Abstract
This study aimed at estimating the extent to which a combination therapy of low-level laser therapy (LLLT) with exercise and orthotic support (usual care) affects functional ability in the patient with plantar fasciitis (PF) when compared to usual care alone. Participants with PF were randomly allocated into two groups: LLLT (n = 27) and control (n = 22). All the participants received home exercise program with orthotic support. In addition, the LLLT group received a gallium-aluminum-arsenide laser with a 850-nm wavelength for ten sessions, three times a week. Functional outcomes were measured by function subscale of American Orthopedic Foot and Ankle Society Score (AOFAS-F) and 12-min walking test including walking speed, cadence, and activity-related pain using visual analog scale (VAS).The scores were recorded at baseline, third week, and third month after the treatment. Analysis was performed using repeated measures ANOVA and an intention to treat approach using multiple imputations. There was a significant improvement in AOFAS-F total score at 3 weeks in both groups (LLLT, p < 0.001; control, p = 0.002), but the improvements were seen only for the LLLT group for AOFAS-F total score (p = 0.04) and two individual items of AOFAS-F (walking distance (p < 0.001) and walking surface (p = 0.01)) at 3 months. The groups were comparable with each other for both walking speed and cadence at all assessment times (p > 0.05). Both groups showed significant reduction in pain over 3 months (LLLT, p < 0.001; control, p = 0.01); however, the LLLT group had lower pain than the control group at 3 months (p = 0.03). The combination therapy of LLLT with usual care is more effective to improve functional outcomes and activity-related pain when compared to usual care alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plantar fasciitis (PF) is one of the most common foot disorders in adult population [1]. It results from degenerative changes in plantar fascia, particularly observed near its attachment to calcaneal tuberosity [2]. Although the exact pathology of PF is not known, it is often sought as a result of pulling force to plantar fascia due to excessive weight bearing (being obese or overuse of the foot in weight-bearing condition), biomechanical abnormalities of the foot, presence of calcaneal spur, and inconvenient shoe usage [3]. The typical symptom of PF is sharp or stabbing pain, felt at the first step in morning or after a period of prolong sitting, limiting daily activities and impairing the walking performance [3]. Thus, treatment for PF potentially targets both reduction in pain occurring in weight-bearing conditions such as standing or walking, and improvement in walking ability [4]. Conservative approaches form the mainstay of the PF management and only around 10% of the patients who report an unsatisfactory response to conservative therapy are referred for surgical release for plantar fascia [5]. For a better management of PF, a combination therapy is suggested including exercise and orthotic support with a conservative approach such as extracorporeal shock wave therapy, laser therapy, and ultrasound [6].
Management of PF mostly focuses on pain management [7, 8]. Although impairment in functional ability is one of the main concerns of patients with PF, therapeutic effect of different conservative approaches on functional abilities including walking performance is not well investigated. Existing studies have investigated physical functioning in PF using only patient-reported outcome measures to examine the effect for few conservative approaches such as extracorporeal shockwave therapy [9]. However, low-level laser therapy (LLLT) is one of the newest approaches used for PF, with higher effectiveness for pain management than ultrasound and extracorporeal shock wave therapy (ESWT) [10], but the evidence supporting the effectiveness of LLLT on functional abilities in PF remains scarce.
In LLLT, visible or invisible laser lights are applied to the surface of the body in order to stimulate or enhance the mitochondrial activity in cell tissues. Several clinical trials done with animals in vivo and vitro demonstrated that photobiostimulation through LLLT leads to an increase in cell proliferation, microcirculation, vascular neoformation, and collagen production [11]; therefore, it is considered to alleviate degeneration in soft tissues such as muscle, fascia, ligament, or cartilage [11, 12]. Recently, several human and animal trials have illustrated positive effect of LLLT on inflammatory markers such as prostaglandin E2 (PGE2), TNF-a, IL-1b, plasminogen activator, cyclooxygenase-1, and cyclooxygenase-2 [13,14,15]. These studies also reported a reduction in edema, hemorrhagic formation, necrosis, and neutrophil cell influx [13].
PF is considered to be a sequel of degenerative or inflammatory changes in plantar fascia; therefore, LLLT has been included in the treatment of PF to decrease the degeneration or inflammation of plantar fascia [10, 16, 17]. LLLT not only modulates the inflammatory processes but also impacts muscle fatigue, muscle contraction, and energy consumption [18]. In this respect, the randomized control trials investigating the effect of photobiomodulation therapy on physical activity demonstrated that athletes receiving laser therapy before or after a physical activity have lower blood lactate levels and muscle fatigue in comparison to other athletes who do not [18,19,20]. But patients with PF are generally prescribed only exercises and orthotic supports to enhance their activity and function levels, and the studies evaluating the effect of LLLT on PF usually focus on the pain-related symptoms rather than physical function levels.
In order to fully ascertain the treatment effects of LLLT in patients with PF, the contribution of LLLT on physical activity should also be investigated. Therefore, this study aimed to estimate the extent to which LLLT contributes to usual care to improve foot function and walking performance in patients with PF when followed up for 3 months. We hypothesized that LLLT in combination with exercises and orthotic support (usual care) improves foot function and walking performance more than usual care alone in the patients with PF.
Methods
Study design
A randomized, prospective, single-blinded controlled trial was conducted in out-patient physiotherapy department at the multicenter public hospital in Konya, Turkey. Participants were consecutively screened, enrolled, and followed up between September 2012 and April 2013 after obtaining ethics approval from Hacettepe University Clinical Research Ethics Boards (file number: 8.302.HAC.0.05.07.00/699). The participants were informed about the test procedures and signed informed consent forms were obtained. This study was carried out in accordance with the World Medical Association Declaration of Helsinki [21]. Assessments were performed at baseline, after completion of the 3-week courses of treatment, and at 12-week follow-up assessment.
Participants
Selective purposive sampling was used to recruit 49 individuals with a diagnosis of PF presenting to the clinics. The participants were included per the following criteria: pain located on medial tubercle or along the medial process of the plantar fascia persisting at least 1 month with a minimum score of 5 on 10-point visual analog scale (VAS), pain felt in the morning at first step over the plantar fascia in the last week before enrolling the study, tenderness to palpation over medial calcaneal tuberosity or along plantar fascia, ≥ 18 years, and agreement to participate and complete treatment and follow-up assessments (without participating in any other therapies including anti-inflammatory drugs and corticosteroid medication). The participants were excluded if they had a history of calcaneal stress fracture, nerve entrapment syndrome, plantar fascia rupture, prior foot surgery, any neurological or systemic diseases including rheumatoid arthritis, tumor, and cancer and are taking local corticosteroid injections in the last 6 months. The sample size was calculated based on a previous study using American Orthopedic Foot and Ankle Society Score (AOFAS) score in the treatment of PF. A sample of 22 participants for each group was calculated for detecting a mean difference of 7.7 points on AOFAS total score with a standard deviation with 80% power and a two-sided 5% significance [22]. After adjusting for a 10% dropout rate, a total sample size of 49 was calculated.
Interventions
Insole and exercise program
All the participants were instructed to wear a full-length silicone insole for 3 months [23] and to practice home exercise for 3 weeks [24, 25]. All the patients were asked to wear a prefabricated full-length silicone insole (Santemol Group Medical) which is a non-custom shock-absorbing insole designed to provide gentle support to the arch of the foot. The patients were guided to wear for 3 months both indoors and outdoors as much as possible [23]. The exercise program included gastrocnemius stretching and plantar fascia stretching exercises [24, 25]. In the gastrocnemius stretching protocol, each patient was instructed to sit on a firm surface and to hold one leg straight in front of him/her. A towel looped around the ball of subject’s foot was used to pull foot toward the shin. Each stretching was sustained for 30 s [24]. Plantar fascia stretching was performed while sitting on a chair or seat. The subjects crossed the affected leg over the contralateral leg and grabbed the base of the toes to pull them toward the shin until feeling a stretch in the arch or plantar fascia. This position was held for 30 s [26]. The participants were asked to do stretching exercises three times in a day with ten repetitions for 3 weeks [27]. Along with the demonstration of each exercise, each subject was given a paper sheet showing the instructed exercises and a template as a record for their home exercise.
Laser therapy
The participants in the LLLT intervention (LLLT group) were treated with a gallium-aluminum-arsenide (GaAlAs) low-level diode laser emitting a divergent 830-nm laser light generating a 100-mW continuous wave output. It consisted of a control console connected to a hand-held delivery probe. The relevant LLLT parameters are presented in Table 1. The patients in this group (27) received LLLT three times in a week with a total ten sessions. It was applied over plantar fascia at tender points (five points). The points where LLLT was applied are presented in Fig. 1. As there was no study addressing the most appropriate dose and duration for GaAlAs low-level laser, we did a pilot study to decide dose, duration, and frequency [28]. In the pilot study, low-level diode laser emitting a divergent 850-nm laser light generating a 100-mW continuous wave output was used for nine sessions three times in a week. In each session, a total 16.8 joule (j) (5.6 j × three points) in a total 240 s (80 s × three points) was applied to plantar fascia (origin of plantar fascia). In the pilot study, LLLT was only applied to origin of plantar fascia at three points, yet in the current study, along with three points in the origin of plantar fascia, we also applied LLLT at two additional points located toward the medium part of plantar fascia using same dose and duration (Fig. 1).
Outcome measures
The main outcome of this study was functional ability and pain during activity. Functional ability was measured using two methods—(a) function subscale of American Orthopedic Foot and Ankle Society Score (AOFAS) [29]. The validity and reliability of AOFAS has been well documented for Turkish-speaking individuals who have foot and ankle injuries [29]. It has three components: pain, function, and alignment. This study used the function subscale that has a total of 50 points distributed to seven questions (activity limitations (0–10 points), walking distance (0–5 points), walking surfaces (0–5 points), gait abnormalities (0–8 points), range of motion (flexion/extension (0–8 points), eversion/inversion (0–6 points), and ankle-hindfoot stability (0–8 points)) [30], where higher score represents better function; and (b) 12-min walking test. The participants were instructed to walk on a 10-m walkway back and forth for 12 min. Walking speed was measured by dividing the distance (meters) walked in the last 2 min by time (2 min); cadence was measured by the number of steps in the last minute of the 12-min walking test. Activity-related pain was evaluated after the 12-min walking test using visual analog scale (VAS) [31]. VAS has a horizontal line 10 cm in length representing severity of the pain where 0 cm indicates no pain and 10 cm indicates worst imaginable pain [31]. The participants were asked to mark experienced intensity of pain on the line. Clinically meaningful change for pain levels on VAS is 3 cm [32] and that for gait speed is between 0.1 and 0.2 m/s among adults with pathologies [33].
Baseline assessment
Each participant was evaluated for medial longitudinal arch (MLA) function and body mass index (BMI) as they are considered risk factors for PF [34]. MLA function was evaluated by Feiss’ line and navicular drop test which is a valid and reliable method to assess the structure and function of MLA [35]. Feiss’ line was evaluated in standing position. The apex of the medial malleolus, navicular tubercle, and first MTP joint was marked, and a line was made from the malleolus to the MTP. The distance from ground to line was measured and divided by three and marked. If navicular tubercle was lower than the line even at one third of distance, it was recorded as pes planus [36]. Navicular drop test was evaluated in both sitting and standing positions. In the sitting position, the navicular tubercle was marked with a pen while the patient was sitting and the distance of the navicular tubercle from the ground was measured and recorded. Then, the patient stood fully weight bearing and relaxed and the distance of the extent to which the navicular dropped was recorded [35]. A navicular drop greater than 10 mm is considered an indicator of excessive pronation [37]. Body mass index (BMI) was calculated as the body mass divided by the square of the body height, and was expressed in units of kilograms per square meter (kg/m2). BMI of the patients then categorized based on the WHO definition under the following categories: normal (18.5–24.99 kg/m2), overweight (25–29.99 kg/m2), obese class I—moderate obese (30–34.99 kg/m2), obese class II—severe obese (35–39.99 kg/m2), and obese class III—very severely obese (≥ 40 kg/m2) [38].
Randomization
Following screening, enrolment, and baseline assessment, the participants were randomized to either the intervention (LLLT) or control group. To conceal randomization, sequentially numbered sealed opaque envelopes were prepared in advance and opened in sequence by an independent advisor blinded to intervention. In forty-nine participants, 27 were assigned to the LLLT group, and 22 were assigned to the control group. The participants were informed about the test procedures and informed consent forms were signed before the data collection. All evaluations were done by an investigator who determined the eligibility of the participants and performed pre-post assessments as well. After the participants were qualified, the subjects were directed to one of the five physiotherapists who were responsible for administering the interventions. The participants and physiotherapists who applied the treatment were blinded to study outcome.
Statistical methods
Descriptive statistics were used to characterize the participants and verify the distribution of variables. The outcome measures were functional ability and pain during walking. The predictor variable was the type of group (LLLT and control groups). Baseline differences were analyzed using chi-square test for the categorical variables, one-way analysis of variance (ANOVA) for the continuous variables that were normally distributed, and Kruskal-Wallis for the continuous variables which were not normally distributed (Table 1). Further, a univariate analysis was done to explore the relationships between baseline variables and main outcome scores (AOFAS-function subscale, walking speed, cadence, and activity-related pain). Repeated measures ANOVAs were used to analyze the effect of three factors (time, group, time × group) on the outcomes. ANOVA diagnostics, including the Shapiro-Wilk statistic and residual by predicted plots, were generated to verify the assumptions of normality and homoscedasticity. When applicable, post hoc pairwise comparisons were used to determine group and time differences. An intention to treat analysis was conducted by including all the participants who completed baseline assessments. Multiple imputation was used to adjust the loss to follow-up. The effect size for each outcome was measured by partial eta square (ɳ 2 p). The interpretations was done as per the classification suggested by Cohen which are small significant effect (ɳ 2 p = 0.01), medium significant effect (ɳ 2 p = 0.06), and large significant effect (ɳ 2 p = 0.14) [39]. The level of significance was set at p < 0.05 for all the analyses. All analyses were done using IBM SPSS Statistics version 23.
Results
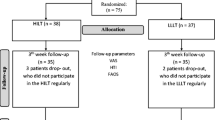
A total of 49 participants were included in the study. One subject from the LLLT group and two subjects from the control group could not participate in the follow-up assessment at 3 months due to scheduling issues (Fig. 2). Table 2 summarizes the demographic information and baseline characteristics of the participants. Mean age of the participants was 45.51 ± 9.90 years. The participants were mostly women (82%). Most participants were either obese (46%) or overweight (40%) and had injuries in both sides (53%). Univariate analysis showed that there were no associations between AOFAS-function subtest total score and age (p = 0.12), side of injury (p = 0.98), gender (p = 0.50), foot pronation (p = 0.29), pes planus (p = 0.67), and BMI (p = 0.09). However, significant associations were found between BMI and AOFAS-function subtest total score (p < 0.05), and BMI was then included as a covariate. Data related to AOFAS-function scale (AOFAS-F), walking speed, cadence, and activity-related pain were presented in Table 3 as mean and standard error (SE) of pooled data obtained after five imputations.
AOFAS-function subscale
Repeated measures ANOVA for AOFAS-F revealed significant main effect for time (p = 0.03) and a significant interaction effect for time × group (p = 0.02). The effect of group (p = 0.76) or BMI (p = 0.12) was not significant (p > 0.05) (Table 4). Post hoc analysis on total score revealed significant improvement in overall foot function for both groups at the third week (LLLT group, p < 0.001; control group, p = 0.002), but only for the LLLT group at 3 months (p = 0.04) (Fig. 3). Further analysis on individual items of AOFAS-F showed that time × group interaction effect was only significant for walking distance (p = 0.03) and walking surface (p = 0.04), yet no main effect of time and group was detected in any item score (p > 0.05) (Table 4). Pairwise analysis on walking distance and walking surface showed that the LLLT group showed significant improvement at 3 weeks for walking distance(p < 0.001) and in both walking distance(p < 0.001) and walking surface (p = 0.01) at 3 months. There were no significant changes in both items in the control (p > 0.05).
Gait performance
For walking speed, only the main of effect of time was significant (p = 0.03), with no significant effects of group or BMI or time × group (p > 0.05) (Table 4). There were no significant main effects of time, group, and BMI or interaction effect for cadence (p > 0.05) (Table 4). For activity-related pain, there was a significant effect of time (p = 0.004) and time × group interaction (p = 0.008), but the main effect of group (p = 0.059) and BMI (p = 0.11) was not significant. Both groups showed significant reduction in pain at all time points (LLLT group, p < 0.001; control group, p = 0.01), but the LLLT group showed significantly greater reduction in pain at the third month when compared to the control (p = 0.03) (Fig. 4).
Discussion
This study evaluated the additive effect of LLLT to usual care on functional ability and activity-related pain in patients with PF when followed over a period of 3 months. Our findings demonstrated that LLLT significantly contributed to usual care to increase functional ability such as walking greater distance or different surfaces as well as to relief pain while walking after the treatment (Figs. 3 and 4).
The current study findings are in line with the previous evidence in showing the positive effect of LLLT on muscle fatigue and walking performance [20, 40]. Previous animals and human studies have suggested that LLLT is beneficial with respect to increasing physical activity and oxygen consumption and decreasing muscle fatigue or injury [6, 14]. An experimental study done in rats with acute skeletal muscle injury showed that rats having LLLT had better walking performance and less inflammatory markers when compared to control [40]. In another study, LLLT significantly delayed the progression of pathological changes in the skeletal muscle tissue and protected the size and number of muscle fibers in the rats with Duchenne muscular dystrophy [41]. Parallel findings were also reported in human experiments. LLLT with exercise significantly reduced oxidative and muscle damage after a high-intensity exercise in 40 healthy individuals [20]. Similarly, in rugby players, laser application during pre-exercise session enhanced the performance and accelerated the recovery of muscle fatigue [18]. However, most of the human experiments included only healthy participants; thus, the effect of LLLT on physical function levels in patients with musculoskeletal conditions still needs to be ascertained.
Few studies used LLLT in the treatment of PF, yet they reported conflicting results and none of which evaluated muscle fatigue or damage as outcome measures. The inconsistencies in results might be due to the variation in the dose and type of LLLT. There are various types of laser such as solid state, gas, and semiconductor having different wavelengths such as ultraviolet light (100–400 nm), visible light (400–750 nm), and infrared (750 nm–1 mm). Generally, two types of laser are used for musculoskeletal disorders: gas laser (visible red light of He-Ne laser having wavelength between 594 and 632 nm) and semiconductor lasers (GaAs or GaAlAs having wavelength between 780 and 905 nm). He-Ne laser for six sessions with a total dose of 1.476 J/cm2 showed significant improvement in pain (90% decline on visual analog scale) and function (70% increase in functional ability) 1 year after the treatment in the patient with PF [17]. However, this study does not provide a strong evidence for the treatment effect of laser therapy since there was no comparison group. A second study that investigated the effectiveness of He-Ne laser therapy included a placebo control group for comparison and used the same dose and same frequency as the previous study [16]. However, the improvements in outcomes were not as high as those found in the previous study that had no control group. The experimental group had only 15% more reduction in pain than placebo group, and the improvement in functional ability was comparable between the groups [16].
He-Ne laser have lower wavelength than semiconductor lasers and have poor tissue penetration [8]. GaAs and GaAlAs lasers are two different types of semiconductor lasers that have deeper penetration causing acceleration in wound healing, alleviation in pain, and reduction in inflammation [42]. GaAs laser has been used only by one study evaluating the changes in plantar fascia thickness after treatment, which reported significant decrements in plantar fascia thickness (1.6 mm) in active treatment group than in placebo control group (0.9 mm) 6 months post treatment [43]. Plantar fascia thickness is one of the indicators of PF, and more than 4-mm thickness is considered as a sign of PF. Thus, the higher reduction in plantar thickness refers to better recovery. The same study also reported a 20% higher reduction in pain in laser group than in placebo group at the sixth week; however, the effect of treatment on functional ability was not measured [43]. In the current study, other type of semiconductor laser, GaAlAs, therapy was used. It was also used by two other studies in the treatment of PF that have conflicting results. Basford et al. (1998) conducted a first randomized trial to investigate the effectiveness of laser therapy on PF using GaAlAs laser and reported no significant treatment effect of laser on PF when compared to placebo [8]. In contrast, a recent study by Ulusoy et al. (2017) showed positive effect of GaAlAs laser on pain, function, and plantar fascia thickness in patients with PF where they compared the GaAlAs laser therapy with ultrasound and extracorporeal shock wave therapy [10]. They reported that success rate of the treatment was higher for the laser group than the shock wave or ultrasound group [10]. The incompatible results between the studies conducted by Ulusoy and Basford may be attributed to the differences in study design, frequency, and doses of therapy [8, 10]. Although the type of laser was the same for both studies, Basford et al. used GaAlAs laser generating a 30-mW continuous wave output for 12 sessions with a total dose of 3 J/cm2 for 33 s per session [8]. On the other hand, Ulusoy et al. used GaAlAs laser generating a 50-mW continuous wave output for 15 sessions, with a total dose of 8 J/cm2 for 200 s per session [10]. As there is no designated treatment protocol for LLLT, application of laser may vary as per the choice of wave output, total dose, duration, and total sessions. In this study, our treatment protocol for LLLT was different than the other protocols used in the literature. We based our treatment protocol on our pilot study (Table 1), yet literature suggested to apply LLLT with higher energy dose (50 j per site) through multiple diodes for muscle performance and post exercise recovery [19]. Our primary target while implementing LLLT was plantar fascia rather than muscle tissue, so the optimal dose for PF may be different from that is used for muscle recovery. As no study provides the best method to apply LLLT for PF, the parameters remain uncertain.
In our study, we only evaluated functional abilities and gait function through a scale and walking test. The functional outcomes were measured by AOFAS-function subscale [10, 25, 44]. Previous studies using AOFAS for different treatment approaches generally reported positive results [25, 44]. One such study illustrated higher improvement in functional ability in LLLT (41%) than ultrasound (38%) and ESWT (32%) [10]. In this study, the improvement in function was 13% in the LLLT group and 4% in the control group, and the improvement continued to be present up to 3 months only in the LLLT group. The difference in percentages between our study and the previous study might be because we only used function subtest of AOFAS without including pain and alignment subscales [10]. We found that both treatments improved walking speed, and adding LLLT to usual care did not result in any further increase in walking speed. On the other hand, LLLT significantly reduced the pain levels after 12-min walking when used with usual care in comparison with usual care alone. The activity-related pain measured in this study was a performance-based measure, where the pain levels were measured just after the 12-min walking test. This method may provide more precise data about activity-related when compared to data from patient-reported pain levels.
The contribution of this study to the existing evidence on effectiveness of laser therapy should be interpreted in the light of some limitations. First, the optimal dose and frequency of laser treatment are not known. Even though we conducted a pilot study to decide dose, duration, and frequency of laser application, it may still not be optimal and needs further validation. Other points about delivery of laser are placing foot in a proper position and exact locations of application. Although there are some recommendations in literature, it still needs to be clarified. Other limitations of this study were the lack of a non-treatment group to account for the natural recovery in patients with PF. To better understand the effect of treatments on walking performance, more sophisticated measures such as motion analysis system are needed. Moreover, measuring the changes in biochemical markers related to recovery of PF after LLLT could give a better understanding of the relationship between the effect of LLLT on cellular tissues and functional ability.
In conclusion, the study findings reveal that LLLT might contribute to better clinical outcomes for activity-related pain and functional ability in patients with PF, when provided in addition to exercises and orthotic support.
References
Kudo P et al (2006) Randomized, placebo-controlled, double-blind clinical trial evaluating the treatment of plantar fasciitis with an extracoporeal shockwave therapy (ESWT) device: a North American confirmatory study. J Orthop Res 24(2):115–123
Buchanan, B. and D. Kushner, Plantar fasciitis, in StatPearls. 2017, StatPearls Publishing LLC: Treasure Island
Muth CC (2017) Plantar fasciitis. JAMA 318(4):400
(2017) Plantar fasciitis: will physical therapy help my foot pain? J Orthop Sports Phys Ther 47(2):56
Rompe JD et al (2007) Shock wave therapy for chronic plantar fasciopathy. Br Med Bull 81(1):183–208
Tomazoni SS et al (2016) Isolated and combined effects of photobiomodulation therapy, topical nonsteroidal anti-inflammatory drugs, and physical activity in the treatment of osteoarthritis induced by papain. J Biomed Opt 21(10):108001
Sun J et al (2017) Extracorporeal shock wave therapy is effective in treating chronic plantar fasciitis: a meta-analysis of RCTs. Medicine (Baltimore) 96(15):e6621
Basford JR et al (1998) A randomized controlled evaluation of low-intensity laser therapy: plantar fasciitis. Arch Phys Med Rehabil 79(3):249–254
Yin MC et al (2014) Is extracorporeal shock wave therapy clinical efficacy for relief of chronic, recalcitrant plantar fasciitis? A systematic review and meta-analysis of randomized placebo or active-treatment controlled trials. Arch Phys Med Rehabil 95(8):1585–1593
Ulusoy A, Cerrahoglu L, Orguc S (2017) Magnetic resonance imaging and clinical outcomes of laser therapy, ultrasound therapy, and extracorporeal shock wave therapy for treatment of plantar fasciitis: a randomized controlled trial. J Foot Ankle Surg 56(4):762–767
da Rosa AS et al (2012) Effects of low-level laser therapy at wavelengths of 660 and 808 nm in experimental model of osteoarthritis. Photochem Photobiol 88(1):161–166
Aparecida Da Silva A et al (2013) Wound-healing effects of low-level laser therapy in diabetic rats involve the modulation of MMP-2 and MMP-9 and the redistribution of collagen types I and III. J Cosmet Laser Ther 15(4):210–216
Marcos RL et al (2012) Low-level laser therapy in collagenase-induced Achilles tendinitis in rats: analyses of biochemical and biomechanical aspects. J Orthop Res 30(12):1945–1951
Tomazoni SS et al (2017) Effects of photobiomodulation therapy and topical non-steroidal anti-inflammatory drug on skeletal muscle injury induced by contusion in rats—part 1: morphological and functional aspects. Lasers Med Sci 32(9):2111–2120
Tomazoni SS et al (2017) Effects of photobiomodulation therapy, pharmacological therapy, and physical exercise as single and/or combined treatment on the inflammatory response induced by experimental osteoarthritis. Lasers Med Sci 32(1):101–108
Macias DM et al (2015) Low-level laser therapy at 635 nm for treatment of chronic plantar fasciitis: a placebo-controlled, randomized study. J Foot Ankle Surg 54(5):768–772
Jastifer JR et al (2014) Low-level laser therapy for the treatment of chronic plantar fasciitis: a prospective study. Foot Ankle Int 35(6):566–571
Pinto HD et al (2016) Photobiomodulation therapy improves performance and accelerates recovery of high-level rugby players in field test: a randomized, crossover, double-blind, placebo-controlled clinical study. J Strength Cond Res 30(12):3329–3338
Aver Vanin A et al (2016) Pre-exercise infrared low-level laser therapy (810 nm) in skeletal muscle performance and postexercise recovery in humans, what is the optimal dose? A randomized, double-blind, placebo-controlled clinical trial. Photomed Laser Surg 34(10):473–482
De Marchi T et al (2017) Does photobiomodulation therapy is better than cryotherapy in muscle recovery after a high-intensity exercise? A randomized, double-blind, placebo-controlled clinical trial. Lasers Med Sci 32(2):429–437
World Medical Association (2016) WMA Declaration of Helsinki—ethical principles for medical research involving human subjects. 2016
Beyzadeoglu T, Gokce A, Bekler H (2007) The effectiveness of dorsiflexion night splint added to conservative treatment for plantar fasciitis. Acta Orthop Traumatol Turc 41(3):220–224
Yucel U et al (2013) Full-length silicone insoles versus ultrasound-guided corticosteroid injection in the management of plantar fasciitis: a randomized clinical trial. Prosthetics Orthot Int 37(6):471–476
Celik D, Kus G, Sirma SO (2016) Joint mobilization and stretching exercise vs steroid injection in the treatment of plantar fasciitis: a randomized controlled study. Foot Ankle Int 37(2):150–156
Chew KT et al (2013) Comparison of autologous conditioned plasma injection, extracorporeal shockwave therapy, and conventional treatment for plantar fasciitis: a randomized trial. Pm r 5(12):1035–1043
Digiovanni BF et al (2006) Plantar fascia-specific stretching exercise improves outcomes in patients with chronic plantar fasciitis. A prospective clinical trial with two-year follow-up. J Bone Joint Surg Am 88(8):1775–1781
DiGiovanni BF et al (2003) Tissue-specific plantar fascia-stretching exercise enhances outcomes in patients with chronic heel pain. A prospective, randomized study. J Bone Joint Surg Am 85-a(7):1270–1277
Cinar E, Uygur F (2013) FRI0582-HPR extracorporeal shock wave therapy versus low intensity laser therapy in the treatment of heel pain. Ann Rheum Dis 72(Suppl 3):A572–A572
Akbaba YA, Celik D, Ogut RT (2016) Translation, cross-cultural adaptation, reliability, and validity of Turkish version of the American Orthopaedic Foot and Ankle Society Ankle-Hindfoot Scale. The Journal of Foot and Ankle Surgery 55(6):1139–1142
Kitaoka HB et al (1994) Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int 15(7):349–353
Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP (2011) Validity of four pain intensity rating scales. Pain 152(10):2399–2404
Lee JS et al (2003) Clinically important change in the visual analog scale after adequate pain control. Acad Emerg Med 10(10):1128–1130
Bohannon RW, Glenney SS (2014) Minimal clinically important difference for change in comfortable gait speed of adults with pathology: a systematic review. J Eval Clin Pract 20(4):295–300
Riddle DL et al (2003) Risk factors for plantar fasciitis: a matched case-control study. J Bone Joint Surg Am 85-a(5):872–877
Spörndly-Nees S et al (2011) The navicular position test—a reliable measure of the navicular bone position during rest and loading. International journal of sports physical therapy 6(3):199
Sporndly-Nees S et al (2011) The navicular position test—a reliable measure of the navicular bone position during rest and loading. Int J Sports Phys Ther 6(3):199–205
Lange B, Chipchase L, Evans A (2004) The effect of low-dye taping on plantar pressures, during gait, in subjects with navicular drop exceeding 10 mm. J Orthop Sports Phys Ther 34(4):201–209
Blew RM et al (2002) Assessing the validity of body mass index standards in early postmenopausal women. Obes Res 10(8):799–808
Lakens D (2013) Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 4:863
de Almeida P et al (2014) What is the best treatment to decrease pro-inflammatory cytokine release in acute skeletal muscle injury induced by trauma in rats: low-level laser therapy, diclofenac, or cryotherapy? Lasers Med Sci 29(2):653–658
Leal-Junior EC et al (2014) Superpulsed low-level laser therapy protects skeletal muscle of mdx mice against damage, inflammation and morphological changes delaying dystrophy progression. PLoS One 9(3):e89453
Kolari PJ (1985) Penetration of unfocused laser light into the skin. Arch Dermatol Res 277(4):342–344
Kiritsi O et al (2010) Ultrasonographic evaluation of plantar fasciitis after low-level laser therapy: results of a double-blind, randomized, placebo-controlled trial. Lasers Med Sci 25(2):275–281
Chuckpaiwong B, Berkson EM, Theodore GH (2009) Extracorporeal shock wave for chronic proximal plantar fasciitis: 225 patients with results and outcome predictors. J Foot Ankle Surg 48(2):148–155
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Cinar, E., Saxena, S. & Uygur, F. Low-level laser therapy in the management of plantar fasciitis: a randomized controlled trial. Lasers Med Sci 33, 949–958 (2018). https://doi.org/10.1007/s10103-017-2423-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-017-2423-3