Abstract
In this study, we present a review of the literature on the impact of photobiomodulation on osteoblast-like cell culture. Searches were performed in the PubMed/MEDLINE (Medical Literature Analysis and Retrieval System Online), SCOPUS, and SPIE digital library databases for original articles regarding the effects of LLLT on osteoblast-like cells in experimental models using LLLT published in English from the last 20 years. The search identified 1439 studies. After the analysis of the abstracts, 1409 studies were excluded and 30 studies were then selected for the full-text analysis, 8 of which were excluded. Thus, 22 studies were included for a critical evaluation of the impact of photobiomodulation on osteoblast-like cell culture. The cell lineages studied were primary rat, primary human, saos-2, Osteo-1, MC3T3, MG63, and OFCOL II. Moreover, a wide variety of experimental models were used to experimentally analyze the impact of photobiomodulation, the most common of which were alkaline phosphatase, MTT, and cell count. This review suggests that osteoblastic-like cells are susceptible to photobiomodulation but that most of the light parameters varied by different authors have little to no influence on proliferation but very high levels of irradiance have demonstrated deleterious effects on proliferation, highlighting the bi-phasic effect of photobiomodulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
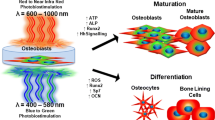
Photobiomodulation involves irradiation of a cell culture and/or tissue at a low irradiance (power over area) with the objective of triggering or enhancing a variety of interrelated mechanisms, which ultimately could result in faster resolution of the inflammatory response, reduction in pain [1], and improved tissue repair [2,3,4,5,6,7,8].
Osteoblasts are single nucleus cells that are not terminally differentiated and whose primary function is to synthesize bone matrix and mineralize bone tissue during its initial formation and later remodeling [9, 10].
Osteoblasts are widely used to evaluate bone formation process because they express bone proteins, besides having the ability to form mineralized bone nodules in vitro. Furthermore, similar to osteoblasts in vivo, cell culture pass through three distinct stages in its development: the proliferation, maturation, and mineralization [11, 12].
Past studies have demonstrated that different osteoblast-like cells react distinctively to light, depending upon several radiometric parameters. Cell analysis included proliferation [3, 5, 13,14,15], adhesion [4, 16], and the expression of many products related to osteogenesis [6, 7, 17,18,19,20,21,22]. Moreover, there are several light parameters that may be adjusted to obtain different results, including irradiance, radiant power, radiant energy, radiant exposure (energy over area), temporal irradiation parameters, polarization, and wavelength. Nevertheless, at present, the influence of these parameters on the impact of photobiomodulation on various osteoblast-like cells remains unclear. Each study has examined different parameters, and even though instructions on how to report radiometric parameters have been published [4,5,6,7,8, 13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36], most authors misreport such information, which makes it impossible to reproduce or analyze the results of some reports.
The photobiomodulation has demonstrated positive effects on bone remodeling with regard to increased cell proliferation [3, 5, 13,14,15] and increased cell differentiation evidenced by increased expression of RunX2 and alkaline phosphatase also in this lineage [6, 8, 14, 17, 19,20,21,22,23]. On the other hand, some authors present contradictory results in this sense as Coombe et al. (2001) and Emes et al. (2013) that demonstrated that the laser did not increase the proliferation or differentiation of osteoblasts compared to the control group.
The mechanism of photobiomodulation to osteoblast-like cells is still not fully understood but the literature emphasizes the participation of cytochrome c oxidase (CCO), the terminal enzyme in the mitochondrial respiratory chain, in the mechanism of action of photobiomodulation. In recent years, it has been demonstrated that the photons received by the cells would act on the cytochrome C oxidase enzyme activity causing the increase in energy (ATP) produced. This hypothesis has already been verified in different studies [37, 38]. Wang et al. [37] submitted 11 healthy participants to the treatment using 1064-nm laser and placebo on their right forearms and the spectroscopic results showed that LLLT induced significant increase in cytochrome c oxidase. Wang et al. [38] in addition verified that the photobiomodulation in human adipose-derived stem cells, treated with 810 and 980 nm lasers, probably occurred in different mechanisms of action and they concluded that in case of 810 nm irradiation, the activation of CCO in mitochondria was responsible for the results showed. The hypothesis proposed by them suggest that the mechanism of action of 980 nm relies on the activation of heat (or light)-gated ion channels and the activation of CCO in mitochondria by 810 nm would be the accepted mechanism.
The correct choice of irradiation parameters such as irradiance, radiant exposure, wavelength, power, frequency of treatment, and pulse rate is crucial to ensure increased proliferation but most authors do not described key parameters to ensure the reproducibility and reliability of the findings.
The aim of this work was to perform a literature review demonstrating the current applications of photobiomodulation on osteoblast-like cells, the influence of different radiometric parameters, and their effects on cell culture.
Methods
Searches were performed in the PubMed/MEDLINE (Medical Literature Analysis and Retrieval System Online), SCOPUS, and SPIE digital library databases for original articles regarding the effects of LLLT on osteoblast-like cells in experimental models using LLLT published in English from the last 20 years.
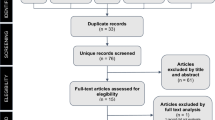
The Medical Subject Headings and SCOPUS were used to find additional key words related to “lasers,” “laser therapy,” “low-level laser therapy,” “low-intensity laser therapy,” “low-level light therapy,” “low-intensity light therapy,” “light therapy,” “phototherapy” or “photobiomodulation,” and “osteoblast.” The bibliographies of all retrieved articles were also examined to identify additional studies (Fig. 1). Two reviewers then independently applied the predetermined eligibility criteria to the full text of the studies retrieved.
The studies selected for analysis were included in the review after meeting the following criteria:
-
(1)
Articles published between April 1996 and April 2016.
-
(2)
Studies involving osteoblast-like cells and photobiomodulation.
-
(3)
Studies that described or allowed the calculation of the following radiometric parameters: wavelength, power, beam spot size, power density, energy density, repetition rate hertz (for pulsed/gated light), pulse duration or duty cycle, exposure duration, frequency of treatments, and total radiant energy (joules).
Studies were excluded based on the following criteria:
-
(1)
Case reports or review studies.
-
(2)
Absence of or incomplete irradiation parameters.
-
(3)
Absence of a control group.
-
(4)
Not focused on osteoblast-like cell culture.
Results
The search identified 1439 potentially relevant studies. Analysis of the abstracts excluded: 1374 works that were unrelated to osteoblast-like cell culture, 31 reviews and 4 duplications (same study in more than one database). Thirty studies were then selected for the full-text analysis, one of which was excluded because of the lack of description of irradiation parameters and seven for not focusing on osteoblast-like cells. Thus, 22 studies were included for a critical evaluation of the impact of photobiomodulation on osteoblast-like cell culture.
In this review, the most important parameter was the target size, as this allowed calculation of the radiant exposure and irradiance. Studies without this information or sufficient data to allow its calculation were excluded.
Seven studies employed pulsed/gated wave and 18 continuous wave (cw) radiation. Twenty-two used radiation in the red region of the spectrum (620 nm < λ ≤ 780 nm) and 12 in the infrared region (λ > 780 nm). Some studied more than one light source.
The cell lineages studied were primary rat (n = 5, Tables 1 and 2); primary human (n = 4, Tables 3 and 4); Osteo-1 (n = 1, Tables 3 and 4); OFCOL II (n = 1, Tables 3 and 4); Saos-2 (n = 6, Tables 5 and 6); MC3T3 (n = 5, Tables 7 and 8); MG63 (n = 2, Tables 7 and 8); and the studies included in this review used different irradiation parameters to modulate the cells. Moreover, a wide variety of experimental models were used to experimentally analyze the impact of photobiomodulation, the most common of which were alkaline phosphatase (n = 14), MTT (n = 11), and cell count (n = 9).
The following tables summarize the primary results and radiometric parameters of the studies included in this review.
Amongst the studies included in this review, there was considerably heterogeneity in the irradiation parameters as well as the methods used to evaluate the results and type of osteoblast-like cell culture, which hinders comparison between studies. Besides the variation in the irradiation parameters, the lack of standardization on how to report radiometric parameters increases the complexity of any analysis of the results. Most authors reported fewer parameters than would be required to perform a deeper evaluation of their work and even the terms used to describe the parameters were inaccurate. In addition, several authors had miscalculated their parameters so, for the purposes of this review, each parameter was carefully recalculated based on the standard size of well plates and manufacturers’ parameters of the light source to allow a more realistic comparison and understanding of photobiomodulation.
Although different authors analyzed different outcomes, the main outcome for the purposes of this review was cell proliferation. Different methods were used to assess proliferation, ranging from a simple count under the microscope [6, 7, 8, 9, 10], to bioconversion by intercellular dehydrogenase of the tetrazolium compound into formazan, with values directly proportional to the number of viable cells in the culture medium [11, 12], and growth curves [13] and MTT [18].
In this section, the papers are divided according to the type of cell culture, to better understand the effect of photobiomodulation.
Culture of primary rat cells
The first studies using this cell culture date back to 1998, when Ozawa et al. [5] studied the effects of a diode laser emitting at 830 nm, cw, on rat primary cells. The radiant exposure was 3.84 J/cm2 applied for 600 s with an irradiance of 6.4 mW/cm2 (500 mW of power). The irradiated cells showed significantly higher rates of proliferation 6 and 9 days post irradiation, in comparison to the control group. Ozawa et al. also demonstrated an increase in the alkaline phosphatase activity of the cells up to 18 days after irradiation.
Ueda and Shimizu [19] used a laser diode emitting at 830 nm in both gated (50% duty cycle, 1, 2 and 8 Hz) and cw regimes, with radiant exposures ranging from 0.48 to 3.84 J/cm2 (exposure times from 180 to 1200 s for gated and 75 to 600 s for cw). The peak power was 500 mW and the irradiance was 6.4 mW/cm2. The authors found an increase in proliferation in all groups between 6 and 9 days. They also reported an increase in alkaline phosphatase expression and activity in all groups.
Xu et al. [14] studied the effects of gated (6 kHz) laser emitting at 650 nm, with radiant power of 2 mW, radiant exposure of 0.23 J/cm2 and 2.28 J/cm2 and exposure duration ranging from 60 to 600 s, and observed an increase in proliferation of primary rat cells in all groups 3 days after irradiation. They also demonstrated an increase in alkaline phosphatase expression in both irradiated groups.
Cankaya et al. [23] conducted a study with primary osteoblast-like cells, LED emitting at λ = 660 nm continuous with irradiance of 20 mW/cm2, applied for 1 min. There was an increase in proliferation between 24 and 48 h after irradiation.
In most studies with primary rat cells, irradiation in the red-infrared spectral regions led to increased proliferation for both cw and gated regimes; red and infrared; and LED and laser. Unlike other authors who tested the effect of low energy laser therapy on rat cap osteoblasts, Emes et al. (2013) compared the effect of this therapy with pulsed electromagnetic field. A magnetic field of 0.06 mT, 0.2 mT and a laser with wavelength of 808 nm were applied and analyzed at 24 and 96 h after the treatment. The authors found that the control group presented greater cellular proliferation than the groups submitted to a pulsed electromagnetic field and in the 96 h period the group submitted to a 0.2 mT electromagnetic field showed a higher rate of proliferation than the other groups. In contrast to most works, Emes et al. did not find greater cell proliferation in cells submitted to low energy laser therapy; however, the comparison is hampered by the difference of parameters used. Unlike other authors, this author applied to high radiant exposure of 47 J/cm2 and high irradiance of 524 mW/cm2. These studies suggest the dichotomous outcome of photobiomodulation, in that higher exposures lead to deleterious effects.
Primary human cells
Khadra et al. [4] studied the effect of diode lasers emitting at λ = 830 nm with radiant power of 84 mW, irradiance of 8.7 mW/cm2 and radiant exposure varying from 1.5 to 3 J/cm2 (172 and 344 s of irradiation) on the proliferation of primary human cells. The authors found an increase in proliferation after 96 h. They also reported a significant increase in cell adhesion from 1 to 96 h after irradiation but alkaline phosphatase expression remained unaltered 10 days after the experiment.
Stein et al. [6] evaluated the proliferation of primary human cells under He-Ne (λ = 633 nm) laser irradiation, with a radiant exposure ranging from 0.14 to 1.43 J/cm2 (1 to 10 s of radiant exposure, 10 mW of radiant power). They found an increase in proliferation at both 24 and 48 h after 3 s of irradiation (0.43 J/cm2), but differences in alkaline phosphatase expression were presented following 1 and 3 s of irradiation.
Petri et al. [16] studied the effect of IR diode laser (λ = 780 nm), cw, with 70 mW and 540 s of exposure. The authors apparently miscalculated their radiant exposure as the target diameter was 12 mm (1.13 cm2 of area), so the radiant exposure would have been about 37.8 J/cm2 and not 3 J/cm2 as reported. There was no change in proliferation 10 and 14 days after irradiation. Phosphatase activity was also unaltered at 10 days but expression significantly increased after 14 days.
As in rat cells, the primary human cell responded to irradiances in the range of tens of mW/cm2. Higher irradiances (of hundreds of mW/cm2) remain untested but the medium range tested by Petri (62 mW/cm2) showed no significant differences in proliferation. Although all authors used different wavelengths, in this range (red-NIR) light absorption is very similar.
Khadra et al. [4] concluded that the irradiation of the laser modulated the activity of the cells and tissues but it is dose-dependent. Stein et al. [6] also found positive results regarding laser therapy, concluding that its application promotes proliferation and maturation of human osteoblasts, only Petri et al [16] found lack of positive results in the application of laser therapy in human osteoblasts, the authors found that the laser did not influence the cellular growth but the parameters used differ from those used by Khadra et al [4] and Stein et al [6].
Primary cells derived from osteosarcoma
Coombe [37] found that proliferation did not differ between groups after irradiation with diode laser (λ = 830 nm, p = 90 mW, irradiance = 629 mW/cm2 0.3 to 4 J), cw, with radiant exposure ranging from 1.7 to 25.1 J/cm2 in a 1–10-day period. Likewise alkaline phosphatase activity was unaffected.
Stein et al. [20] conducted a study using a λ = 670 nm cw laser with 400 mW of power, irradiation duration of 30 and 60 s (radiant exposure of 1 J/cm2 and 2 J/cm2, irradiance = 42 mW/cm2). The authors found no difference in the proliferation or alkaline phosphatase activity of the irradiated group compared to the control group.
Arisu et al. [15] studied the effect of Nd:YAG, pulsed, emitting at 1064 nm with different repetition rates (10, 15, 20, and 30 Hz), pulse energy (20, 60, 80, and 120 mJ) and irradiation duration of 10 s for all parameters. They also analyzed the influence of cw HeNe laser (λ = 633 nm, p = 100 mW and t = 10 s). They found that HeNe laser and 20 mJ pulse energy, 10 Hz, 10 s Nd:YAG laser irradiation had a stimulatory effect on proliferation but that any increment in the pulse energy, pulse repetition rate, and power output had an inhibitory effect on proliferation.
Chellini et al. [17] studied the effect of a Nd:YAG laser 1064 nm pulsed (pulse duration 100 μs; pulse energy: 20 mJ; repetition rate 50 and 70 Hz; average power: 1 and 1.4 W; irradiation duration: 10 s with radiant exposure of 1.5 J/cm2). The authors observed an increase in proliferation in relation to the control group for both irradiation parameters. They also reported an increase in the expression of alkaline phosphatase as well as RUNX2.
Bloise et al. [21] demonstrated an increase in the proliferation of Saos-2 cells 2 days after a single irradiation session of λ = 659 nm laser with 10 mW of radiant power (5 mW/cm2), for both 1 and 1.5 J/cm2 of radiant exposure. After increasing the exposure to four sessions of irradiation on days 1, 2, 3 and 7 (with the same parameters), the authors also observed increased proliferation on days 2, 3 and 7. With a single irradiation of 1.5 J/cm2, the alkaline phosphatase activity was also increased.
Coombe [37] demonstrated that cell proliferation was not significantly affected by any of the energy levels or different exposure regimes studied, as did Arisu et al. [15] who realized that large increased energy, pulse repetition rate, and power has a negative effect on cell viability and proliferation. On the other hand, Stein et al [20], Chellini et al [17] and Bloise et al [21] observed a biostimulating effect on the cells. Stein et al [20] observed a biostimulating effect on cells after 72 h of irradiation at 2 J/cm2. Unlike Arisu et al [15], Chellini et al [17], concluded that low pulse energy and high repetition rate irradiation may have a biostimulating effect on different cells of the oral microbiote, especially osteoblasts. Bloise et al. [21] observed that laser irradiation increased cell proliferation without altering their morphological characteristics.
Osteo-1 cell culture
Fujihara et al. [13] applied 10 mW of radiant power, 12 s of exposure and 0.12 J of radiant energy and observed increased proliferation of cells 3, 5 and 7 days after irradiation. The authors found that the irradiated group presented higher cell numbers than the corresponding non-irradiated group but that adhesion remained unchanged.
OFCOL II culture
Oliveira et al. [25] conducted a study examining the effect of a λ = 830 nm laser with radiant power of 50 mW and 3 J/cm2 (36 s of exposure) on the proliferation of Ofcol II cells. The authors observed an increase in proliferation after 24, 42 and 72 h.
Cell lines MC3T3 and MG63
Renno et al. [8] studied the influence of different wavelengths (830, 780, and 670 nm) on the MTS results of MC3T3 and MG63 cells. The radiant power was 30, 50, and 10 mW for the 830, 780, and 670 nm laser, respectively. The radiant exposure varied as follows: 0.5, 1, 5 and 10 J/cm2 and the MTS essay was performed 24 h after irradiation.
Following irradiation with the 780 nm laser, the authors found a significant reduction in proliferation of the osteoblast cell line for radiant exposures greater than 0.5 J/cm2 and 156 mW/cm2. However, the same amount of irradiation significantly increased the proliferation of the osteosarcoma cell line. The 830 nm laser only increased the proliferation of the osteoblast cells at the higher radiant exposure of 10 J/cm2 and the 670 nm irradiation led to increased proliferation only for the osteosarcoma cells for the radiant exposure of 5 J/cm2 (no data is available for 10 J/cm2). The alkaline phosphatase activity remained unaltered for these parameters in all cultures.
Saracino et al. [22] conducted a study exposing MG63 cells to superpulsed laser irradiation, using the following protocol: every 24 h for the first 5 days, then every 48 h until day 20. The nominal wavelength was 904–910 nm, pulse width 200 ns of pulse duration, peak power of 33 W, average out power of 200 mW, frequency 30 kHz, exposure time 300 s, radiant energy per irradiation 60 J and total energy of 720 J for each well (target size 9 cm2). The radiant exposure per irradiation was 6.7 J/cm2. The authors observed inhibition of proliferation of MG63 cells between 10 and 20 days, but they also reported an increase in alkaline phosphatase expression.
Aleksic [7] conducted a study with an Er:YAG pulsed laser in which several parameters were varied: Energy-output-dependent effect: the laser was fixed at 30 Hz and 30 s, and pulse energy at 23, 39, 50 and 68 mJ (total radiant exposure of 2.1, 3.6, 4.7 and 6.4 J/cm2, respectively). Time-dependent effect: the laser was fixed at 30 Hz and 23 mJ/pulse, and the irradiation time was set at 30, 60, 90 and 120 s (radiant exposure 2.1, 4.3, 6.7 and 8.6 J/cm2, respectively). Pulse-rate-dependent effect: the laser was fixed at 23 mJ/pulse and 30 s and the pulse rate was set at 10, 20, 30, 40 and 50 Hz (radiant exposure 0.7, 1.4, 2.1, 2.9 and 3.6 J/cm2, respectively). Pulse-rate-dependent effect: energy of 50 mJ/pulse and a shorter irradiation time of 20 s were used with the pulse rate set at 10, 20 and 30 Hz (radiant exposure 1.0, 2.1 and 3.1 J/cm2, respectively). Time-dependent effect in the presence of medium: irradiation was performed with the cell surface covered with a minimal amount (0.5 ml) of α-MEM without phenol red (Invitrogen, Carlsbad, CA). The energy level was set at 23 mJ/pulse, pulse rate at 30 Hz and irradiation time at 1, 2, 2.5, 3, 3.5 and 4 min (radiant exposure 4.3, 8.6, 10.8, 12.9, 15.1 and 17.2 J/cm2, respectively).
Amongst all these combinations of parameters, the authors found some that significantly increased cell proliferation:
-
30 Hz, 30 s, 39 mJ/pulse and 3.6 J/cm2;
-
30 Hz, 23 mJ/pulse, 30 s and 60 s, 2.1 and 4.3 J/cm2 (respectively); irradiation for 90 and 120 s (radiant exposure 6.7 and 8.6 J/cm2, respectively), however, resulted in significantly lower proliferation rates;
23 mJ/pulse, 30 s, pulse rate of 40 and 50 Hz (RE: 2.9 and 3.6 J/cm2, respectively);
-
50 mJ/pulse, 20 s, pulse rate of 10 and 20 Hz (RE 1.0 and 2.1 J/cm2, respectively);
-
23 mJ/pulse and 30 Hz, respectively, in the presence of medium, irradiation for 3 and 3.5 min (RE 12.9 and 15.1 J/cm2, respectively).
Renno et al. [8] concluded that each cell type responds differently to each wavelength and irradiation parameters, ie by changing the parameters, the cellular response changes. Saracino et al. [22] used a different radiation, - superpulsed light -, realized that this type of irradiation decreases cell growth and thus concluded that it has heterogeneous properties because it induces the expression of mediating molecules of bone formation and increases calcium deposits. Already, Aleksic [7] found greater cell proliferation at approximately 1–15.1 J/cm2 doses, and induction of extracellular signal regulated protein kinase phosphorylation (MAPK/ERK) 5 at 30 min after irradiation, which confirms results obtained In other studies that the Er:YAG laser can promote bone healing after periodontal and perimplant therapy.
Effect of photobiomodulation on osteoblastic cell proliferation
Most authors used diode lasers or HeNe lasers in the red to near-infrared spectral region but three studies used expensive solid state pulsed lasers Nd:YAG and Er:YAG. Both types of laser produced positive results despite being pulsed. Thus from cw to a few Hz to KHz, there is a lack of data to indicate that the pulse rate affects cell proliferation [20].
Another important parameter is the wavelength. The studies reviewed herein varied the wavelength from the red 633 nm to the mid-IR 2700 nm Er:YAG laser. This latter wavelength is strongly absorbed by water and thus the researcher must be careful to avoid a long optical path in which the photons are absorbed by the culture medium. Nevertheless, one or more authors reported positive results with each of the analyzed wavelengths; thus, this parameter seems to have a limited influence on osteoblast proliferation.
In general, most studies were conducted in the radiant exposure range of 1–3 J/cm2. The radiant power itself only has a small influence on the outcome, but the irradiance plays a major role. The radiant exposure is related to the overall amount of radiant energy deposited on the surface of the culture and the irradiance is related to how fast this energy is delivered. Irradiance in the range of a few tens of mW/cm2 increased proliferation in most cultures, while lower or much higher levels of irradiance resulted in a lack of statistical differences or even deleterious effects [24].
Effect of photobiomodulation on alkaline phosphatase expression and activity in osteoblastic cells
Fewer authors studied the expression and activity of alkaline phosphatase (compared to proliferation); nonetheless, of the 11 studies that investigated these parameters regardless of the type of cell, ten reported an increase (three activity and seven expression) with one or more irradiation parameters. Seven authors reported a lack of significant differences but no one reported deleterious effects, even for very high exposures.
Conclusions
The main drawback of this review is the variation in the irradiation parameters in the different studies, which, in addition to the lack of standardization on how to report radiometric parameters, make it difficult to draw general conclusions about the effects of photobiomodulation on osteoblastic-like cells. Recalculating the radiometric parameters based on the information given by the authors was also difficult due to the authors’ mistakes.
The works analyzed for this review suggest that osteoblastic-like cells are susceptible to photobiomodulation but that most of the light parameters varied by different authors have little to no influence on proliferation.
Different temporal regimes (pulsed/gated or cw), radiant exposures and wavelengths—even the unusual 2700 nm erbium laser—have had positive effects on proliferation and/or alkaline phosphatase activity but very high levels of irradiance have demonstrated deleterious effects on proliferation, highlighting the bi-phasic effect of photobiomodulation. The same parameters were unable to significantly decrease alkaline phosphatase activity, meaning that it is less susceptible to the deleterious effects of light irradiation.
References
Frare JC, Nicolau RA (2008) Clinical analysis of the effect of laser photobiomodulation (GaAs-904 nm) on temporomandibular joint dysfunction. Braz J Phys Ther 12(1):37–42
Schalch TD, Ferrari RAM, Souza NHC, Albarelo PM, França CM, Bussadori SK et al (2013) Effect of steroid nandrolone decanoate on osteoblast-like cells. Med Sci Technol 54:107–111
Asai T, Suzuki H, Kitayama M, Matsumoto K, Kimoto A, Shigeoka M, Komori T (2014) The long-term effects of red light-emitting diode irradiation on the proliferation and differentiation of osteoblast-like MC3T3-E1 cells. J Med Sci 60(1):E12-E18
Khadraa M, Lyngstadaasb SP, Haanæsa HR, Mustafac K et al (2005) Effect of laser therapy on attachment, proliferation and differentiation of human osteoblast-like cells cultured on titanium implant material. Biomaterials 26:3503–3509
Ozawa Y, Shimizu N, Kariya G, Abiko Y (1998) Low-energy laser irradiation stimulates bone nodule formation at early stages of cell culture in rat calvarial cells. Bone 22(4):347–354
Stein A, Benayahu D, Maltz L, Oron U (2005) Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in vitro. Photomed Laser Surg 23:161–166
Aleksic V, Aoki A, Iwasaki K, Takasaki AA, Wang CY, Abiko Y, Ishikawa I et al (2010) Low-level Er:YAG laser irradiation enhances osteoblast proliferation through activation of MAPK/ERK. Lasers Med Sci 25:559–569. https://doi.org/10.1007/s10103-010-0761-5
Renno ACM, Donnell PONA, Laakso EL (2010) The effects of laser irradiation on osteoblast and osteosarcoma cell proliferation and differentiation in vitro. Photomed Laser Surg 25(4):275–280. https://doi.org/10.1089/pho.2007.2055
Mackie EJ (2003) Osteoblasts: novel roles in orchestration of skeletal architecture. Int J Biochem Cell Biol 35:1301–1305
Dimitriou R, Jones E, McGonagle D, Giannoudis PV (2011) Bone regeneration: current concepts and future directions. BMC Med 31(9):66. https://doi.org/10.1186/1741-7015-9-66
Harris SA, Enger JR, Riggs BL, Spelsberg TC (1995) Development and characterization of a conditionally immortalized human fetal osteoblastic cell line. J Bone Miner Res 10(2):178–186
Coombe AR, Ho CT, Darendeliler MA, Hunter N, Philips JR, Chapple CC, Yum LW (2001) The effects of low level laser irradiation on osteoblastic cells. Clin Orthod Res 4(1):3–14
Fujihara NA, Hiraki KRN, Marques MM (2006) Irradiation at 780 nm increases proliferation rate of osteoblasts independently of dexamethasone presence. Lasers Surg Med 38:332–336
Xu M, Deng T, Mo F, Deng B, Lam W, Deng P, Zhang X, Liu S (2009) Low-intensity pulsed laser irradiation affects RANKL and OPG mRNA expression in rat calvarial cells. Photomed Laser Surg 27(2):309–315. https://doi.org/10.1089/pho.2008.2283
Arisu HD, Türköz E, Bala O (2006) Effects of Nd:Yag laser irradiation on osteoblast cell cultures. Lasers Med Sci 21:175–180. https://doi.org/10.1007/s10103-006-0398-6
Petri AD, Teixeira LN, Crippa GE, Beloti MM, Oliveira PT, Rosa AL (2010) Effects of low-level laser therapy on human osteoblastic cells grown on titanium. Braz Dent J 21(6):491–498
Chellini F, Sassoli C, Nosi D, Deledda C, Tonelli P, Orlandini SZ, Formigli L, Giannelli M (2010) Low pulse energy Nd:YAG laser irradiation exerts a biostimulative effect on different cells of the oral microenvironment: “an in vitro study”. Lasers Surg Med 42:527–539
Oliveira DAAP, Oliveira RF, Zangaro RA, Soares CP (2008) Evaluation of low-level laser therapy of osteoblastic cells. Photomed Laser Surg 26(4):401–404. https://doi.org/10.1089/pho.2007.2101
Ueda Y, Shimizu N (2003) Effects of pulse frequency of low-level laser therapy (LLLT) on bone nodule formation in rat calvarial cells. J Clin Laser Med Surg 21(5):271–277
Stein E, Koehn J, Sutter W, Wendtlandt G, Wanschitz F, Thurnher D, Baghestanian M, Turhani D (2008) Initial effects of low-level laser therapy on growth and differentiation of human osteoblast-like cells. Wien Klin Wochenschr 120(3):112–117. https://doi.org/10.1007/s00508-008-0932-6
Bloise N, Ceccarelli G, Minzioni P, Vercellino M, Benedetti L, MGC DA, Imbriani M et al (2013) Investigation of low-level laser therapy potentiality on proliferation and differentiation of human osteoblast-like cells in the absence/presence of osteogenic factors. J Biomed Opt 18(12):128006
Saracino S, Mozzati M, Martinasso G, Pol R, Canuto RA, Muzio G (2009) Superpulsed laser irradiation increases osteoblast activity via modulation of bone morphogenetic factors. Lasers Surg Med 41:298–304
Cankaya, Erdem MA, Erdem AP, Erguven M, Aybar B, Kasapoglu C et al (2011) Evaluation of light-emitting diode (LED-660 nm) application over primary osteoblast-like cells on titanium surfaces: an in vitro study. Int J Med Sci 8(7):584–593
Emes Y, Akça K, Aybar B, Yalçın S, Çavuşoğlu Y, Baysal U, Işsever H, Atalay B, Vural P, Ergüven M, Çehrel MC, Bilir A (2013) Low-level laser therapy vs. pulsed electromagnetic field on neonatal rat calvarial osteoblast-like cells. Lasers Med Sci 28:901–909. https://doi.org/10.1007/s10103-012-1165-5
Ke D, Zhang LY, Yang Z, Xu ZJ (2013) A promising injectable scaffold: the biocompatibility and effect on osteogenic differentiation of mesenchymal stem cells. Biotechnol Bioprocess Eng 18:155–163. https://doi.org/10.1007/s12257-012-0429-z
Shen CY, Li L, Feng T, Rong J, Yu M, Lu Q, Li H (2015) Dental pulp stem cells derived conditioned medium promotes angiogenesis in hindlimb ischemia. Tissue Eng Regen Med 12(1):59–68. https://doi.org/10.1007/s13770-014-9053-7
Silva APRB, Petri AD, Crippa GE, Stuani AS, Stuani AS, Rosa AD, Stuani MBS (2012) Effect of low-level laser therapy after rapid maxillary expansion on proliferation and differentiation of osteoblastic cells. Lasers Med Sci 27:777–783. https://doi.org/10.1007/s10103-011-0968-0
Schwartz-Filho HO, Reimer AC, Marcantonio C, Marcantonio EM Jr, RAC M (2011) Effects of low-level laser therapy (685 nm) at different doses in osteogenic cell cultures. Lasers Med Sci 26:539–543. https://doi.org/10.1007/s10103-011-0902-5
Danti S, Serino LP, D’Alessandro D, Moscato S, Danti S, Trombi L, Dinucci D, Chiellini F, Pietrabissa A, Lisanti M, Berrettini S, Petrini M (2013) Growing bone tissue-engineered niches with graded osteogenicity: an in vitro method for biomimetic construct assembly. Tissue Eng Part C Methods 19(12). https://doi.org/10.1089/ten.tec.2012.0445
Huertas RD, Luna-Bertos E, Torrecillas JR, Leyva FM, Ruiz C, Martı ́nez OG (2014) Effect and clinical implications of the low-energy diode laser on bone cell proliferation. Biol Res Nurs 16:191–196. https://doi.org/10.1177/1099800413482695
He YF, Ma Y, Gao C, Zhao G, Zhang LL, Li GF et al (2013) Iron overload inhibits osteoblast biological activity through oxidative stress. Biol Trace Elem Res 152:292–296. https://doi.org/10.1007/s12011-013-9605-z
Kwon H, Lim W, Kim J, Jeon S, Kim S, Karna S et al (2013) Effect of 635 nm irradiation on high glucose-boosted inflammatory responses in LPS-induced MC3T3-E1 cells. Lasers Med Sci 28:717–724. https://doi.org/10.1007/s10103-012-1122-3
Zeng XB, Hu H, Xie LQ, Lan F, Jiang W et al (2012) Magnetic responsive hydroxyapatite composite scaffolds construction for bone defect reparation. Int J Nanomedicine 7:3365–3378
Ma WH, Liu YJ, Wang W, Zhang YZ (2015) Neuropeptide Y, substance P, and human boné morphogenetic protein 2 stimulate human osteoblast osteogenic activity by enhancing gap junction intercellular communication. Braz J Med Biol Res 48(4):299–307. https://doi.org/10.1590/1414-431X20144226 ISSN 1414-431X
Peplow PV, Chung TY, Baxter PD (2010) Laser photobiomodulation of proliferation of cells in culture: a review of human and animal studies. Photomed Laser Surg 28(1):S3–S40. https://doi.org/10.1089/pho.2010.2771
Zhao KW, Murray EJB, Murray SS (2015) Spp24 derivatives stimulate a Gi-protein coupled receptor-Erk1/2 signaling pathway and modulate gene expressions in W-20-17 cells. J Cell Biochem 116:767–777
Wang X, Tian F, Soni SS, Gonzalez-Lima F, Liu H (2016) Interplay between up-regulation of cytochrome-c-oxidase and hemoglobin oxygenation induced by near-infrared laser. Sci Rep 6:30540
Wang Y, Huang YY, Wang Y, Lyu P, Hamblin MR (Feb 2017) Photobiomodulation of human adipose-derived stem cells using 810nm and 980nm lasers operates via different mechanisms of action. Biochim Biophys Acta 1861:441–449
Funding
There was no funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This work is a review; therefore, no ethical committee approval is required.
Informed consent
This work is a review; therefore, no informed consent was necessary.
Rights and permissions
About this article
Cite this article
Deana, A.M., de Souza, A.M., Teixeira, V.P. et al. The impact of photobiomodulation on osteoblast-like cell: a review. Lasers Med Sci 33, 1147–1158 (2018). https://doi.org/10.1007/s10103-018-2486-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-018-2486-9