Abstract
Induction of matrix synthesis by low-level laser has been demonstrated extensively. However, the question of dose- or power intensity-dependency is under-investigated. To address this issue we chose human osteoblast cell cultures and measured their alkaline phosphatase (ALP) activity after laser irradiation. The cell cultures were irradiated periodically by 690 nm radiation via optical transmission fiber-based laser needles, reaching into the culture dishes. The osteoblasts showed no induction of ALP activity when we used a single laser needle stimulation with a laser irradiance of 51 mW/cm2, an increase of approximately 43% at 102 mW/cm2 irradiance (two needles per well) and a ninefold increase at 204 mW/cm2 irradiance (four needles per well), leaving the temperature of the culture medium unaffected. We concluded that the osteoblastic response in ALP activity to a laser stimulus shows a logarithmic relationship, with a distinct threshold, rather than a linear dose-dependency. Secondly, the laser irradiance, rather than the dose, is relevant for the impact of the laser.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of non-ablative lasers in medicine to treat connective tissues has become increasingly popular. Applications include the treatment of skin, such as in psoriasis [1, 2]) and wound healing [3–5]. The aim of non-invasive laser therapy is reduction of inflammation, thus lessening pain and the reconstitution of tissue by fibroblast proliferation and extracellular matrix build-up. In contrast to the topical use of low-level laser therapy (LLLT), where the exposure to light is obvious, the effect on hard tissues such as bone and cartilage is far less taken for granted. The purpose of this study was to investigate the effect of LLLT on cells derived from hard tissue: osteoblasts. Their osteo-anabolic activity can be monitored easily in the form of the key enzyme in bone formation, alkaline phosphatase. A stimulatory effect of LLLT on osteoblast alkaline phosphatase activity has serious clinical implications, both in fracture healing [6, 7] and inflammatory bone disease. As pointed out, the penetration of the target tissue by laser emitted light is crucial for its therapeutic effect. The main questions for the LLLT practitioner are what laser irradiance to use and for how long to treat. In this context we thus addressed this question, analyzing the importance of laser irradiance during the stimulation of human osteoblast cell cultures in relation to the irradiation dose.
Materials and methods
Cell culture
Human primary osteoblasts were derived from the femoral heads of patients undergoing hip replacement. Parallel experiments were carried out with the osteosarcoma cell line SaOS-2. All cells were kept under standard conditions (37°C, humidified incubators, with 5% CO2) in phenol-red-free Dulbecco’s Modified Medium supplemented with 10% calf serum. Thirty-thousand cells were seeded per well in 48-well-dishes. They became adherent over night and were deprived of serum (0.5%) for another 24 h before treatment.
Laser treatment
After a change of serum-deprived medium, the osteoblasts were exposed to laser light of 690 nm wavelength via optical transmission fibers as used in therapy with laser needle technology.
The 500 μm wide laser needles are new medical instruments (Laserneedle, Wehrden, Germany), which emit laser light of high area density and low beam divergence. They are used for non-invasive irradiation of articular spaces.
Different levels of irradiance were accomplished by the use of one (51 mW/cm2), two (102 mW/cm2) or four (204 mW/cm2) needles reaching into the culture dishes with specially prepared dish covers. The distance to the cell layer and the lens design of the needle enabled an even irradiation of the treated cell surfaces. The osteoblast were treated for the first 30 min every 4 h for 24 h in total.
SaOS-2 cells were laser treated under the same conditions as the primary osteoblasts. However, the alkaline phosphatase (ALP) activity after a periodic irradiation pattern (30 min on, 3.5 h off, 51 mW/cm2 irradiance) was compared with that of a single irradiation (30 min) after 24 h. To assess a threshold value for the effectiveness of the irradiance on ALP activity, we plotted ln(ALP [U/mg protein]) over 1/radiant exposure [mJ/cm2]. The subsequent intersection of the linear phase of this concave Arrhenius plot with the abscissa was taken as a threshold value for the effectiveness of laser irradiance on ALP activity.
ALP assay
After each experiment, the culture medium was removed, the cells were rinsed in phosphate-buffered saline solution, lysed in 250 μl 25 mM Tris-phosphate (pH 7.8) buffer containing 1% Triton-X, and kept frozen until required for the assessment of ALP activity. Cell extracts were diluted and mixed with an ALP substrate (p-nitrophenylphosphate),leading to the formation of a yellow chromogen (p-nitrophenyl), measured photometrically (405 nm). ALP activity was expressed according to the time of product formation and total protein content of each well, derived from a Pierce assay in units per milligram of protein.
Temperature measurement
In a separate series of experiments, the temperature course in the culture medium was measured during laser treatment with a highly accurate Ni-NiCr thermocouple.
Statistical analysis
We calculated the mean values with standard errors of means, using the Mann–Whitney test to express significance, employing Prism 3.0 (Graphpad).
Results
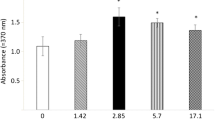
Human osteoblasts did not alter (P > 0.05) their ALP activity under irradiance of 51 mW/cm2 (0.15 ± 0.02 U/mg protein vs 0.17 ± 0.03 U/mg protein in controls). The doubling of the irradiance to 102 mW/cm2 resulted in a significant (P < 0.005), but fairly small, increase of 43% (0.14 ± 0.01 U/mg protein vs 0.10 ± 0.02 U/mg protein in controls). Using four LASERneedles, and thus using 204 mW/cm2 irradiance, however, we found that the ALP activity of the cells increased (P < 0.005) approximately eightfold (1.47 ± 0.03 U/mg protein vs 0.18 ± 0.04 U/mg protein in controls). On the basis of these findings, shown in Fig. 1, we estimated an optical activation energy threshold at a radiant exposure of 178 mJ/cm2 according to the linear section of a concave Arrhenius plot. Protein content remained constant during the experiment in controls and in laser-treated groups.
Figure 2 illustrates the effect of radiant exposure compared with that of irradiance on ALP activity. A repetitive laser treatment (six times 30 min within 24 h) with 51 mW/cm2 irradiance adds up to a higher dose of energy exposed to the cells (550.8 mJ) than does a single treatment (30 min) with an irradiance of 204 mW/cm2 (367.2 mJ). SaOS-2 cells demonstrated higher constitutive levels of ALP activity than did primary osteoblasts. The low irradiance, in combination with a high total dose of irradiation, even led to a 20% reduction in ALP activity, whereas the high irradiance with a low total dose of irradiation increased ALP activity by 40%.
Figure 3 shows the temperature course during irradiation of the cell cultures for 40 min. One LASERneedle (51 mW/cm2) left the target temperature of 37°C almost unaltered (+0.1°C); the maximum irradiance used led to a slight increase over time (+0.4°C).
Discussion
The primary intent of this study was to prove the ability of the laser needle irradiation system to have an effect on bone cells. Primary human osteoblast cultures periodically irradiated with increasing irradiance (51 mW/cm2, 102 mW/cm2 and 204 mW/cm2) were analyzed for ALP activity. As shown in Fig. 1, the osteoblast cultures responded with an over eightfold stimulation of ALP activity compared with non-irradiated controls. However, maximum irradiance was necessary to achieve this. One-half and one-quarter of the maximal irradiance left ALP activity of the primary osteoblast unaltered. This pattern of reaction to laser needle irradiation strongly implies the existence of a threshold value of irradiance, below which the osteo-anabolic activity of the cells remains quiescent. The linear rise of irradiance in correlation with the logarithmic gain of ALP activity demonstrates a behavior that is described in the Weber–Fechner law. According to this law, a stimulus cannot be perceived as distinct below a certain threshold. To manifest this threshold numerically we estimated an optical activation radiant exposure of 178 mJ/cm2 for this particular system.
In contrast to bone tissue in vivo, the in vitro osteoblasts were freely exposed to the laser needle irradiation, covered only by colorless growth medium. The question arose as to whether the total radiant exposure (irradiance over time) could compensate the lack of irradiance for the osteo-anabolic effect in the lower irradiance groups. To investigate this we chose a human osteosarcoma cell line (SaOS-2) and compared the effect of periodic treatment (30 min every 4 h with an irradiance of 51 mW/cm2, resulting in a total dose of 550.8 J) with that of a singular treatment (30 min with an irradiance of 204 mW/cm2, resulting in a total dose of 367.2 J). After 24 h, only high irradiance increased the ALP activity in SaOS-2 cells, although the total dose of irradiation was much higher in the low-irradiance group (Fig. 2). The rate of increase of ALP activity in this experiment was much lower, due to the high constitutive levels of ALP in the SaOS-2 cells. This finding adds up to the fundamental role of irradiance in the effect of laser needle irradiation on bone tissue culture.
With regard to laser needle irradiation applied in vivo or in vitro, particularly using high irradiances with red color, the question of thermal effects constantly arises. To address this issue, we used a very precise thermal couple and measured the temperature during an interval in the laser treatment (Fig. 3). Even with maximum irradiance, the temperature gain remained negligible.
Further studies will reveal whether a given irradiance threshold value for the induction of ALP activity will also account for other effects, such as collagen formation. Future analysis of transcription factors and cytokines will shed more light on the effect of laser needle irradiation on bone cells.
Conclusion
Our experiments clearly demonstrated that laser needle irradiation had an osteo-anabolic effect, assessed as stimulation of alkaline phosphatase activity, on both human primary osteoblasts and cells of a human osteosarcoma cell line. The linear increase in irradiance, resulting in a logarithmic rise in alkaline phosphatase activity, implies a threshold value of irradiance according to the Weber–Fechner law. In accordance with that, the irradiance plays a superior role to that of the total irradiation dose.
References
Hern S, Stanton AW, Mellor RH, Harland CC, Levick JR, Mortimer PS (2005) In vivo quantification of the structural abnormalities in psoriatic microvessels before and after pulsed dye laser treatment. Br J Dermatol 152:505–511
Feldman SR (2002) Remissions of psoriasis with excimer laser treatment. J Drugs Dermatol 1:13–14
Posten W, Wrone DA, Dover JS, Arndt KA, Silapunt S, Alam M (2005) Low-level laser therapy for wound healing: mechanism and efficacy. Dermatol Surg 31:334–340
Almeida-Lopes L, Rigau J, Zangaro RA, Guidugli-Neto J, Jaeger MM (2001) Comparison of the low level laser therapy effects on cultured human gingival fibroblasts proliferation using different irradiance and same fluence. Lasers Surg Med 29:179–184
Herascu N, Velciu B, Calin M, Savastru D, Talianu C (2005) Low-level laser therapy (LLLT) efficacy in post-operative wounds. Photomed Laser Surg 23:70–73
da Silva RV, Camilli JA (2006) Repair of bone defects treated with autogenous bone graft and low-power laser. J Craniofac Surg 17:297–301
Baibekov IM, Khanapiyaev UK (2001) Healing of bone fractures of rat shin and some immunological indices during magnetic laser therapy and osteosynthesis by the Ilizarov method. Bull Exp Biol Med 131:399–402
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haxsen, V., Schikora, D., Sommer, U. et al. Relevance of laser irradiance threshold in the induction of alkaline phosphatase in human osteoblast cultures. Lasers Med Sci 23, 381–384 (2008). https://doi.org/10.1007/s10103-007-0511-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-007-0511-5