Abstract
The purpose of this in vitro study was to evaluate the effect of etching time on the tensile bond strength (TBS) of a conventional adhesive bonded to dentin previously irradiated with erbium:yttrium–aluminum–garnet (Er:YAG) and erbium, chromium:yttrium–scandium–gallium–garnet (Er,Cr:YSGG) lasers. Buccal and lingual surfaces of 45 third molars were flattened until the dentin was exposed and randomly assigned to three groups (n = 30) according to the dentin treatment: control (not irradiated), irradiated with Er:YAG (1 W; 250 mJ; 4 Hz; 80.6 J/cm2) laser or Er,Cr:YSGG (4 W; 200 mJ; 20 Hz; 71.4 J/cm2) laser, and into three subgroups (n = 10) according to acid etching time (15 s, 30 s or 60 s) for each experimental group. After acid etching, the adhesive was applied, followed by the construction of an inverted cone of composite resin. The samples were immersed in distilled water (37°C for 24 h) and subjected to TBS test [50 kilogram-force (kgf), 0.5 mm/min]. Data were analyzed by analysis of variance (ANOVA) and Tukey statistical tests (P ≤ 0.05). Control group samples presented significant higher TBS values than those of all lased groups. Both irradiated groups exhibited similar TBS values. Samples subjected to the different etching times in each experimental group presented similar TBS. Based on the conditions of this in vitro study we concluded that Er:YAG and Er,Cr:YSGG laser irradiation of the dentin weakens the bond strength of the adhesive. Moreover, increased etching time is not able to modify the bonding strength of the adhesive to irradiated dentin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total etching of enamel and dentin is a very well known technique used with adhesive systems for composite resin bonding. These adhesive materials were originally developed to be applied to dentin conventionally prepared by diamond burs.
However, the use of equipment capable of removing carious lesions and hard dental tissues, such as the erbium:yttrium–aluminum–garnet (Er:YAG) and the erbium, chromium:yttrium–scandium–gallium–garnet (Er,Cr:YSGG) lasers [1–3], have been shown to produce surfaces differing from those prepared by mechanical drilling.
When Er:YAG or Er,Cr:YSGG lasers are used to irradiate the dentin, the ablation phenomenon occurs, removing the smear layer, exposing dentinal tubules and creating a surface with a micromechanical retention pattern apparently favorable to bonding procedures [4–12]. However, phosphoric acid etching is still recommended for bonding with total etch adhesive systems after laser cavity preparation [13–15].
Studies have described that the application of phosphoric acid to erbium laser-irradiated surfaces (enamel and dentin) can show some advantages, such as decreased marginal leakage [16] and enhanced bond strength of laser-irradiated dentin when different resin bond systems are used [13, 17]. However, other studies report significantly lower bond strength values for Er:YAG laser-treated surfaces than for acid-etched dentin [18–22].
Although there is literature available with regard to strength of the bond to erbium laser-treated dentin, the results are not yet conclusive. Based on the hypothesis that a longer etching time on laser treated surfaces could promote a higher quality of adhesive interface, the purpose of this in vitro study was to evaluate the effects of different etching times on the tensile bond strength of an adhesive bonded to dentin treated with Er:YAG and Er,Cr:YSGG lasers.
Materials and methods
The study protocol was approved by the Research Ethics Committee of the School of Dentistry, University of São Paulo. Forty-five freshly extracted human third molars were kept in a 0.5% chloramine solution at 4°C. The roots were sectioned 2 mm from the enamel–cementum junction, and the crowns were divided into halves with a slow-speed diamond saw in a sectioning machine, the Labcut 1010 (Extec, Excel Technologies Inc., Enfield, CT, USA) under cooling. Each half was embedded in acrylic resin to facilitate its manipulation (Fig. 1, steps A and B). The buccal surfaces were polished with 120 grit, 240 grit and 400 grit sandpaper disks under running water in a polishing machine until the dentin surface was exposed. A 600 grit sandpaper disk was used for 60 s in the same machine under cooling to standardize the smear layer. A 4 mm x 4 mm square was delimited on the dentin surface in order to determine the laser irradiation area (Fig. 1c).
Steps in the preparation of the samples. A root section and mesio-distal section of the crowns; B crowns embedded in a self-curing acrylic resin; C dentin exposure and determination of the irradiation area of 4 mm side; D laser irradiation (10 s in each direction); E delimitation of the bonding area; F total etch adhesive procedure; G placement of two-piece Teflon matrix; H inverted cone of composite resin (PA 35% phosphoric acid, AS adhesive system
The samples were randomly divided into three groups (n = 30), according to dentin treatment, and into three subgroups, according to the different acid etching times (n = 10) in each experimental group, as shown in Table 1.
In Group L1 the samples were irradiated with the Er:YAG laser (Kavo Key Laser III, Biberach, Germany) at a wavelength of 2.94 µm. A single irradiation was uniformly and perpendicularly performed with a #2051 handpiece in non-contact mode, 12 mm distant from the surface (focused mode laser beam approximately 630 µm in diameter) and scanning the surface for 20 s (10 s longitudinally and 10 s vertically) (Fig. 1, step D). Water cooling was constantly used during irradiation, with a flow rate of 6 ml/min.
In group L2, an Er,Cr:YSGG laser (Waterlase Millennium, Biolase Technology, San Clement, CA, USA) with a wavelength of 2.78 µm was used. Dentin was uniformly and perpendicularly irradiated with a 600 µm diameter sapphire tip positioned 1 mm (focused mode) from the dentin surface for 10 s, longitudinally (Fig. 1), step D. Water cooling was used constantly during irradiation, with a water–air spray (75% and 65%, respectively).
Er:YAG and Er,Cr:YSGG laser energy parameters are presented in Table 1.
An area 3 mm in diameter was delimited on the dentin surface for the adhesive procedure (Fig. 1, step E). In all groups (control and experimental), 35% phosphoric acid (3M ESPE, São Paulo, SP, Brazil) was applied to the dentinal surface, according to the different groups (15 s, 30 s, 60 s of exposure to acid) and rinsed for the same time. An adhesive (Single Bond, 3M ESPE, batch number OEH) was applied in accordance with the manufacturer’s instructions: two consecutive layers, gently air dried for 5 s and polymerized with light for 10 s (Fig. 1, step F) using halogen light curing apparatus (460 mW/cm2) (Optilux 501, Kerr/Demetron, Danbury, CT, USA).
In order to build an inverted resin cone, we fixed the samples in a metal clamping device (developed by Houston Biomaterials Research Center, Dental Branch, TX, USA), and a Teflon matrix was placed over the prepared surface (Fig. 1, step G). Three increments of composite resin (Z250, 3M ESPE, batch number 1KK) were placed in the cavity formed by the matrix, each one polymerized with light for 20 s. After this, the sample was removed from the clamping device and the two-piece matrix was opened and separated, leaving a 4 mm-high inverted composite resin cone, 6 mm in diameter and tapering to a 3 mm diameter bonding area adhered to the dentin surface (Fig. 1, step H).
After 24 h of storage in distilled water at 37ºC, the samples were tested for tensile bond strength in a universal testing machine (model 4442, Instron Inc., Canton, MA, USA), belonging to the Laboratory of Restorative Dentistry at the University of São Paulo, at a crosshead speed of 0.5 mm/min until fracture occurred.
For statistical analysis, the data were presented as means (MPa) ± standard deviations (SDs); for normal distribution of the data, ANOVA and Tukey tests were performed (P ≤ 0.05).
Results
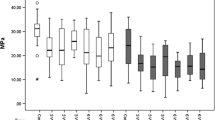
The mean TBS and SD values are summarized in Table 2.
The results showed that between the experimental groups (L1 and L2) there were no statistically significant differences when different times of exposure to acid etching were considered (P > 0.05). However, the values for tensile bond strength for these groups were shown to be statistically lower than those for the samples that were not irradiated (control groups) (P < 0.05).
All acid etching times associated with L1 or L2 groups indicated significantly lower bond strength values than those for the respective times in the control groups (P < 0.05) (Table 2). Group L1 indicated a reduction of 26%, 27% and 33% in bond strength for 15 s, 30 s and 60 s of etching time, respectively. For the L2 group, this reduction was 45%, 50% and 53%, respectively.
Within each dentin treatment group (C, L1 and L2), the different acid etching times produced no significant difference (P > 0.05) (Table 2).
Discussion
The hypothesis that a longer etching time would enhance the bond strength of adhesion to dentinal surfaces treated with both Er:YAG and Er,Cr:YSGG lasers was rejected in this in vitro study. A loss of adhesiveness was observed with increasing acid etching time.
Special attention was given to correct procedures, in particular the preparation of all samples and the application of the adhesive in a standardized way. Since it is almost impossible to produce flattened surfaces with laser irradiation, superficial dentin was exposed with abrasive paper disks, and, consequently, only a thin layer was removed by laser irradiation at the parameters tested [17]. Erbium laser irradiation was performed perpendicularly to the dentinal surface. According to Harashima et al. [6], when irradiation is done perpendicularly to cavity walls, the surfaces are very clean, almost free of debris, and have open dentinal tubules. However, in regions irradiated at different angles, most of the wall surfaces present a scratched appearance with interspersed open dentinal tubules in areas covered by melted surfaces.
Nowadays, the most acceptable technique for dental surface treatment is total etching of enamel and dentin, for 15 s and 10 s, respectively. Increased acid etching time for a dentin surface prepared by conventional drills is not recommended, since it leads to lower bond strengths [23]. In agreement with the findings of Abu-Hanna and Gordan [24], our results did not show this adverse effect in the control groups when the acid etching time was increased, at least not to a statistically significant extent.
As regards each acid etching time, there was a significant and proportional loss of bond strength in the laser groups when compared with the control group. Probably, these results were related to morphological alterations in the dentin.
Erbium lasers have wavelengths with optimal absorption of hydroxyapatite and water and low absorption in dental hard tissues [25, 26], allowing the ablation phenomenon to occur, which consists of vaporization of the water present in the tissue, leading to microexplosions and the removal of the organic and inorganic portion of enamel and dentin. Another hypothesis for the mechanism of cutting dental hard tissue is the hydrokinetic tissue-cutting effect based on the strong absorption of laser energy by fine water droplets, resulting in a violent, yet controlled, microexpansion, which induces strong mechanical forces on the targeted tissue surface. As a result, the hydrokinetic forces produce mechanical separation of the calcified tissue surface and cause quick and clean tissue removal [27].
When Er:YAG or Er,Cr:YSGG laser is applied to dentin, ablation occurs, removing the smear layer, exposing dentinal tubules and promoting irregularities [4–12]. The literature suggests that there is little difference in the morphology of cavities prepared with the two types of erbium lasers. The small differences found are due the fact that the 2,940 nm wavelength of the Er:YAG laser is very close to that of the Er,Cr:YSGG laser (2,780 nm). However, even with the similarity in the wavelength, it seems that surfaces irradiated with Er,Cr:YSGG laser are more thermally affected than those irradiated with Er:YAG laser. Ablation begins at temperatures of approximately 300°C for the Er:YAG laser and 800°C for the Er,Cr:YSGG laser, well below the melting and vaporization temperatures of the carbonated hydroxyapatite mineral component [melting point (m.p). = 1,200°C] [28].
Harashima et al. [6] showed that cavities prepared by Er:YAG laser presented a characteristic rough surface, similar to an acid-etched surface, and most of the open dentinal tubules were visible. On the other hand, the cavities prepared by Er,Cr:YSGG laser presented a characteristic surface that was scale-like, and open dentinal tubules were more clearly visible than those prepared by Er:YAG laser. Smaller width and stripped surfaces were also observed in the cavities prepared by Er,Cr:YSGG laser.
When surfaces produced with an Er,Cr:YSGG laser and phosphoric acid were compared, the tubular openings seen with laser irradiation were smaller and the roughness was nine-times greater than that seen on the acid-etched surface [10]. In addition, the orifices of the dentinal tubules were opened without widening, and the peritubular dentin protruded slightly from the surrounding intertubular dentin [10]. As suggested by Cardoso et al. [29], salient irregularities on the lased dentine surface may reduce the bond strength by preventing uniform stress distribution at the adhesive–dentin interface. Moreover, the presence of irregularities on the dentinal surface leads to a non-uniform thickness of the adhesive layer, thus resulting in diminished bonding effectiveness.
Although previous studies have reported that lased surfaces appear to be favorable to bonding procedures, since they show opened dentinal tubules, micro-irregularities and a smear layer-free surface, there are thermal effects associated with erbium laser irradiation of dental tissues.
According to Malta et al. [30], the residual thermal energy resulting from the erbium laser ablation process can influence bonding by denaturing the collagen fibers and hindering the formation of a regular and defined hybrid layer along the adhesive interface. Erbium laser-irradiated collagen appears to be packed and melted, with an aspect similar to that of glazing [31]. Aranha et al. [7], using the same adhesive system and similar Er,Cr:YSGG laser parameters as used in our study, showed the formation of a hybrid layer exhibiting wider and funnel-shaped resin tags that were dispersed and fewer in quantity, with the larger base sealing the tubule entrances and funneling into them, in comparison with the control group. These structural changes in the collagen and hybrid layer itself are expected to have a negative influence on the micromechanical penetration and micro-retention of adhesive materials. Thus, the idea of this study was to use a longer acid etching time that could reach a greater depth where there would be sound collagen suitable for adhesion and that would promote a better bond to the dentin. However, this hypothesis was not confirmed in this study. Probably, an altered layer of collagen still remains on the surface, even after acid etching has been performed.
Previous studies have reported that erbium laser irradiation can enhance the strength of the composite resin bond to dentin [13, 17] or produce bond strength values similar to those of bur-prepared cavities [10, 32]. However, our study showed different results. Different laser irradiation parameters on a dentin surface treated with phosphoric acid led to lower bond strength values between the two substrates. Similar results were observed by other authors [18, 19, 22, 33, 34].
In our study the irradiation parameters were in accordance with the manufacturer’s instructions for both lasers. Other energy power settings have been evaluated in the literature, with special attention being paid to the application of different repetition rates and energy densities [10, 13, 35]. Camerlingo et al. [35] suggested that laser treatment can determine different chemical environments in the treated dentin, depending on the duration of the laser pulse. The authors concluded that treatment with very long pulses promoted a dentin surface very similar to that obtained with burs, while very short laser pulses could produce severe modification of the organic components. These observations are also in line with the findings of de Souza and colleagues [13] and Gonçalves et al. [17], since they showed better bond strength using lower repetition rates (2 Hz, 4 Hz). It is important to point out that the Er,Cr:YSGG laser operates at a fixed repetition rate (20 Hz) and that this could be one of the main reasons for the lower bond strengths found in this study. In addition, Freitas and co-workers [9] concluded that the increase in repetition rate and decrease in output energy of the laser system could be an effective alternative for hard tissue ablation. However, according to Corona et al. [11], the increase in energy and/or pulse repetition rate promotes a stronger influence on the rate of mass loss and on morphological changes. In our study a lower repetition rate (20 Hz) was used for the Er,Cr:YSGG laser than for the Er:YAG laser groups (4 Hz). The high repetition rate in the Er,Cr:YSGG laser groups could explain the lowest bond strength presented.
Further studies are necessary on the use of other laser energy parameters and other strategies to treat erbium-lased substrate in order to achieve a more suitable surface for bonding. Morphological and biochemical studies are also important, in order to verify and compare the hybrid layer pattern after dentin irradiation with the two erbium lasers.
Conclusion
Based on the conditions of this in vitro study, we concluded that Er:YAG and Er,Cr:YSGG laser irradiation of the dentin weakens the bonding strength of an adhesive. Moreover, increased etching time is not able to modify the strength of the bond of the adhesive to irradiated dentin.
References
Eversole LR, Rizoiu I, Kimmel AI (1997) Pulpal response to cavity preparation by an erbium, chromium:YSGG laser-powered hydrokinetic system. J Am Dent Assoc 128:1099–1106
Hibst R, Keller U (1989) Experimental studies of the application of the Er:YAG laser on dental hard substances: I. Measurement of the ablation rate. Lasers Surg Med 9:338–344
Keller U, Hibst R (1989) Experimental studies of the application of the Er:YAG laser on dental hard substances: II. Light microscopic and SEM investigations. Lasers Surg Med 9:345–351
Hossain M, Nakamura Y, Tamaki Y, Yamada Y, Murakami Y, Matsumoto K (2003) Atomic analysis and knoop hardness measurement of the cavity floor prepared by Er, Cr:YSGG laser irradiation in vitro. J Oral Rehabil 30:515–521
Schein MT, Bocangel JS, Nogueira GE, Schein PA (2003) SEM evaluation of the interaction pattern between dentin and resin after cavity preparation using ER:YAG laser. J Dent 31:127–135
Harashima T, Kinoshita J, Kimura Y, Brugnera A, Zanin F, Pecora JD, Matsumoto K (2005) Morphological comparative study on ablation of dental hard tissues at cavity preparation by Er:YAG and Er, Cr:YSGG lasers. Photomed Laser Surg 23:52–55
Aranha AC, De Paula Eduardo C, Gutknecht N, Marques MM, Ramalho KM, Apel C (2007) Analysis of the interfacial micromorphology of adhesive systems in cavities prepared with Er, Cr:YSGG, Er:YAG laser and bur. Microsc Res Tech 70:745–751
Esteves-Oliveira M, Zezell DM, Apel C, Turbino ML, Aranha AC, Eduardo Cde P, Gutknecht N (2007) Bond strength of self-etching primer to bur cut, Er, Cr:YSGG, and Er:YAG lased dental surfaces. Photomed Laser Surg 25:373–380
Freitas PM, Navarro RS, Barros JA, de Paula Eduardo C (2007) The use of Er:YAG laser for cavity preparation: an SEM evaluation. Microsc Res Tech 70:803–808
Chou JC, Chen CC, Ding SJ (2009) Effect of Er,Cr:YSGG laser parameters on shear bond strength and microstructure of dentine. Photomed Laser Surg 27:481–486
Corona SA, Souza-Gabriel AE, Chinelatti MA, Pecora JD, Borsatto MC, Palma-Dibb RG (2008) Influence of energy and pulse repetition rate of Er:YAG laser on enamel ablation ability and morphological analysis of the laser-irradiated surface. J Biomed Mater Res A 84:569–575
Youssef M, Quinelato A, Youssef F, Pelino JEP, Salvadori MC, Mori M (2008) Dentinal surface-cutting efficiency using a high-speed diamond bur, ultrasound and laser. Laser Physics 18:472–477
de Souza AE, Corona SA, Dibb RG, Borsatto MC, Pecora JD (2004) Influence of Er:YAG laser on tensile bond strength of a self-etching system and a flowable resin in different dentin depths. J Dent 32:269–275
Giachetti L, Scaminaci Russo D, Scarpelli F, Vitale M (2004) SEM analysis of dentin treated with the Er:YAG laser: a pilot study of the consequences resulting from laser use on adhesion mechanisms. J Clin Laser Med Surg 22:35–41
de Carvalho RC, de Freitas PM, Otsuki M, de Eduardo CP, Tagami J (2008) Micro-shear bond strength of Er:YAG-laser-treated dentin. Lasers Med Sci 23:117–124
Ceballos L, Osorio R, Toledano M, Marshall GW (2001) Microleakage of composite restorations after acid or Er-YAG laser cavity treatments. Dent Mater 17:340–346
Gonçalves M, Corona SA, Borsatto MC, Silva PC, Pecora JD (2002) Tensile bond strength of dentin-resinous system interfaces conditioned with Er:YAG laser irradiation. J Clin Laser Med Surg 20:89–93
Martínez-Insua A, Da Silva Dominguez L, Rivera FG, Santana-Penín UA (2000) Differences in bonding to acid-etched or Er:YAG-laser-treated enamel and dentin surfaces. J Prosthet Dent 84:280–288
De Munck J, Van Meerbeek B, Yudhira R, Lambrechts P, Vanherle G (2002) Micro-tensile bond strength of two adhesives to erbium:YAG-lased vs bur-cut enamel and dentin. Eur J Oral Sci 110:322–329
Dunn WJ, Davis JT, Bush AC (2005) Shear bond strength and SEM evaluation of composite bonded to Er:YAG laser-prepared dentin and enamel. Dent Mater 21:616–624
Cardoso MV, Coutinho E, Ermis RB, Poitevin A, Van Landuyt K, De Munck J, Carvalho RC, Lambrechts P, Van Meerbeek B (2008) Influence of Er, Cr:YSGG laser treatment on the microtensile bond strength of adhesives to dentin. J Adhes Dent 10:25–33
Botta SB, da Ana PA, Zezell DM, Powers JM, Matos AB (2009) Adhesion after erbium, chromium:yttrium-scandium-gallium-garnet laser application at three different irradiation conditions. Lasers Med Sci 24:67–73
Hashimoto M, Ohno H, Kaga M, Sano H, Tay FR, Oguchi H, Araki Y, Kubota M (2002) Over-etching effects on micro-tensile bond strength and failure patterns for two dentin bonding systems. J Dent 30:99–105
Abu-Hanna A, Gordan VV (2004) Evaluation of etching time on dentin bond strength using single bottle bonding systems. J Adhes Dent 6:105–110
Kayano T, Ochiai S, Kiyono K, Yamamoto H, Nakajima S, Mochizuki T (1989) Effects of Er:YAG laser irradiation on human extracted teeth. J Stomatol Soc Jpn 56:381–392
Kumazaki M, Fujiwara H, Matsuda T, Zennyu K, Kumazaki M, Toyoda K, Fujii B (1992) Excision of dental caries. J Jpn Soc Laser Dent 3:23–27
Rizoiu I, Kohanghadosh F, Kimmel AI, Eversole LR (1998) Pulpal thermal responses to an erbium, chromium: YSGG pulsed laser hydrokinetic system. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 86:220–223
Fried D, Featherstone JD, Visuri SR, Seka WD, Walsh JT (1996) The caries inhibition potential of Er:YAG and Er:YSGG laser radiation. Proc SPIE 2672:73–78
Cardoso MV, Coutinho E, Ermis RB, Poitevin A, Van Landuyt K, De Munck J, Carvalho RC, Van Meerbeek B (2008) Influence of dentin cavity surface finishing on micro-tensile bond strength of adhesives. Dent Mater 24:492–501
Malta DAMP, Costa MM, Pelino JEP, de Andrade MF, Lizarelli RFZ (2008) Bond strength of an adhesive system irradiated with Nd:YAG laser in dentin treated with Er:YAG laser. Laser Phys Lett 5:144–150
Benazzato P, Stefani A (2003) The effect of Er:YAG laser treatment on dentin collagen: an SEM investigation. J Oral Laser Appl 3:79–81
Manhaes L, Oliveira DC, Marques MM, Matos AB (2005) Influence of Er:YAG laser surface treatment and primer application methods on microtensile bond strength self-etching systems. Photomed Laser Surg 23:304–312
Armengol V, Jean A, Rohanizadeh R, Hamel H (1999) Scanning electron microscopic analysis of diseased and healthy dental hard tissues after Er:YAG laser irradiation: in vitro study. J Endod 25:543–546
Kameyama A, Kawada E, Takizawa M, Oda Y, Hirai Y (2000) Influence of different acid conditioners on the tensile bond strength of 4-META/MMA-TBB resin to Er:YAG laser-irradiated bovine dentin. J Adhes Dent 2:297–304
Camerlingo C, Lepore M, Gaeta GM, Riccio R, Riccio C, De Rosa A, De Rosa M (2004) Er:YAG laser treatments on dentine surface: micro-Raman spectroscopy and SEM analysis. J Dent 32:399–405
Acknowledgements
The authors wish to express their gratitude to the Special Laboratory of Lasers in Dentistry (LELO) and the exchange program with Aachen University (Germany). They also thank FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) for its financial support, grants 97/10823-0 and CEPID/CEPOF 98/14270-8).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferreira, L.S., Apel, C., Francci, C. et al. Influence of etching time on bond strength in dentin irradiated with erbium lasers. Lasers Med Sci 25, 849–854 (2010). https://doi.org/10.1007/s10103-009-0715-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-009-0715-y