Abstract
One of the most essential methods for preserving water resources is water reuse. The present study has been aimed at adjusting a gray water treatment system with high purification efficiency, low cost, easy maintenance, and high availability in every environment. To this goal, a new simple hybrid model for gray water treatment was made. In this model after conducting the settlement and aeration processes, seven different combinations of polypropylene activated carbon and anthracite filters beside resins were utilized for removing the contaminants in single and combined forms with three repetitions, and the results of each step were compared with the available standards. The different flow rates of 5, 2, 1, 0.5, 0.25, and 0.1 l/min were passed through the selected system to assess their effects on the reduction efficiencies of the pollutants in the system. The instantaneous ozonation method was used at the ozone concentrations of 1, 2.5, and 5 g/h. The results suggested the weakness of each filter alone for reducing the pollutants. After investigating the seven combinations, the combined filter of “polypropylene + resin + activated carbon” was recognized as the best filter for nondrinking purposes. The pH accounted for the lowest change of 10%, and chlorine and BOD5 accounted for the highest omissions of 90% and 80%, respectively. The system displayed the best performance at the flow intensity of 0.25 l/min or 15 l/h. The minimum rate of ozone production 1 g/h was able to remove all the coliform within any periods. This treated gray water is suitable for nondrinking uses such as irrigation and washing places.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urban sewage contains a variety of chemical pollutants, such as toxic substances, heavy metals, organic matter, and living microorganisms (viruses, bacteria, fecal coliform, and fungi). The discharge of raw sewage into the environment and its contact with surface water, groundwater, and soil lead to the contamination of these valuable resources, and if consumed by humans, the risks of the spread of various diseases among people will increase. Until achieving a degree of assurance as one of the objectives of the purification process, wastewater will be still generated via sewage treatment, which can be beneficial for estimating part of the needs of the community to water resources for agricultural use, green space irrigation, aquaculture, recreational purposes, and industrial use. According to the Iranian publication, No. 117-3 (Anonymous 2015), the average household per capita consumption of water without green space has been approximately 150 L per person over 24 h in 2016, of which 30 L has been used for bathroom and toilet. Based on the estimations, the remaining per capita consumption includes 20 L, which has been lost as evaporation and other non-recyclable and aggregate wastes. Therefore, an amount of 100 L per day has turned into sewage with less microbial load, which is called gray water.
Gray water is domestic wastewater collected from washbasins, showers and/or baths, and washing machines (Eriksson et al. 2002) that has a much lower degree of pollution in terms of pathogenicity compared to blackwater (bathroom and toilet sewage); however, depending on its source, it can contain other more or fewer impurities than blackwater (Almeida et al. 1999; Jamrah et al. 2006; Cheng Leong et al. 2018). Removal of common impurities from water or sewage would not seem to be problematic in the current situation about the existing technologies, while gray water treatment systems can now enjoy sufficient stabilities to withstand the shock loads of pollutants (Hejranfar et al. 2014).
Using sewage for washing or irrigating the environment leads to human contact with the wastewater, which must be disinfected. Moreover, some plants and flowers are quite sensitive to microbes and pathogenic agents, while the presence of these factors in their irrigation water can disrupt their health or growth. In a study, the researcher considered the permitted use of sewage for irrigating plants, such as local fruit trees, berry trees, and coastal plants (e.g., maple, willow, birch trees, and aquatic plants) (Moradi Nafchi and Morshed 2015). Nevertheless, plants with edible roots or trees that are dried but still persistent are not advised to be irrigated with this water if not disinfected. Salt and sodium compounds, boron, and chlorine are the substances to be necessarily removed from gray water.
A hybrid treatment mechanism was applied to the gray water produced in rural areas, and the results indicated the effectiveness of this method. After employing cascade weir, aeration, mixing, and filtration, 83, 70, 83, 50, 97, 46, and 49% of COD, total dissolved solids (TDS), TSS, total hardness, oils, anions, and cations were removed, respectively (Pangarkar et al. 2010). Also, two types of gray water treatment systems were utilized in a study on a two- and four-floor building with and without a soil-and-earthworm layer. The presence of the mentioned layer significantly increased efficiency and decreased BOD and COD, while the water acidity was regulated in the neutral range as well. The suspended solids were placed above this layer and decomposed by the earthworms (Kharwadea and Khedikarb 2011). Using biological methods, a household gray water treatment and its application in a flush toilet were studied. To this purpose, they used sludge for natural digestion and determined the life of the used sludge to be 60 days. Then, they found that the treated gray water in this system almost lacked organic matter, while observing only 10% of COD in the output of this system, thus concluding its suitable application (Hocaoglu et al. 2013).
Gray water treatment was studied by building a 20-L tank containing three layers of gravel and sand. Based on the results, by passing the sewage through the three-layered filter, the dissolved oxygen in the water increased and parameters such as turbidity, COD, acidity, TSS, water hardness, and electrical conductivity (EC) were seen to decrease (Kundu et al. 2015). The alternating sequential filters (ASFs) of chitosan and alginate can well purify gray water of the bathroom for nondrinking uses (Oha et al. 2016). After purification, its turbidity was observed to be less than 2 NTU, while the amount of total soluble solids (TSS) was less than 30 ppm. Approximately 20% of chemical oxygen demand (COD) was also analyzed in this way (Oha et al. 2016). Three adsorbents (activated carbon (AC), Iranian natural zeolite (Z), and stabilized nano-zero-valent iron (nZVI)) in single and combined forms were applied to treat gray water from student hostel of Fasa University. The results also indicate that single adsorbents alone are not adequate to guarantee a sufficient reduction in COD, TDS, and turbidity, but the combination of AC, Z, and nZVI had the best performance in the gray water treatment (Amiri et al. 2019).
Based on the previous studies, various methods can be used to treat gray waters of varying degrees, but the investigation of a complete hybrid package (from aeration to disinfection) for the home user was not observed. Hence, the present research has been aimed at adjusting a gray water purification system with high treatment efficiency and easy maintenance to be available in any homemade, residential, official, or industrial environments.
Materials and methods
Characteristics of the studied gray water
Gray water is commonly produced at different percentages via various sources (washing machines, dishwashers, kitchen sinks, basins, house runnels, and baths). The gray water in this study belonged to an Iranian household living in Ahvaz, and the study period was 6 months, from May to the middle of October.
Gray water sampling
In this research, the three sources of the washing machine, dishwasher, and bath were used to take the gray water samples. Since there was no separate pipework, the sewage of each source was separately collected, and then, the mixing percentages were achieved based on the data taken from Journal 117-3 of the Plan and Budget Organization of the Iranian Ministry of Energy as presented in Table 1. Therefore, it could be said that in each volume of the gray water, there were ten, four, and three shares of the sewage generated in the bath and by the washing and dishwashing machines, respectively. The obtained ratios of the samples in all the experiments and repetitions were reported in this research.
Model development
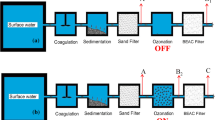
The algorithm shown in Fig. 1 was used to develop the proposed model in this study. Of course, all the filters were included in this algorithm. Regarding the purpose of the test, each filter was first investigated separately, and then, the seven combinations of the filters were examined with three repetitions. Figure 1 presents the complete filtration system.
Screens were applied to remove heavy waste materials from the sewage of the washing and dishwashing machines and the bathrooms (e.g., hair). A glass settlement and aeration tank were selected with the dimensions of 30 × 40 × 60 cm and an approximate volume of 70 L. Foam and oil isolations from the solutions were associated with the aeration and oxidation of some elements due to their compositions with oxygen. The purpose of this unit was to provide their sedimentations. The next unit included a physical purification with a polypropylene filter to remove the suspended particles like sand, silt, mud, algae, rust, and decayed particles separated from the inside layers of the pipes.
Depending on the testing type, other filters, such as resins, activated carbon, and anthracite, were added to the system. Cation and anion resins in this study were applied to reduce the amounts of TDS, EC, BOD, TSS, and some other organic and mineral materials, which were granular and branded as Purolite. The characteristics of the two cationic and anionic types are presented in Table 2.
Activated carbon in this study was used due to its high potential for mineral absorption and chlorine, color, and odor removal.
Disinfection is a crucial stage in the purification process. Ozone was utilized in the disinfection process in this system. The Venturi tube had to be employed for the instantaneous injection of ozone to the sewage. The oxygen capsule was attached to the ozone generator input, and the generated ozone was entered into the Venturi tube from the generator outflow to be thoroughly mixed with the gray water. The oxygen cylinder used in this system produced a concentration of 99.99% and the output pressure of 5000 kPa as indicated on the measuring gauge at 50 bar (approximately 732 psi), while the rotameter outflow was 10 l/min.
The last step of the purification system was to use an anthracite column to remove nitrate and further turbidity. The particle size of anthracite in this research was 0.8 mm.
Propylene activated carbon, cation and anion resins, and anthracite were poured into the cylindrical tank with a diameter of 7.5 cm and a height (cartridge) of 23 cm. After putting the cartridges in the compartments (housings), all the housings were attached to a chassis. The housing for polypropylene was transparent, while the other housings were non-transparent.
All the joints, tubes, and interfaces in the present study were 0.25 inches (6 mm). The gray water inside the tank was pumped through the purification system by using two pumps with a power of 30 W providing the maximum flow rate of 1500 l/h, and a single pump of height head 2.3 m could generate the same purification power as well.
Experiments and the measured parameters
Gray water was investigated and measured in terms of TSS, TDS, BOD, EC, pH, chloride, boron, calcium, magnesium, sodium, potassium, ammonia, phosphate, carbonate, bicarbonate, phosphate, nitrate, and coliform as the indices of pathogenic contaminations. The values obtained from the results were assessed for irrigating urban green spaces according to the standard values of Environmental Criteria for Treated Wastewater and Return Flow Reuse (Anonymous 2010). All the measurements were made based on the standard method (Anonymous 1998).
Measurements of EC, TDS, sodium, boron, pH, and turbidity were done by using an EC meter, a TDS meter, a flame photometer (Jenway PFP7 Flame Photometer), a BS-AA320N flame atomic absorption spectrometer (made by BIOBASE), a pH meter, and a WTW turbidity meter (Germany), respectively. A titration technique was employed to measure carbonate, bicarbonate, and chlorine. Nitrate, ammonia, and phosphate measurements were conducted by the DR 5000™ UV–Vis spectrophotometer (DR 5000, Hach Co., USA). The amount of biological oxygen demand (BOD) was measured with a particular BOD measurement machine (BSB/BOD Sensomat (AQUALYTIC, Germany) and BD (model 600)). Coliform measurement was done through the VRBA medium.
Results and discussion
Measurements of the amounts of the contaminants before purification
Twenty gray water samples were collected and prepared based on the intended compositions (Table 1) on different days, and the required measurements were done based on the standard methods for each contaminant type (Table 3).
All the measurements taken from the initial gray water samples suggested that the values of some significant elements, such as TSS, TDS, and BOD5, were higher than the maximum values permitted by the standards (Table 4). To reduce the concentrations of the contaminants, those types of filters were used, which were available, economically feasible, and applicable for more extended periods.
Measurements of the amounts of the pollutants after purification
Polypropylene fibers, granular activated carbon, weak anionic resins, weak cationic resins, and anthracite were the materials used as the filters. First, the effects of each filter on the reductions in the intended parameters were investigated (Table 4). Based on the results, pH, EC, boron, nitrate, ammonia, and TDS had a variation of 0–1% when passing through the polypropylene filter, which could be considered unchanged after a proper approximation. However, the TSS rate dropped by 60%, which was justifiable considering the size of the filter pores. This physical elimination resulted in a 38% reduction in turbidity. Sodium, chlorine, and bicarbonate decrease also resulted from the initial aeration and sedimentation processes or the presence of the chemical substances in the filter structure. The reduction in the BOD amount could be more related to the aeration than filter effects. This result is consistent with that of the study conducted by Azizi et al. (2015), emphasizing the impact of deep aeration on BOD alleviation.
The results of the activated carbon filter alone (Table 4) indicated no changes in the levels of pH, boron, and phosphate. The low amount of turbidity and TSS reduction resulting from using this filter were due to the quite large pores of the activated carbon compared to the polypropylene filter. EC and TDS decreases by 18% and 20% were related to both the surface and internal adsorption powers of activated carbon, respectively. Since the activated carbon structure consists of C4+, the reductions in some of the soluble materials in the gray water could be attributed to the absorption of the ions of opposite charges in the activated carbon. Chlorine is easily absorbed by activated carbon due to its negative charge in the adsorption phenomenon. Too much adsorption of the granular activated carbon led to chlorine declination by 70%.
Due to having an opposite electric charge to that of the activated carbon, bicarbonate, nitrate, and ammonia were absorbed and thus lowered; however, it seemed that the adsorption phenomenon did not occur identically for the different components and elements, which could be resulted due to the factor of “tendency to combine” and some other factors.
The above results are consistent with those obtained from the study conducted by Alizadeh and Borghaei (2009), who further discovered the effectiveness of activated carbon in the alleviations of BOD5 amount and turbidity by 92% and 84% in the sewage treatment of textile industry at the times of high stagnation. One of the most critical applications of activated carbon is chlorine removal, the fact that has been evidenced in many studies. In their studies, Chiang et al. (1997) found that activated carbon has currently provided one of the adequate approaches to the purification of priority organic pollutants and harmful compounds besides being widely utilized for softening hard water and removing excessively remaining chlorine, as well as desalting and deodorizing water (Chiang et al. 1997). In the study carried out by Prathapar et al. (2006), activated carbon led to the reductions in TSS and organic matter amounts by 50% and 40%, respectively, which are consistent with those of the present research. Also, the filter of granular activated carbon showed removal efficiencies for COD (56%), color (67%), TSS (33%), turbidity (38), and Zn (62%) from gray water (Cheng Leong et al. 2018).
The resin filter in this study was referred to as the simultaneous use of anion and cation resins. Resins led to the reductions in the amounts of many pollutants and ions (Table 4). The reductions in the amounts of EC, sodium, chlorine, bicarbonate, phosphate, nitrate, ammonia, and TDS could be ascribed to their decreases in the ion-exchange processes of the resins. The reduced TSS and turbidity were also due to the gray water passing through fine resin grains acting as a filter against the suspended particles. The strengths of the resins in exchanging ions and various compounds and removing them from the gray water were the basis of lowering the BOD amount, which was considered to be reasonable. The obtained results are well consistent with those of the previous studies. In one study, it was found that ion-exchange resins have good yields of removing the ions that intensify water hardness. This study showed that resins could well remove such ions as sulfate and nitrate from water. Calcium and magnesium, and consequently, water hardness were alleviated as well (Sadeghi et al. 2017). In the other research, the resin was used as a suitable agent to reduce water hardness, total minerals, water salinity (desalting), and hydrogen, which is congruent with the result achieved in this investigation (Ahmadpari et al. 2017). In one study, ion-exchange resins could best reduce up to 85% of BOD5, which is consistent with the result of the current study (Heydari et al. 2017).
Anthracite is one of the most widely used filters in water and wastewater treatments for reducing turbidity. The reasons behind the reductions achieved through an anthracite filter are highly similar to those of an activated carbon filter with the difference that the smaller anthracite grains convert anthracite into a more active filter for physically removing the pollutants. It should be noted that anthracite has a high tendency to absorb nitrate. In terms of reducing sodium, anthracite indicated similar performance to that of the activated carbon. The results of the previous studies on anthracite are properly in agreement with these results. In a study performed on an anthracite sample in the past, water turbidity had decreased by 90%. Also, anthracite significantly reduced BOD5 (Shah Mansouri and Alipour 2004).
Based on the results, each filter alone was not able to make the desired parameters reach the standard levels; hence, it was impossible to ignore the combined roles of the filters, and thus, the components of the filters were investigated. The seven components such as “primary filter + anthracite,” “primary filter + anthracite + activated carbon,” “primary filter + activated carbon + anthracite,” “primary filter + activated carbon + resin,” “primary filter + resin + activated carbon,” “primary filter + anthracite + resin,” and “primary filter + resin + anthracite” were studied, the results of which are presented in Table 4. These filters were used for removing the suspended matter and reducing the relative turbidity under the mentioned conditions. The samples were first aerated, and then, the solutions were left to reside. In the single and combined filters, the volumes of each filter were equal to three housings and one housing, respectively.
Applications of the different combinations yielded better results than a single filter. For selecting the best combination, the effects of each combined filter on the numbers of the reduced pollutants were evaluated by comparing them with the standard levels (Table 5).
According to Table 5, the best performance pertains to the combined filter of “polypropylene + resin + activated carbon,” which has reduced eight standard pollutants, including TSS and BOD5. The mechanisms selected in this study for the low-cost purification of gray water incorporated the trash racks of the settling and suspended materials, aeration and settling tanks, polypropylene filter, anion and cation resin filters, and activated carbon filter. After selecting the above combination, the gray water was treated, and the reduced amounts of the pollutants were measured after each stage. Table 6 exhibits the share of each filter in lowering the desired parameters. For instance, the 80% reduction in BOD5 was the result of 15, 15, and 50% decreases through the settlement and polypropylene filter (primary fiber), activated carbon, and resins. Based on this table, resins play a significant role in relieving the studied parameters. Friedler et al. (2005) combines the biological treatment (RBC) with physicochemical treatment (sand filtration and disinfection) in the pilot plant and could remove TSS, turbidity, BOD, and COD by 82%, 98%, 96%, and 75% respectively.
The pilot-scale hybrid treatment system was (a multimedia filter, a granular activated carbon filter, and ozone disinfection) applied by Cheng Leong et al. (2018) for gray water treatment (sourced from a mixture of showers/baths and laundry). This system removed 52% COD, 53% BOD5, 14% NH3-N, 67% PO4-P, 81% color, 81% turbidity, 50% total suspended solids (TSS), 53% of total coliform, 63% copper (Cu), and 29% zinc (Zn).
Selecting an adequate debit for the system
It was necessary to adjust the inlet flow rate of the system after selecting the purification algorithm. To this goal, the flow rates of 5, 2, 1, 0.5, 0.25, and 0.1 l/min were passed through the system, and the reduction efficiencies of the pollutants (e.g., BOD5 reduction as shown in Fig. 2) were obtained. In the present study, a proper flow rate played a quite significant role in the performance of the filters, while low flow rates in some cases led to their ineffective performance. The system showed the best performance at the flow rate of 0.25 l/min or 15 l/h. If the flow rate were high, the adsorbents and filters inside the system would lack sufficient time to exchange the ions and eliminate the wastes, thus being almost inefficient. In a rather low flow rate, the low pressure would cause flow stagnation, especially in the final filters, and it could be said that the system would not have an acceptable treated volume at the outlet, thus being unreliable.
Disinfection
Disinfection was conducted to ensure the safety of the treated water for nondrinking uses involving human contact. In this research, instantaneous ozonation was performed for disinfection. To evaluate the effects of ozone concentration and its adjustment, the concentrations of 1, 2.5, and 5 g/h were used for ozone production and injection. The coliform culture was indicative of negative results in all the three cases, confirming that the minimum amount of ozone (1 g/h) in this method could instantly eliminate all the gray water coliform and disinfect them.
Ozone has antimicrobial effects on a range of gram-positive and gram-negative bacteria and spores (Parish et al. 2003). With the progressive oxidation of vital compounds, ozone destroys microbe cells and prevents their growths. The results of this research demonstrated how ozone could destroy coliform. The above study corroborates the findings of the present investigation. Kim et al. (2009) treated gray water through a microfiltration membrane and oxidation process. This system could effectively remove COD, turbidity, color, and suspended solids, while OP was useful for the removal of E. coli, total coliform, Salmonella, and Staphylococcus.
Conclusion
The current investigation indicated that a combination of the most straightforward common filters in the market, i.e., polypropylene filter, resins, activated carbon, and anthracite, could well purify the studied gray water to the standard level. However, each filter was able to reduce only a certain number of the intended parameters and could not thus affect all the parameters. Filter arrangements and appropriate flow rates significantly changed the results of the present study. After examination, the filter of “polypropylene + resin + activated carbon” was found to have the best performance. This combination was more effective on the reductions in sodium, chlorine, phosphate, nitrate, ammonia, BOD5, TDS, and EC. The mentioned combined filter could determine if the amounts of the pollutants were within the permissible and standard ranges. For the best performance, the flow rate of 0.25 l/min or 15 l/h was regarded as the appropriate flow rate of the system. Any lower and higher flow rates would slow down the system performance and lower the purification efficiency, respectively. In this system, the disinfection process occurred through instantaneous ozonation, which led to the destruction of all the coliform in the gray water at the concentration of 1 g/h. This treated gray water is suitable for nondrinking usage.
References
Ahmadpari H, Hashemi Garmdarreh SE, Mohseni Fard M (2017) Investigation on ion exchange technology for water purification, 4th Scientific Congress on the development and promotion of agricultural sciences, natural resources, and the environment, Tehran (In Persian)
Alizadeh R, Borghaei SM (2009) Use of granular activated carbon in a carboniferous process to remove organic materials and textile waste drains. J Iran Chem Chem Chem Eng 25(4):21–28 (In Persian)
Almeida MC, Butler D, Friedler E (1999) At-source domestic wastewater quality. Urban Water 1(1):49–55
Amiri MJ, Bahrami M, Badkouby M, Ioannis K, Kalavrouziotis K (2019) Greywater treatment using single and combined adsorbents for landscape irrigation. Environ Process 6(1):43–63
Anonymous (1998) Standard methods for the examination of water and wastewater, 20th edn., APHA, AWWA, WEF
Anonymous (2010) Environmental criteria of treated wastewater and return flow reuse, vice presidency for strategic planning and supervision. J Islamic Republic Iran (In Persian)
Anonymous (2015) Concepts and criteria for designing urban water supply plans. J 117–3. 2015 Tehran: Iran planning and budget organization, Center for economic and social documents and publications (In Persian)
Azizi M, Amini S, Hashemi H (2015) Evaluation of the role of the deep aeration system in reducing the amount of BOD and COD required in the biological treatment of sewage. In: 3rd National conference on new technologies in chemistry and chemical engineering, Ghouchan (In Persian)
Cheng Leong JY, Chong MN, Poh PE (2018) Assessment of greywater quality and performance of a pilot-scale decentralized hybrid rainwater-greywater system. J Clean Prod 172:81–91
Chiang PC, Chang EE, Wu JS (1997) Comparison of chemical and thermal regeneration of aromatic compounds on exhausted activated carbon. J Water Sci Technol 35(7):279–285
Eriksson E, Auffarth K, Henze M, Ledin A (2002) Characteristics of grey wastewater. Urban Water 4:85–104
Friedler E, Galil NI, Kovalio R (2005) On-site greywater treatment and reuse in multi-story buildings. Water Sci Technol 51(10):187–194
Hejranfar A, Mohammadi Hariri S, Alijani, MH (2014) Investigating the possibility of purification of grey water by nanofiltration, disinfection, and re-circulation. National Water Recycling Conference, an important solution for water crisis management, Tehran (In Persian)
Heydari F, Asafvari Sh, Abbasi M (2017) Investigating the methods of separation and purification of pollutants in grey water for reuse. In: 4th international conference on management and environmental planning, Tehran (In Persian)
Hocaoglu M, Atasoy E, Baban A, Orhon O (2013) Modeling biodegradation characteristics of grey water in a membrane bioreactor. J Membr Sci 429:139–146
Jamrah A, Al-Omari A, Al-Qasem L, Abdel Ghani N (2006) Assessment of availability and characteristics of Greywater in Amman. Water Int 31(2):210–220
Kharwadea AM, Khedikarb I (2011) Laboratory-scale studies on domestic grey water through vermifilter and non-vermifilter. J Eng Res Stud 2(4):35–39
Kim J, Song I, Oh H, Jong J, Park J, Choung Y (2009) A laboratory-scale greywater treatment system based on membrane filtration and oxidation process -characteristics of greywater from a residential complex. Desalination 238:347–357
Kundu S, Khedikar I, Sudame A (2015) Laboratory scale study for the reuse of greywater. J Mech Civil Eng 12(3):40–47
Moradi Nafchi, F, Ahmadi Nadoushan A, Mahmoudi Raad S, Morshed, M, Massah F, Mohammadi F (2015) Grey water recycling to reduce drinking water consumption. In: Third national climate conference, building, and energy conservation, Isfahan Province Engineering Organization (In Persian)
Oha K, Poh PE, Chong MN, Chan ES, Lau EV, Saint CP (2016) Bathroom greywater recycling using polyelectrolyte-complex bilayer membrane: advanced study of membrane structure and treatment efficiency. Carbohydr Polym J 148:161–170
Pangarkar B, Parjane S, Sane MG (2010) Design and economic performance of greywater treatment plant in a rural region. Int J Civil Environ Eng 2(1):1–5
Parish M, Beuchat LR, Suslow TV (2003) Methods to reduce/eliminate pathogens from fresh and fresh-cut produce. Comprehen Rev Food Sci Food Safety 2:161–173
Prathapar S, Ahmed M, Adawi SA, Al Sidiari S (2006) Design, construction, and evaluation of an ablution water treatment unit in Oman: a case study. Int J Environ Stud 63(3):283–292
Sadeghi S, Abbasi M, Asafvari Sh (2017) Investigating the possibility of using ion exchange resins to reduce hardness and TDS of drinking water to prevent corrosion and sedimentation. In: 4th National conference on science and engineering isolation, Babol (In Persian)
Shah Mansouri M, Alipour V (2004) Comparison of the efficiency of anthracite of indoor production and imported types in water treatment operations. J Shahid Sadoughi Univ Med Sci Health Serv Yazd 1:48–53 (In Persian)
Acknowledgements
The authors are grateful to the Research Council of the Shahid Chamran University of Ahvaz for financial support (GN.95.3.02.31400).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Naghsh Javaheri, M., Tishehzan, P. & Moazed, H. Development of a complete and straightforward hybrid model for gray water treatment. Clean Techn Environ Policy 22, 1745–1753 (2020). https://doi.org/10.1007/s10098-020-01913-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-020-01913-z