non-steroidal anti-inflammatory drugs, local steroid injections, heel cups, orthotics, electrotherapy, extracorporeal shock wave therapy and physiotherapy with stretching exercises are used [15–20]. In the 10% of patients that remain, surgical intervention is recommended [21]. Low-power lasers have gained popularity over the past 30 years in the management of soft tissue injuries and painful conditions. Over the years their clinical use has grown, although acceptance and scientific support remain somewhat mixed [22]. Nowadays, low-level lasers are used to accelerate wound healing, lessen pain, decrease inflammation and speed recovery from musculoskeletal injury [9, 23–28]. Despite this use, efficacy still remains contentious [22].
We conducted a randomized, double-blind, placebo-controlled trial to evaluate the efficacy of low-level laser therapy (LLLT) in patients with plantar fasciitis. The aim of this study was to investigate the effect of LLLT on plantar fasciitis documented by the ultrasonographic appearance of the aponeurosis and by patients’ pain scores.
Methods
From January 2006 till August 2007, a randomized, double-blind, placebo-controlled trial was performed to assess with ultrasound and a pain visual analogue scale the effectiveness of low-intensity laser in the treatment of idiopathic plantar fasciitis. Participants were recruited from private practice-referring physiatrists, orthopaedic surgeons and sports medicine physicians. All procedures were in accordance with the ethical protocols proposed by our national ethics committee. Informed consent was given by all the participants, according to the Declaration of Helsinki, prior to participation in the study.
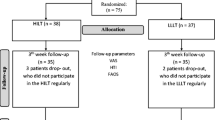
Thirty individuals (15 women, 15 men; mean age 40 ± 14 years; range 22–65 years), with a diagnosis of unilateral plantar fasciitis, were enrolled in the trial. Entry criteria included (1) unilateral plantar heel pain, mainly during the first few steps upon arising in the morning, which worsened with increased weight-bearing activity throughout the day; (2) duration of pain of more than 6 weeks; (3) tenderness at the insertion site of the plantar aponeurosis, on the medial calcaneal tubercle, which increased with dorsal flexion of the toes. Potential participants were excluded if (1) the plantar pain was diffuse or bilateral; (2) there was a history of recent trauma or foot surgery; (3) the subjects had enrolled during the past 6–12 months in another heel pain therapeutic protocol; (4) there was a previous diagnosis of rheumatoid arthritis, seronegative spondyloarthritis, calcaneal stress fracture, osteomyelitis, plantar fascia neoplasm or plantar aponeurosis rupture. The mean duration between the onset of the pain and admission to the trial was 85 ± 123 days. Three patients had mild diabetes mellitus, controlled only by diet, and six had hypertension. No other co-morbidities were reported. In addition, none of the patients was overweight. The contralateral fascia of the asymptomatic foot was used as a control.
After enrolment, symptomatic individuals were randomly assigned to receive LLLT (n = 15), three times weekly for 6 weeks (giving a total of 18 sessions), or identical placebo therapy (n = 15, and after the third week n = 10, since five individuals dropped out for economic reasons). Randomization was achieved with a computer-generated list, and each patient was given a badge number. Low-level laser therapy was administered with a gallium–arsenide (GaAs) laser with an infrared wavelength of 904 nm (Irradia medical laser, M/D Laser Professional, Stockholm, Sweden) with four infrared diodes by the same blinded experienced sports medicine physician (third author). On the laser probe, an A/B switch determined whether active (A) or sham (B) irradiation would be given. During operation the laser appeared to be identical for both active and sham irradiation. Standard treatment was given, which consisted of continuous irradiation over the origin of the plantar fascia on the medial calcaneal tubercle (first point, stationary mode) and then two continuous sweeps of the probe along the proximal medial border of the fascia (second point, scanning mode). The output of the laser averaged 4 × 60 mW = 240 mW (the Irradia medical laser has a built-in sensor for auto-calibration of the optical output before each application). The frequency of the pulse was 5,000 Hz. The spot area was almost 1.5 cm2 over the tendon insertion and 3 cm2 along the medial border of the fascia, giving a power density of 0.16 W/cm2 and 0.08 W/cm2, respectively. Each patient was treated for 157.5 s per session, and the dose of active treatment was 8.4 J over the tendon insertion site followed by 8.4 J along the medial border of the fascia, giving a total of 680.4 J. Both plantar fascias were treated. Which side should be treated with the laser probe in switch position A or B was decided for each patient by the opening of an opaque envelope, one for the right leg and one for the left, containing the patient’s badge number and a written character A or B. The A/B switch on the laser was then switched to the appropriate letter by a technician, and treatment was started for the right leg first. Thus, the allocation of patients to groups was concealed from the patients, the sports physician and the observer (fourth author). The code of the A/B switch positions on the laser probe was known only to the technician who was responsible for opening the envelopes.
A clinical investigation, including ultrasonography, was carried out initially and after completion of the therapy, either LLLT or placebo. Ultrasound was performed by the same radiologist (senior author), who was unaware of the subject’s clinical data and was unaware of the group allocation, using a 7.5 MHz linear array transducer. Both heels of the participants were scanned in two-dimensional (2D) real-time B mode. The subjects lay prone, with their knees fully extended, feet hanging freely over the end of the examination table, and ankles in a neutral position (0° of plantar and dorsal flexion). We took great care to obtain strictly comparable views of the contralateral sides. Acoustic coupling gel was applied to the plantar surface of the foot. The focus was adjusted to the depth of the fascia. Sagittal imaging of the plantar fascia was performed with the transducer aligned along the longitudinal axis of the aponeurosis. Quantitative assessment of plantar fasciitis was achieved by measurement of its thickness at a standard reference point, where the plantar fascia crossed the anterior aspect of the inferior border of the calcaneous [4, 14]. We took three measurements per heel in an attempt to avoid error due to transducer obliquity, and the average of the three readings was recorded. In addition, qualitative changes were evaluated. The plantar fascia was examined for its echogenic appearance (homogeneous/inhomogeneous, diffuse/localized, isoechoic with surrounding fat/hypoechoic, size of hypoechoic areas) and its fibrillary pattern (maintenance/loss of striated appearance). Ultrasonographic images were also assessed for changes in the underlying calcaneal bone surface (bony spurs), perifascial oedema/fluid collections (appearing as hypoechoic/anechoic areas in the perifascial fat) and calcifications. A beam perpendicular to the aponeurosis was always maintained to avoid artefactually reduced echogenicity due to anisotropy.
The subjective subcalcaneal heel pain of the 25 symptomatic individuals was recorded at baseline and after either therapy. Maximum pain at different situations (after night rest, daily activities) was assessed by a subjective, 100 mm, visual analogue scale (VAS), ranging from no pain (0) to maximum pain (100) [29].
Statistical analysis was performed with Statistical Package for the Social Sciences (SPSS) software, version 16.0 for Windows (SPSS, Chicago, IL, USA) (second author). A study statistician reviewed unblinded data for safety and efficacy. A paired-samples t-test was used to discover significant changes in both plantar fascia thickness and subjective subcalcaneal pain over the experimental period, and an independent t-test was used for detecting any significant differences in these two parameters between the two groups. The chi-squared test was used for statistical differences in the qualitative assessment of plantar fascia. P values less than 0.05 denoted the presence of a statistically significant difference. The Pearson product–moment correlation coefficient was employed to investigate the relationship between fascia thickness and visual analogue scale score. Preliminary analyses were performed to ensure no violation of the assumptions of normality, linearity and homoscedasticity.
Results
Thirty individuals were enrolled in the study, but 25 symptomatic subjects completed the 6 weeks’ therapeutic protocol (15 irradiated, ten placebo) and appeared for examination (ultrasound of the plantar fascia, pain estimation) at baseline and after treatment, either with laser or placebo.
The demographic characteristics of the groups (laser, placebo) are outlined in Table 1. None of the participants was overweight—a significant outcome, since obesity appears to be an independent risk factor for plantar fasciitis [10]. The two groups in the beginning did not differ significantly regarding the age and gender ratio (P = 0.000).
At baseline, the thickness of the plantar fascia was increased in 22 of 25 (92%) painful heels of the symptomatic subjects (mean 5.316 ± 1.2472 mm; range 3.5–7.9 mm) in comparison with that of their asymptomatic sides (mean 3.028 ± 0.4421 mm; range 2.4–3.8 mm) (Table 2). This difference was statistically significant (P = 0.000). In addition, there was no significant difference (P = 0.572) in mean plantar fascia thickness between those subjects irradiated with laser (mean 5.287 ± 1.3233 mm; range 3.5–7.9 mm) and those treated by placebo (mean 5.360 ± 1.1918 mm; range 3.8–7.8 mm). There was no significant difference in mean plantar fascia thickness between men and women, regardless of heel pain (P = 0.391).
Initially, in 2D real-time B mode, the proximal plantar fascia was focally hypoechoic in 18 of the 25 (78%) symptomatic heels. There was a normal striated appearance in 11 of the 25 (44%) painful heels. Perifascial oedema was seen in two of the 25 (8%) symptomatic feet, and a calcaneal spur was identified in two cases. Almost all the asymptomatic feet did not show such abnormalities. Only one asymptomatic heel was diffusely hypoechoic.
Six weeks after LLLT or identical placebo treatment, the plantar fascia thickness in both groups showed statistically significant changes over the experimental period (P = 0.000). Furthermore, when the difference in plantar fascia thickness was compared between the two groups, the change was statistically significant (P = 0.007). In addition, after LLLT or identical placebo treatment, the thickness of the plantar aponeurosis had normalized in seven out of 15, and only in one out of ten patients, respectively. However, the post-treatment thickness of the plantar fascia was insignificant in the laser-treated group (mean 3.627 ± 0.977 mm; range 2.5–5.7 mm) in comparison with that in the placebo-treated group (mean 4.380 ± 1.0042 mm; range 3.2–6.5 mm) (P = 0.121) (Table 3).
After therapy in 2D real-time B mode, there were no significant differences in dichotomized qualitative data of the plantar fascia [hypoechoic findings disappeared in 10/15, and 4/10 gave non-significant relative risk at 1.67 (0.72–2.62)]. Both groups had a tendency towards normalization of the fascia structure on qualitative ultrasound, with no significant differences between them (P = 0.088).
Pain estimation on the visual analogue scale had improved significantly in all test situations (after night rest, daily activities) after LLLT compared with that of the placebo-treated group. (P = 0.006 and P = 0.01, respectively). Additionally, when the difference in pain scores was compared between the two groups, the change was statistically significant (after night rest P = 0.000; daily activities, P = 0.001) (Table 3). Six weeks after LLLT the pain had decreased by 59% in the irradiated group and by 26% in the placebo-treated group. Finally, those subjects with little pain (a VAS score less than 25) during everyday life showed significantly (Pearson correlation = 0.901; P = 0.002) thinner fascias (3.044 ± 0.4304 mm) than did those with more persisting pain (4.500 ± 0.73212 mm).
The experiment was well tolerated by all the patients. None of the patients experienced any side effects or adverse reactions, such as pain increase or skin irritation.
Discussion
Real-time B mode ultrasonography is a non-invasive, inexpensive, easy to perform technique, which makes it an ideal imaging modality for the diagnosis of plantar fasciitis, with good sensitivity and specificity [12, 30]. Several studies have focused on the identification of the characteristic findings of plantar fasciitis [4, 5, 9, 11, 12, 30, 31]. Thickening of the plantar fascia (more than 4 mm) is a well-established sonographic criterion for the diagnosis of plantar fasciitis [4, 9, 32, 33]. In all studies a significant increase in the plantar aponeurosis thickness was observed in symptomatic feet compared with the contralateral asymptomatic side of the patients, or with the heels of individuals who had never experienced plantar heel pain (Fig. 1). Normally, the plantar fascia is hyperechoic and uniformly fibrillary [4, 9]. A hypoechoic fascia is a frequent finding in plantar fasciitis in several studies [4, 5, 9, 11, 12, 30, 31]. It is related to reparative processes after microtears, fibre degeneration and oedema [26]. Our results regarding the qualitative assessment of plantar fasciitis (echogenicity, fibrillary pattern, perifascial oedema, calcaneal spur) are similar to the outcomes of previous studies. The proximal plantar fascia was focally hypoechoic in 18 of the 25 (78%) symptomatic heels (Fig. 1).
Huerta and Alarcón García showed that gender is an independent predictor of plantar fascia thickness 1 cm proximal to the insertion only in univariate regression analysis [10]. According to our results, there was no significant difference in mean plantar fascia thickness, between men and women, where the plantar fascia crossed the anterior aspect of the inferior border of the calcaneous.
To our knowledge the ultrasonographic appearance of plantar fasciitis after LLLT has not yet been described.
There is only one study, published almost a decade ago, where Basford et al., using subjective pain rating, had assessed the effectiveness of low-intensity laser therapy in the treatment of plantar fasciitis [22]. The authors had concluded that laser therapy is ineffective in the treatment of plantar fasciitis. In their study, 28 subjects were irradiated with 0.83 μm gallium–aluminium–arsenide (GaAlAs) laser three times per week, for 1 month. Subjective pain was assessed at baseline and at follow-up. A placebo-irradiated group was used as control. Basford et al. concluded that no significant differences were observed between groups during treatment or follow up [22].
The purpose of our study was twofold: first, to investigate the efficacy of LLLT in plantar fasciitis by ultrasonography and second to measure its effectiveness with a 100 mm visual analogue scale. According to our results, after either LLLT or placebo therapy, plantar aponeurosis thickness showed a statistically significant change over the experiment period in each group. In addition, the difference in plantar fascia thickness (thickness before treatment minus that after treatment) between the two cohorts was found to be significant. Furthermore, the number of patients with normalized fascia thickness was greater in the LLLT group (seven out of 15 patients). We should state that we used the patient’s contralateral asymptomatic side as a measure of normalization of the aponeurosis thickness. According to Bjordal et al., intersubject tendon variations were far larger and less reliable than intrasubject side difference. For this reason, they suggested that using a fixed tendon thickness value as a diagnostic criterion seemed inadequate [34]. Individual side differences in tendon thickness are likely to be considerably more precise for the diagnosis of pathological thickening of plantar aponeurosis. However, after LLLT, the thickness values and the qualitative assessment of plantar fasciitis in the 15 irradiated subjects were found to be insignificant in comparison with those in the placebo-treated individuals (Fig. 2). It should be stated that the qualitative sonographic data are not very important, as none was statistically significant in terms of relative risks for improvement.
Pain estimation on the 100 mm visual analogue scale had improved significantly in all test situations (after rest, daily activities) after LLLT when compared with that of the placebo-treated group. Additionally, when the difference in pain scores was compared between the two groups, the change was statistically significant (Table 3). Six weeks after LLLT the pain had decreased by 59% in the irradiated group and by 26% in the placebo group. Finally, those subjects with little pain (VAS score less than 25) during everyday life showed significantly thinner fascias than those with more persisting pain.
Our outcomes are almost completely opposite to those in the report by Basford and colleagues (except data from qualitative assessment of plantar fasciitis and absolute post-treatment values of fascia thickness). It is possible that the negative outcome reported by Basford et al. was due to the very low dosage. One less possible explanation for the slight discrepancy in our results is that pain estimation on the visual analogue scale is very subjective and, thus, variable, and its quantification by inexperienced patients is extremely difficult. On the other hand, it is possible that LLLT may advocate pain reduction by modulating pain-regulating mechanisms. Laser therapy has been thought to play a role in the treatment of musculoskeletal disorders through its analgesic effects [23–26]. In a recent study Bjordal et al. concluded that low-level laser therapy could reduce inflammation and pain in Achilles tendinitis [35]. In another study Walker reported relief of chronic pain with laser irradiation [28]. The pain reduction was accompanied by an increase in the urinary excretion of 5-hydroxyindolacetic acid, a by-product of serotonin metabolism. The researcher concluded that laser irradiation might have a potential effect on serotonin metabolism, thereby acting as a pain suppressor. However, there is a possibility that LLLT might not affect the ultrastructure of the plantar fascia but only cell metabolism, thereby producing early changes not detectable by ultrasound. It is possible that LLLT triggers the regeneration of the fibrous tissue and accelerates the reparation process. After 6 weeks of laser or identical placebo therapy, the difference over time in the plantar fascia thickness was more profound in the laser-treated group. This outcome, which was in agreement with our previous results regarding significant differences in plantar fascia thickness between the experimental groups before and after treatment, leads to the hypothesis that LLLT may contribute to plantar fasciitis healing, serving as a cell stimulator and possibly triggering and accelerating the normalizing process. During the past decade, laser therapy has been thought to be useful in the treatment of musculoskeletal disorders through its tissue healing and biostimulation effects [23–26] or by stimulating tissue repair [27].
Our study had several limitations. First, due to the preliminary nature of the study, the sample size was relatively small. Second, both the number of sessions per week and the duration of the follow-up could have been longer. However, it would have been either expensive or impossible to recruit the participants for so long, without multiple drop-outs. In addition, our scanning mode was both stationary and sweeping. It is possible that this combined procedure yielded a less positive result concerning the effectiveness of LLLT in the rehabilitation of plantar fasciitis. (For the record, the scanning procedure was stationary in almost all the experimental designs of the effect of LLLT in tendinopathies [36]) Finally, one parameter for the evaluation of laser therapy (pain estimation on the VAS) was not validated in our population. The findings of our study should be interpreted in the context of this particular protocol, and further clinical and imaging studies are required to provide the data that could be used to optimize treatment protocols.
In summary, we believe that 904 nm GaAs infrared (IR) laser therapy may contribute to plantar fasciitis healing and pain reduction. At this point, we should state that LLLT warrants further study as a treatment for plantar fasciitis.
References
Wearing SC, Smeathers JE, Urry SR, Hennig EM, Hills AP (2006) The pathomechanics of plantar fasciitis. Sports Med 36:585–611
Mitchell IR, Meyer C, Krueger WA (1991) Deep fascia of the foot: anatomical and clinical considerations. J Am Podiatr Med Assoc 81:373–378
Sarratia SK (1983) Anatomy of the foot and ankle: descriptive, tomographic, functional. Lippincott, New York
Gibbon WW, Long G (1999) Ultrasound of the plantar aponeurosis (fascia). Skeletal Radiol 28:21–26
Akfirat M, Sen C, Günes T (2003) Ultrasonographic appearance of the plantar fasciitis. Clin Imaging 27:353–357
Baxter DE (1994) The heel in sports. Clin Sports Med 13:683–693
Hill JJ, Cutting PJ (1989) Heel pain and body weight. Foot Ankle 9:254–256
Berkowitz JF, Kier R, Rudicel S (1991) Plantar fasciitis: MR imaging. Radiology 179:665–667
Cardinal E, Chhem RK, Beauregard CG et al (1996) Plantar fasciitis: sonographic evaluation. Radiology 201:257–259
Huerta PJ, Alarcón García JM (2007) Effect of gender, age and anthropometric variables on plantar fascia thickness at different locations in asymptomatic subjects. Eur J Radiol 62:449–453
Kamel M, Kotob H (2000) The role of ultrasound in the diagnosis and management of idiopathic plantar fasciitis. Rheumatology 27:2139–2141
Kane D, Greaney T, Shanahan M et al (2001) The role of ultrasonography in the diagnosis and management of idiopathic plantar fasciitis. Rheumatology (Oxford) 40:1002–1008
Uzel M, Cetinus E, Ekerbicer HC, Karaoguz A (2006) The influence of athletic activity on the plantar fascia in healthy young adults. J Clin Ultrasound. 34:17–21
Gill LH (1997) Plantar fasciitis: diagnosis and conservative management. J Am Acad Orthop Surg 5:190–197
Babcock MS, Foster L, Pasquina P, Jabbari B (2005) Treatment of pain attributed to plantar fasciitis with botulinum toxin a: a short-term, randomized, placebo-controlled, double-blind study. Am J Phys Med Rehabil 84:649–654
Digiovanni BF, Nawoczenski DA, Malay DP et al (2006) Plantar fascia-specific stretching exercise improves outcomes in patients with chronic plantar fasciitis. A prospective clinical trial with two-year follow-up. J Bone Joint Surg Am 88:1775–1781
Malay DS, Pressman MM, Assili A et al (2006) Extracorporeal shockwave therapy versus placebo for the treatment of chronic proximal plantar fasciitis: results of a randomized, placebo-controlled, double-blinded, multicenter intervention trial. J Foot Ankle Surg 45:196–210
May TJ, Judy TA, Conti M, Cowan JE (2002) Current treatment of plantar fasciitis. Curr Sports Med Rep 1:278–284
Radford JA, Landorf KB, Buchbinder R, Cook C (2007) Effectiveness of calf muscle stretching for the short-term treatment of plantar heel pain: a randomised trial. BMC Musculoskelet Disord 8:36–42
Tsai WC, Wang CL, Tang FT et al (2000) Treatment of proximal plantar fasciitis with ultrasound-guided steroid injection. Arch Phys Med Rehabil 81:1416–1421
Hammer DS, Adam F, Kreutz A et al (2005) Ultrasonographic evaluation at 6-month follow-up of plantar fasciitis after extracorporeal shock wave therapy. Arch Orthop Trauma Surg 125:6–9
Basford RJ, Malanga AG, Krause AD, Harmsen SW (1998) A randomized controlled evaluation of low intensity laser therapy: Plantar fasciitis. Arch Phys Med Rehab 79:249–254
Chow RT, Barnsley L (2005) Systematic review of the literature of low-level laser therapy (LLLT) in the management of neck pain. Lasers Surg Med 37:46–52
Djavid GE, Mortazavi SMJ, Basirnia A et al (2003) Low level laser therapy in musculoskeletal pain syndromes: pain relief and disability reduction. Lasers Surg Med Suppl 15:43–43
Gam AN, Thorsen H, Lonnberg F (1993) The effect of low-level laser therapy on musculoskeletal pain: a meta-analysis. Pain 52:63–66
Jacobsen FM, Couppe C, Hilden J (1997) Comments on the use of low-level laser therapy (LLLT) in painful musculo-skeletal disorders. Pain 73:110–111
Reddy GK, Stehno-Bittel L, Enwemeka CS (1998) Laser photostimulation of collagen production in healing rabbit Achilles tendons. Lasers Surg Med 22:281–284
Walker J (1983) Relief from chronic pain by low power laser irradiation. Neurosci Lett 43:339–344
Downie WW, Leatham PA, Rhind VM et al (1978) Studies with pain rating scale. Am Rheum Dis 37:378–381
Sabir N, Demirlenk S, Yogei B et al (2005) Clinical utility of sonography diagnosing plantar fasciitis. J ultrasound Med 24:1041–1048
Ozdemir H, Yilmaz E, Murat A (2005) Sonographic evaluation of plantar fasciitis and relation to body mass index. Eur J Radiol 54:443–447
Wall JR, Harkness MA, Crawford A (1993) Ultrasound diagnosis of plantar fasciitis. Foot Ankle 14:465–470
Yu JS (2000) Pathologic and post-operative conditions of the plantar fascia: review of MR imaging appearances. Skeletal Radiol 29:491–501
Bjordal JM, Demmink J, Ljunggren A (2003) Tendon thickness and depth. An ultrasonography study on healthy subjects. Physiotherapy 89:375–383
Bjordal JM, Lopes-Martins RAB, Iversen VV (2006) A randomised, placebo controlled trial of low level laser therapy for activated Achilles tendinitis with microdialysis measurement of peritendinous prostaglandin E2 concentrations. Br J Sports Med 40:76–80
Tumilty S, Munn J, McDonough S, Hurley DA, Basford JR, Baxter GD (2009) Low level laser treatment of tendinopathy: a systematic review with meta-analysis. Photomed Laser Surg Aug 26. [Epub ahead of print]
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kiritsi, O., Tsitas, K., Malliaropoulos, N. et al. Ultrasonographic evaluation of plantar fasciitis after low-level laser therapy: results of a double-blind, randomized, placebo-controlled trial. Lasers Med Sci 25, 275–281 (2010). https://doi.org/10.1007/s10103-009-0737-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-009-0737-5