Abstract
Different ideas have been presented to describe the mechanism of augmented laser ablation of dental enamel with different shapes by adding water to the working environment. In this study, the influence of water–laser interaction on the surface of enamel during ablation was investigated at a wavelength of 2.94 μm with different distances between the laser tip and the enamel surface. A motion-control system was used to produce linear incisions uniformly on flat enamel surfaces of bovine anterior teeth, with free-running Er:YAG laser very short pulses (pulse length = 90–120 μs, repetition rate = 10 pulses per second). Four different output energies (100, 200, 300 and 400 mJ) were radiated on samples under distilled water from different distances (0.5, 0.75, 1, 1.25, 1.75 and 2.00 mm). The tooth slices were prepared with a cutting machine, and the surfaces of the ablated areas were measured with software under a light microscope. The average and standard deviation of all cut areas in different groups were reported. There was no significant difference when using a different pulse ablation speed (cm3/J) and a water-layer thickness between the tip and enamel surface of 0.5–1.25 mm with energy densities of 30–60 J/cm2 (200–400 mJ). However, using an output energy of 15 J/cm2 (100 mJ) and a thicker water layer than 1 mm, a linear ablation did not take place. This information led to a clearer view of the efficiency of Er:YAG laser in the conditions of this study. There are several hypotheses which describe a hydrokinetic effect of Er,Cr:YSGG. These basic studies could guide us to have a correct attitude regarding hydro-mechanical effects of water, although the wavelength of 2.78 μm has a better absorption in hydroxyl branch of water molecules. Therefore, our results do not directly interrupt with the series of investigations done with Er,Cr:YSGG. Water propagation and channel formation under water are investigated during the ablation of tooth enamel with the Er:YAG laser from different distances. Comparing the results of this study with the same research done with water/air spray concludes that the bubble formation and channel propagation in water with this wavelength leads to a more symmetric (linear) ablation process with cavity-preparation-recommended parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, a growing interest is seen in using the benefits of hydro-mechanical properties of water droplets for more efficient ablation of dental hard tissue with Er-YAG (2,940 nm), Er-YSGG (2,790 nm) and Er,Cr-YSGG (2,780 nm) laser irradiation.
The effect of 2,940 nm laser irradiation on tooth enamel and dentin preparation is improved by using suitable amounts of aerosol. Also, the thermal effects are reduced by these aerosols with different percentages of additional water/air spray along with the laser beam. The laser should be applied in a way that the rise in temperature in the pulp cavity can be limited to 3°C [1].
The actual mechanism of augmented ablation in this condition is not yet clear, and different ideas are presented to be evaluated. Majaron et al. [2, 3] investigated the volume of craters produced by Er:YAG pulses on enamel. They found out that the dependence of the craters’ surface cross-sections on the laser fluence can be described by a linear approximation.
Different investigators have chosen several methods to find out the influence of additional water on the enamel ablation process, but there has not yet been a study reporting on tooth and laser tip completely submerged in water.
When the Er:YAG laser is irradiating freely under water because of the channel propagation of strongly absorbed laser radiation, the absorption depth is much smaller than the beam waist. Therefore, it can be assumed that the incident laser energy is absorbed instantaneously, without losses due to heat conduction or light scattering to the surrounding material. As we know, heating of a surface layer by absorption leads to evaporation. So, the forces considered to be relevant to the channel propagation are the vapour pressure inside the channel, the recoil pressure on the surface of the evaporation region, the liquid viscosity and the surface tension [4, 5].
Furthermore, in a series of studies, the mechanisms of channel formation in water and influence of pulse duration on the ablation of bio-tissue under water have been evaluated. It is mentioned that although erbium-laser radiation is strongly absorbed by water, it is possible to ablate tissue in a non-contact mode under water. The laser radiation is transmitted through a water-vapour channel initiated around the fibre tip by the leading part of the laser pulse. This is possible because steam, in contrast to liquid water, first of all has a 100–1,000 times lower density and second of all a low absorption coefficient.
The laser radiation is transmitted through a water-vapour channel created by the leading part of the laser pulse. Strong pressure transients with amplitudes up to a few hundreds of bars measured with a needle hydrophone were found to accompany the channel-formation process. Additional pressure transients in the range of kilobars were observed after the laser pulse, associated with the collapse of vapour-channel transmission measurements; this revealed that the duration of the laser-induced channel stays open and therefore the energy transmittable through it is substantially determined by the laser-pulse duration. The optimum pulse duration was found to be in the range between 250 and 350 μs. Increasing the pulse duration from 300 to 800 μs leads to a decrease in crater depth from 1,600 to 300 μm.
The zone of thermally damaged tissue along the laser incisions after underwater treatment decreases from 40 μm to less than 10 μm with increasing crater depth, whereas after treatment through the air the zone of thermally damaged tissue has a constant thickness of about 60 μm. The smaller thermal damage is due to the cooling effect of the surrounding water [6].
Three aspects of the cavitation bubble dynamics can contribute to tissue ablation in a liquid environment: (a) the material erosion by the elastic rebound of the tissue that is deformed during bubble expansion, (b) the suction force exerted by the collapsing bubble, and (c) the impact of the high-velocity jet generated during bubble collapse. Figure 1 presents results of numerical simulations of the bubble dynamics proximal to an elastic boundary which shows the material ejection upon bubble collapse. While the suction force enhances the material removal only for very soft tissues, the elastic rebound can also remove tissues with moderate strength, and the jet impact can erode even hard materials with high mechanical strength.
Numerical simulations showing the dynamics of the cavitation bubble generated during pulsed laser ablation of an elastic tissue-like material together with the bubble-induced material response. The elapsed time after the instantaneous energy deposition is indicated below each frame. Ablation is enhanced by jet-like material ejection during the bubble collapse
The influence of the cavitation bubble dynamics on ablation in a liquid environment is equivalent to the role played by the hydrodynamics of the ablation plume in a gaseous environment.
In both cases, the precision and efficiency of the ablation are substantially influenced by events occurring long after the direct interaction between laser radiation and tissue [7].
Although the effect of a secondary bombardment by particles and water droplets is proposed by other investigators, some studies reveal that using a suspension of sapphire particles in water allows a threefold increase in efficiency of enamel removal compared to the use of an Er-YAG laser with water spray alone. Leaving the point of beam–tissue interaction, the enamel particles are accelerated by the process of ablation, and these particles are also able to remove enamel very effectively [8, 9].
It is interesting to consider that several studies of hard-tissue ablation with Er:YAG lasers have shown that adding an optically thick water layer (1 mm) on the surface of dental enamel before each incident laser pulse profoundly influences the rate and efficiency of ablation and the resulting surface morphology. It is described that the water prevents the formation of non-apatite calcium phosphate phases on the enamel surface [10].
In this study, the authors are going to investigate the effects of this hydro-mechanical basis on the velocity of tooth-enamel ablation in different distances between tooth-enamel surface and laser tip, which both are submerged in pure water.
The chemical components present in hard tissue such as water, phosphate, carbonate and organic material strongly absorb infrared radiation. Transmission and reflection spectra in the infrared range from 2.5 to 25 μm of the enamel as well as dentine tissues from human and bovine teeth were acquired in a study by Bachman et al. According to their investigation, no differences were found between the mineral matrix of human and bovine tissues [11].
Materials and methods
The extracted bovine anterior teeth were cleaned and saved in normal saline for 24 h. The enamel surface of the teeth was gritted with gritting paper number 800 by using a rotary machine.
An Er:YAG dental laser (Fidelis Plus II; Fotona; Slovania) with a very short pulse (pulse length ~90–120 μs) was used for cutting the teeth enamel under pure water. The beam profiles on the tooth surface from different distances consisting of 0.25, 0.5, 0.75 and 1–5 mm were measured according to the pulse over lap factor with help of the knife edge method. (Fig. 1) The knife edge method was performed as the following: The beam path was fitted with a beam splitter, so that the energy could be measured (two-channel energy meter and detectors by laserprobe) simultaneously during each irradiation process. The energy density of the laser beam at the location of the sample was determined in every second measurement. This was achieved by using the ‘knife-edge’ method, in which a razor blade is moved perpendicularly through the beam on a micro-positioner. The energy profile behind the blade is recorded by a detector. The first differentiation of the measured curve corresponds to the profile of the laser beam (determined at 1/e2 intensity level), provided that a Gaussian distribution and radial symmetry are ensured. Both prerequisites are made by using a fluoride glass fibre. Figure 2 shows the measurement of the beam profile in the plane of irradiation. This made it possible to determine the energy density of the laser beam on the surface of the sample and to determine the ablation threshold values [12].
All of the teeth were irradiated with the Er:YAG, using very short pulses (pulse length = 120 μs), a repetition rate (frequency) of 10 Hz, and energy fixed according to the requirement.
Before beginning any procedure, the laser output energy was measured by an energy meter (LaserProbe, Utica, NY, USA).
The different output energies which were used are the following: 100, 200, 300 and 400 mJ.
Every tooth enamel surface was fixed parallel to the tip surface. Each tooth was placed in a retainer and fixed with flexible paste. Then the whole complex was placed in a transparent glass, and distilled water was poured into the glass box. The box was moved by a motor (OWIS GmbH, Staufen, Germany) which was programmed and controlled by a computer (software developed by Dr. R. Franzen, AALZ GmbH, Aachen, Germany). The speed of the motor was set to 460 μm/s according to the knife edge method results (Fig. 1). The motor was programmed from position 0 to 11,000 μm. This means that the cuts were made continuously on the tooth with a width of 11,000 μm.
Moreover, the laser tip was also submerged into distilled water. The distance between the enamel surface and laser tip was set by a micrometer movement plate. The following distances were investigated: 0.5, 0.75, 1.00, 1.25, 1.50, 1.75 and 2.00 mm.
A diode laser was also applied to the tip of the handpiece to guide the operator in focusing the Er:YAG laser beam on the tooth surface (Fig. 3). On gritted area of every tooth enamel, three cuts, starting from the incisal tip of the tooth, were ablated by the laser energy. After completing the cuts on each tooth, the samples were saved in normal saline until proceeding to the histological examination process.
The teeth were cut axially to prepare thin slices with diameters near to the laser-beam diameter on the enamel surface. For this purpose, a cutting machine (Exakt Trennschleifsystem, Norderstadt, Germany) was used.
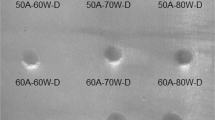
The slices were saved in normal saline, so that they could afterwards be fixed to the lams (slides) and also polished with a gritting machine using gritting paper no. 2400 (DP-U4, Struers, Denmark). The adequate thickness was obtained in the same way down to 70–120 μm to obtain a good transmission view under the microscope. The samples dried on a whipping tissue, and for every slice, an identification number was carved into the related lam. To measure the ablated area of the enamel which was positioned under the light microscope (Leica DMRX, Leica Mikroscopie und Systeme GmbH,Germany) the DISKUS software (DISKUS ver. 4.30.539, developed by Department of Dentistry IT support, Aachen hospital, Rheinisch-Westfälische Technische Hochschule, Germany) was used. A digital camera (Hitachi VH-C20A, Hitachi Kokusai Electric, Japan) was set on the microscope to transfer the field of view to the computer which was equipped with the above-mentioned software. The objective lens with a fivefold magnification was selected for the best view. The software area measurement tool was applied on all input images, and the output pictures were saved (Fig. 4). Furthermore, all measurements were checked again by a second examiner, and the collected data was reported and saved in MS-Excel data sheets for statistical analyses.
Results
The biggest amount of ablation is achieved with an output energy of 400 mJ and when the thickness of the water layer between the laser tip and tooth surface is 0.5 mm. Yet, the smallest ablation is reported with 100 mJ and a water layer thickness of 2 mm (Table 1).
However, when the data were analysed using the multiple analysis of variance test (P > 0.05) there was no significant difference found in the results, so only a comparison between the average and standard deviation of this collected data, according to the different output energy levels and water layer thicknesses, could be shown (n = 13; Fig. 5). Regarding the graph, the standard deviation especially in two of the groups is very high which shows that the samples are distributed in a wide range.
Therefore, to eliminate the interrupting variable of different energy outputs, the entire data was divided into these values after calculating the volume of cuts per pulse. The volume of cuts per pulse was easily reached by multiplying the data to the speed of motion (0.46 mm/s) to find the volume of a cut in 1 s. Moreover, to extract the volume of a cut per pulse, the current data were divided into 10 as the repetition rate for all experiments. In conclusion, the volume of cuts per pulse depending on the different water layer thickness was statistically analysed. The connection between the groups with a water layer thickness of 0.5 to 1.25 mm and energy outputs of 200, 300 and 400 mJ was statistically established (Fig. 6).
The amount of cuts (volume of cut per pulse) is called “ablation speed”; i.e. the speed of cut in 0.1 s. Although we imagine this information as a complex unit (mm3/J),the ablation process is a continuos procedure, so the real unit should be mm3/0.1 s.
After the information in the mentioned groups were correlated, the average and standard deviation of the volume of a cut per pulse according to the water layer thickness were depicted in a graph to show the linear correlation between the increase of the water layer thickness (mm) and volume of a cut per pulse (mm3/pulse). In this way, the standard deviation is controlled by eliminating the effect of different energy outputs as an interrupting factor (Fig. 7).
However, the “specific ablation energy (J/mm3)”, depending on the “integral energy density” (J/cm2) and the different water layer thickness, could be presented to declare such a co-efficiency.
Discussion
As is seen in Table 1 and Fig. 5, the standard deviation of different output energies is high, so according to statistics, we conclude that more samples are needed for so many groups. But by limiting the effect of different energy output amounts in this study, a suitable correlation between the data resulted to undertake a statistical analysis.
According to Mehl et al. [13], they found a 0.017 mm3/pulse average dentine and enamel ablation amount with the Er:YAG (250–400 mJ, 3–15 pps and 20–180 s proceeding time, beam diameter = 500 μm) in combination with water-spray (0.19 ml/s). This was the result of a 20 to 180-s lasting irradiation to a fix spot (diameter was about 500 μm) [13]. Our study revealed a similar mean ablation amount of enamel per pulse with the same range of energy output (0.025 mm3/pulse). Although the difference cannot be analysed statistically, it can be assumed that the volume of the cut under water is bigger than when using 0.19 ml/s water spray, especially when considering that Mehl et al. measured the average in enamel and dentin. However, in our study, the ablation amount only resulted from the enamel cuts, which is harder to ablate.
Two different but related mechanisms were investigated in different studies to describe the procedure of enamel ablation. First, the channel propagation by infrared lasers in a water layer and then the interaction between the water-layer and the laser radiation on the enamel surface were investigated which resulted in augmented ablation. Some studies are trying to establish a hydro-kinetic effect, in which water droplets in water spray are accelerated by the laser photons. The proposed mechanism of this hydro-kinetic system is that the Er,Cr-YSGG pulsed laser source delivers photons into an air–water spray matrix resulting in micro-explosive forces on the water droplets. This process is hypothesised to contribute significantly to the mechanism of hard tissue cutting [14–18].
The working length of this crystal emission is 2,780 nm and resulted in a good absorption of a hydroxyl branch of water molecules, so our future studies are focused on investigating the interactions of a 2,780-nm wavelength with water.
The results of this study performed with the Er:YAG are not directly comparable with the results of the Er:YSSG. Furthermore, keeping the samples and beam output tip in water dedicates a different hydro-mechanical condition that may only provide some guidelines for investigating the interaction of infrared radiation with water droplets and enamel surface.
There was no thermal damage seen on samples in any of the groups except when using an energy density of 100 mJ in a water-layer thickness of more than 1 mm. In these cases, the ablation did not take place linearly and symmetrically. However, this was not the result of energy densities lower than the threshold but rather the influence of the absorption of laser energy in water cavity in the first millimetre of water, preventing exposing rest of the energy to the surface.
Moreover, VSP (very short pulse = 90–120 μs) may be a good reason for preventing the zone of thermal damage; yet this still needs to be confirmed in further studies.
References
Gutknecht N (1999) Laser applications in dental practice (in German). Quintessence Publishing, Germany
Majaron B, Lukac M (1999) Thermo-mechanical laser ablation of hard biological tissue:modeling the micro-explosions. SPIE 3593:184–195
Majaron B, Sustercic D, Lukac M (1997) Influence of water spray on Er:YAG ablation of hard dental tisssues. In: Laffitte F et al (eds) Medical applications of lasers in dermatology, ophtalmology, dentistry and endoscopy. SPIE 3192
Forrer M, Frenz M, Romano V, Weber HP (1993) Channel propagation in water and gelatin by a free-running erbium laser. J Appl Phys 74(1):720–727
Brennen CE (1995) Cavitation and bubble dynamics. Oxford University Press, USA
Ith M, Pratisto H, Altermatt HJ, Frenz M, Weber HP (1994) Dynamics of laser-induce channel formation in water and influence of pulse duration on the ablation of bio-tissue under water with pulsed erbium- laser radiation. Appl Phys B 59:621–629
Vogel A, Venugopalan V (2003) Mechanisms of pulsed laser ablation of biological tissues. Chem Rev 103:577–644
Altshuler GB, Belikov AV, Sinelnik YA (2001) A laser-abrasive method for the cutting of enamel and dentine. Laser Surg Med 28:435–444
Altshuler GB, Belikov AV, Erofeev AV (2001) Comparative study of contact and non contact operation mode of hard tooth tissues Er-laser processing. 5th International Congress of the International Society of Laser Dentistry, 21–25 (Jarusalem, Israel, 1996)
Staninec M, Xie J, Le CQ, Fried D (2003) Influence of an optically thick water layer on the bond-strength of composite resin to dental enamel after IR laser ablation. Lasers Surg Med 33(4):264–269
Bachman L, Diebolder R, Hibst R, Zezell DM (2003) Infrared absorption bands of enamel and dentine tissues from human and bovine teeth. Appl Spect Rev 38(1):1–14
Apel C, Meister J, Ioana RS, Franzen R, Hering P, Gutknecht N (2002) The ablation threshold of Er:YAG and Er:YSGG. Lasers Med Sci 17:246–252
Mehl A, Kremers L, Salzmann K, Hickel R (1997) 3D volume-ablation rate and thermal side effects with the Er:YAG and Nd:YAG. Dental Mater 13:246–251
Rizoiu I, Kohanghadosh F, Kimmel AI, Eversole LR (1998) Pulpal thermal responses to an Er, Cr:YSGG pulsed laser hydrokinetic system. Oral Surg Med Path 86(2):220–223
Eversole LR, Rizoiu I, Kimmel AI (1997) Pulpal response to cavity preparations by erbium chromium: YSGG laser powered hydrokinetic system. JADA 128:1099–1106
Eversole LR, Rizoiu I (1995) Preliminary investigations on the utility of an erbium chromium:YSGG laser. CDA J 41–47
Rizoiu IM, DeShazer LG (1994) New laser matter interaction concept to enhance hard tissue cutting efficiency. Laser–Tissue Interaction V 2134A:309–317
Rizoiu I, Kimmel AI, Eversole LR (1996) The effect of an Er,Cr-YSGG laser on canine oral tissues. Laser applications in medicine and dentistry. SPIE 2922:74–83
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mir, M., Meister, J., Franzen, R. et al. Influence of water-layer thickness on Er:YAG laser ablation of enamel of bovine anterior teeth. Lasers Med Sci 23, 451–457 (2008). https://doi.org/10.1007/s10103-007-0508-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-007-0508-0