Abstract
A novel approach for the complete utilization of a low-grade banded hematite jasper ore assaying ~ 47% Fe has been taken in order to address the issue of rapid consumption of high-grade iron ores and the rising concerns of waste disposal. The process includes the enrichment of Fe through reduction roasting followed by magnetic separation, and smelting of the non-magnetic fraction to produce ferrosilicon alloy. The optimum values of iron grade of ~ 66% Fe and recovery of ~ 72% as determined by the Taguchi-based statistical design of experiments have been achieved at a temperature of 900 °C, time of 90 min, coal-to-feed ratio of 0.15, coal of size − 3.35 + 1 mm and ore of size − 1 mm. The ore and the roasted products have been subjected to characterization techniques such as optical microscopy and X-ray diffraction that reveal the phase transformation under different conditions. Further, the smelting of the silica-rich non-magnetic part in a laboratory-scale electric arc furnace has resulted in a ferrosilicon alloy with ~ 20% Si as indicated by scanning electron microscopic studies. This innovative approach of recovering the maximum iron values using reduction roasting and exploiting the non-magnetic reject as a silica source for ferrosilicon production has the potential to reduce industry’s reliance on high-grade iron as well as silica resources.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increasing demand of iron and steel along with the gradual depletion of high-grade iron ores necessitates the utilization of low-grade ores and rejects such as slimes and fines. So far as the Indian context is concerned, the national steel policy has set a crude steel production target of 300 Million Tonnes Per Annum (MTPA) by 2030 (Mazumdar 2017). In order to achieve this target, the Indian steel industry would need at least 450 MTPA of high-quality iron ore. However, with the existing hematite iron ore reserves, the steel industry can survive up to 30–35 years. Hence, it is time industries looked into the utilization of low-grade iron ore resources. The key difficulty in subjecting these low-grade ores to iron making is the presence of impurities like silica and alumina that need to be removed through beneficiation. The complex mineral phase association in most of these ores poses a hindrance in the physical separation processes. Therefore, new alternative beneficiation strategies are now receiving attention for treating such poorly liberated ores. Moreover, beneficiation processes end up creating a huge mass of tailings that are left unused. For this reason, a complete utilization would be achieved only if the waste generated can be converted to a valuable product.
Banded iron ores constitute a major portion of such low-grade iron resources. The low iron-bearing banded iron formations in India have been designated as banded hematite quartzite (BHQ), banded hematite jasper (BHJ) and banded magnetite quartzite (BMQ). All these varieties of rocks occur as separate bands/veins/patches/pocket deposits in and around the iron ore mines. They mostly occur in association with the iron ores either as the host rocks or as overburden materials and hence get generated during mining. There are several successful investigations on the beneficiation of BHQ ores. Most of these attempts including flotation (Rath et al. 2013; Kar et al. 2013) and magnetic separation (Das et al. 2010) could end up with a product having more than 63–64% Fe with an appreciable yield of over 60–65%. Similarly, a typical BMQ ore has been subjected to flotation successfully (Sahoo et al. 2014). However, most of the reports related to the beneficiation of BHJ ores suggest that they are relatively more difficult to beneficiate. For example, a BHJ ore of 41.9% Fe could result in a product grade of 51.4% Fe with an iron recovery of 69.9% using dry high-intensity magnetic separation and 57.3% Fe at an iron recovery of 68.5% through jigging (Makhija et al. 2013). Gurulaxmi et al. (2012) attributed the difficulty in beneficiation to the complex mineralogical association as their quantitative mineralogical studies of a BHJ ore assaying 40% Fe showed 80% liberation only for particles below 10 μm size. Similarly, Ghosh et al. (2012) studied four different low-grade BHJ ores from the Jharkhand–Odisha region and found highly compact occurrence of silica mineral with hematite, which is a hindrance in the physical separation processes. As the jasper phase is very fine-grained, it creates a problem in beneficiation even after grinding the ore to a very fine size (Das et al. 2012; Das and Mishra 2013). In another study, the electron microscopic analysis of jasper revealed the presence of a high amount of Fe in the form of submicron-sized hematite grains, which further explains why the physical beneficiation operations cannot separate the iron and silica phases efficiently (Sahoo et al. 2016).
As the conventional beneficiation of BHJ ores does not lead to a satisfactory grade or recovery, several alternative strategies have been applied for its upgradation. For example, Singh et al. (2015) used colloidal magnetic coating to enhance the magnetic separation of BHJ ore and achieved 60.2% Fe with 56% Fe recovery from a feed grade of 47.8% Fe as compared to 60.8% Fe with 51% Fe recovery in the case of magnetic separation without any coating. A recent report shows the application of reduction roasting followed by low-intensity magnetic separation where a BHJ ore containing 43.06% Fe could be upgraded to 60% Fe at a Fe recovery of about 85% (Das et al. 2018). However, most of the beneficiation processes as discussed above or already implemented in industries try to recover maximum iron values so as to utilize them in pellet making or in blending with the blast furnace feed. As a result, they produce a huge volume of siliceous or aluminous waste as the beneficiation tailings. In this connection, new processes for making useful products out of these tailings should be explored.
The siliceous tailings generated during beneficiation of low-grade iron ores can be thought of as a valuable resource for the production of ferrosilicon alloy. It may be noted that ferrosilicon alloy is conventionally produced through the smelting of various blends of quartz/quartzite ores, iron ore/iron chips/mill scale and fluxes. Due to its high corrosion resistance and good magnetic properties, ferrosilicon is well suited to heavy medium separation. For example, ferrosilicon is used as the media in the dense medium separation of the diamond-bearing gravels found along South Africa’s West Coast in order to reclaim the alluvial diamonds. At the end of each operation, ferrosilicon can be easily reclaimed from the process stream using a magnetic separator (Waanders and Rabatho 2005). Ferrosilicon acts as a source of silicon to reduce metals from the respective oxides and as a deoxidizing agent for the production of steel and other ferrous alloys. Similarly, it is also a raw material in the manufacture of corrosion-resistant and high-temperature-resistant alloys used in electromotors and transformer cores (Littmann 1982; Commission and Lukes 1984; Tangstad 2013; Farzana et al. 2016). In cast iron manufacture, ferrosilicon is employed for inoculation of the iron to accelerate graphitization (Zhukov et al. 1989). Other than this, ferrosilicon finds application in some electrode coatings used in arc welding and in the manufacture of pre-alloys like magnesium ferrosilicon (Sales 1977; Skaland 2004).
In the case of BHJ ore, both the iron and silicon are closely associated with each other, which may be beneficial for the production of low-grade ferrosilicon. So, a scientific attempt is necessary to transform it into an alternative silica source, thereby saving on the cost of input materials. In the present investigation, a BHJ ore has been subjected to reduction roasting followed by low-intensity magnetic separation (LIMS) in order to achieve a high-grade iron ore concentrate. The non-magnetic fraction thus obtained as the tailing has been subjected to smelting in an electric arc furnace (EAF) to produce ferrosilicon alloy. Further, characterization studies have been employed to understand the phase changes during reduction roasting and smelting.
Materials and methods
Materials
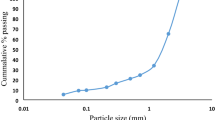
The BHJ ore was received from Barbil, Odisha, India. The chemical composition of the ore is given in Table 1. The total iron content of the ore is around 47% with the other major component being SiO2 with small amounts of Al2O3. The bulk density, specific gravity and moisture of the sample were found to be 2.51 g/cc, 3.64 and 1.51%, respectively. The iron content along with the weight percentages of the size fractions of the sample crushed to below 20 mm size is shown in Fig. 1. It is observed that the iron content is almost equally distributed in all the size fractions except in the finest size fraction of − 45 μm, which contains 39.93% Fe. Coal obtained from Australia was used as the reductant in this study. The proximate analysis of the coal is summarized in Table 2. The gross calorific value (GCV) of the coal was calculated as 6819 cal/g.
Beneficiation studies
The as-received sample was crushed to below 10 mm and thoroughly mixed, and the representative samples were drawn using the coning and quartering method. Several beneficiation studies like hydrocycloning, flotation and magnetic separation were carried out with the representative samples after crushing and grinding them to the required size. The direct flotation studies were carried out using oleic acid as the collector and sodium silicate as the dispersant, whereas dodecylamine and soluble starch were applied as the collector and depressant, respectively, for the reverse flotation. Methyl isobutyl carbinol (MIBC) was used as the frother. All the flotation experiments were carried out at natural pH (7–7.5).
Experimental design
In the present investigation, the Taguchi-based L16 orthogonal array was used to conduct four levels of reduction roasting experiments with five factors, namely ore size, coal size, temperature, time and coal-to-ore ratio. It is noteworthy that Taguchi method offers a great advantage in terms of experimental time and cost, considering that it requires minimum number of experiments to be carried out in order to study the effect of different factors. For the current work, the factors with their corresponding levels are described in Table 3. The iron grade and recovery as obtained using the magnetic separation of the roasted product were treated as the response variables. Moreover, each test was carried out thrice to avoid experimental error and the average responses were used for statistical analysis.
Reduction roasting
The iron ore and coal sample were subjected to crushing followed by classification as per the requirement of the experimental design. Reduction roasting of the iron ore was carried out in a laboratory muffle furnace by using high-quality refractory crucibles. A batch of 200 g of the iron ore sample mixed thoroughly with the calculated amount of coal was subjected to heating in the furnace at a particular temperature for a given residence time. The roasted mass was ground to − 75 µm particle size and subjected to LIMS at a magnetic intensity of ~ 0.18 T. The total iron content of both magnetic and non-magnetic fraction obtained from the LIMS was analyzed using wet chemical method. The iron content of the magnetic part which is commonly termed as “grade” was considered for statistical analysis. The percentage iron recovery calculated based on the iron content of the feed sample and magnetic fraction is described in Eq. (1).
where \(W_{\text{c}}\) and \(W_{\text{f}}\) are the weights of concentrate and feed, respectively, whereas \(I_{\text{c}}\) and \(I_{\text{f}}\) are their respective iron contents.
Ferrosilicon alloy synthesis
The non-magnetic fraction was subjected to smelting reduction in a laboratory-scale arc furnace. Activated charcoal with 93.5% fixed carbon was chosen as the reductant because it contains high amount of carbon as well as an acceptably low level of impurities (such as sulfur and phosphorus). The mixture of the non-magnetic fraction and charcoal put in a graphite crucible was kept inside the furnace. The arc was struck, and the current of 300 A was maintained with an arc voltage of about 55 V. It was continued for 30 min to complete the smelting. The temperature of the molten bath was measured using an optical pyrometer. After the completion of the reaction, the power supply to the arc furnace was switched off and the product (ferrosilicon alloy) was allowed to cool to room temperature in the crucible.
Characterization techniques
Characterization studies were undertaken for the iron ore and selected roasted and magnetic products. X-ray diffraction (XRD) studies were carried out using a PANalytical instrument (X’Pert PRO model) with Cu–Kα radiation operated at 40 kV and 30 mA in the range of 10–80 °C with a scanning speed of 2°/min. Electron microscopic studies were carried using a ZEISS (EVO) make scanning electron microscope attached with an energy-dispersive spectroscopy (SEM–EDS). The optical microscopic studies were carried out using a Leitz instrument. The raw ore and roasted samples were cold-mounted in resin with a hardener and then polished for optical microscopic studies, while powder samples were subjected to XRD analysis. The polished ferrosilicon alloy samples were carbon-coated for SEM–EDS studies.
Results and discussion
Beneficiation studies
Several attempts involving hydrocyclone followed by spiral, flotation and wet high-intensity magnetic separation (WHIMS) were taken to enrich the iron content of the BHJ ore. The best results of different unit operations are depicted in Fig. 2. It is observed that the maximum Fe grade of only 60.9% Fe with a yield of 42% was obtained using WHIMS, whereas the combination of hydrocyclone and spiral concentrator could not give rise to a good product. Moreover, with double-stage reverse flotation, it was possible to achieve a concentrate with 62.8% Fe at 10.2% yield only. The beneficiation studies revealed that it is difficult to upgrade this sample to a satisfactory Fe grade. This may be attributed to the poor liberation of the ore owing to its complex mineralogy and textural features.
Statistical analysis
The Taguchi design makes use of the signal-to-noise (\(S/N\)) ratio approach in order to estimate the deviation of the output characteristic from the desired values. Here, the signal represents the desirable value of the output, and the word noise refers to the undesirable value of the output. The \(S/N\) ratio characteristics can be broadly divided into three categories: the larger-the-better (LTB), the smaller-the-better (STB) and the nominal-the-better (NTB). In a reduction roasting study, one of the most important targets is to identify the optimum conditions under which maximum iron grade and recovery would occur. Therefore, the larger-the-better (LTB) analysis was used in this study.
The following equation was used to calculate the \(S/N\) ratio according to the LTB analysis:
where \(Y_{i}\) represents an observed value determined by a trial and n is the number of repetitions under the same experimental conditions. The optimum combination of the experimental factors is determined using the parametric levels of the highest \(S/N\) ratio in the orthogonal array using the following equation:
where \(\left( M \right)\begin{array}{*{20}c} {{\text{level}} = l} \\ {{\text{factor}} = f} \\ \end{array}\) is the mean of the \(S/N\) ratios of \({\text{factor}}\, f\) at \({\text{level}}\, l\), and \(n_{fl}\) represents the number of occurrences of \({\text{factor}}\, f\) at \({\text{level}}\, l\).
The experimental design used in this work consisted of 16 tests, each test comprising three replications. The iron grade and recovery calculated for each test were used as the responses in the statistical design, as shown in Table 4. The measured values are substituted in Eq. (2) to obtain \(S/N\) ratio for each test. The values of \(S/N\) ratio are then substituted into Eq. (3) to determine the mean \(S/N\) ratio of each factor in different levels. In this study, a higher \(S/N\) ratio was considered to be preferable for better response. The effects of the main experimental factors (i.e., ore size, coal size, temperature, time and coal-to-ore ratio) on the performance output (mean \(S/N\) ratios) for the responses are shown in Fig. 3.
Figure 3a and b show the effect of various factors on the \(S/N\) ratios for grade and recovery, respectively. The highest value of \(S/N\) ratio of a factor is the representative of the optimum level (Mia et al. 2017). Temperature, time, coal size and coal-to-ore ratio at level 4 and ore size at level 1 imply the optimum levels of the factors for obtaining the highest grade. Considering the corresponding delta values of the factors for grade as depicted in Fig. 3d, temperature has the most significant effect as compared to other factors. These delta values denote the relative significance of the factors toward the achievement of the maximum values of the response variable (Rath and Rao 2017). Similarly, the temperature, time and coal size at level 3, ore size at level 4 and coal-to-ore ratio at level 2 exhibit maximum recovery (Fig. 3b). The delta values of 4.86 and 4.45 (Fig. 3d) for temperature and ore size, respectively, indicate that both the factors are equally important for better recovery.
The results shown in Fig. 3a, b highlight the conditions under which grade and recovery can be maximized individually. However, both grade and recovery need to be at their optimum levels for a successful processing. Therefore, further statistical analysis was carried out while considering both grade and recovery as the responses and the results are displayed in Fig. 3c. It is observed from the figure that the \(S/N\) ratio increases rapidly in the temperature range from 700 to 800 °C; the rate slows down from 800 to 900 °C and thereafter decreases above 900 °C. In a similar way, the \(S/N\) ratio increases with time up to 90 min and then decreases abruptly at 120 min. The reduction in \(S/N\) ratio can be accredited to the formation of weekly magnetic phases at high temperature and time that results in poor Fe recovery in the magnetic product. On the other side, with the decrease in both the ore size and coal size, the \(S/N\) ratio increases initially and then remains almost constant. It is also noticeable that the \(S/N\) ratio of coal-to-ore ratio reaches the maximum at level 2 (coal-to-ore ratio of 0.15). This may be credited to the fact that when the concentration of carbon monoxide, which is ultimately responsible for the reduction, increases beyond a certain point, it favors further reduction of magnetite to wustite, thereby causing a loss of iron values in the magnetic fraction. The optimum process variables for the grade and recovery obtained are temperature at level 3 (900 °C), ore size at level 4 (− 1 mm), coal size at level 3 (− 3.35 + 1 mm), coal-to-ore ratio at level 2 (0.15) and time at level 3 (90 min). In addition, it is found from Fig. 3d that temperature possesses the highest delta value followed by ore size. Therefore, temperature has the greatest influence on the grade and recovery. The least delta value has been possessed by time, indicating that compared to other factors it has less impact on the process. A concentrate having an average grade of 66.12% Fe and iron recovery of 71.48% was generated by performing tests thrice at the optimum levels as concluded by the statistical analysis.
Characterization
Optical microscopic studies of both the raw iron ore and the ore roasted at the optimum conditions obtained from the statistical analysis were carried out in order to identify the various mineral phases. Figure 4a clearly depicts the microstructure of a typical BHJ iron ore sample banded with alternating layers of iron oxide and silica. The iron-rich bands mostly comprise of hematite (H), while the silica-rich bands are found to contain both jasper (J) and quartz (Q). The alternative bands are formed due to different stages of sedimentation and diagenesis. The bands are generally found to be parallel, while the concentration of iron ore minerals in an iron-rich band is more or less uniform; in a silica-rich band it is highly erratic. The ore shows complex interlocking texture between hematite and jasper. A secondary quartz vein (arrow mark in Fig. 4a) is also being observed within BHJ. These quartz veins, which show crosscut relationships with BHJ, are of supergene types. Salt-and-pepper texture, as observed in Fig. 4a, is a very common feature in BHJ formed due to small disseminated quartz within the hematite matrix and vice versa (Roy and Venkatesh 2009).
The optical microstructure of the BHJ sample roasted at optimum condition is shown in Fig. 4b. The newly developed euhedral grains of hematite may be attributed to the recrystallization of the hematite present in the feed sample by the hydrothermal fluids generated at high temperature. The presence of magnetite (M) bordering hematite (H) suggests the peripheral reduction of the latter. Again, some of the silica (Q/J) grains are seen to have formed iron silicates (S) as a result of intra-phase reactions.
The presence of the mineral phases described above in both the raw and roasted samples was also confirmed through their diagnostic XRD peaks (Fig. 5). In raw BHJ sample, the major phases found are hematite and quartz along with some goethite. On the other hand, clear peaks of magnetite can be seen in the XRD pattern of the sample roasted at optimum conditions. The absence of goethite peak in the roasted sample implies that it has dehydrated to form hematite or might have further reduced to magnetite.
The roasted product from test number 4 (Table 4) having the highest grade of ~ 68% and a low recovery of ~ 48% was subjected to characterization in order to find out the reasons for the same. The microstructure of the concerned roasted sample, as depicted in Fig. 6, reveals the presence of a number of wustite grains with some amounts of fayalite. The formation of the feebly magnetic phases like wustite and fayalite at such high temperature (1000 °C) and time (120 min) has also been reported earlier (van den Berg and Dippenaar 1989; Bai et al. 2018). These phases are reflected in the non-magnetic part of the XRD pattern (Fig. 7). In addition, the magnetic part shows magnetite as the major phase. This describes the reason behind the highest grade and moderately low recovery of the roasted sample in test number 4.
Reflected light micrographs of roasted BHJ sample as obtained from test no. 4 (Table 4) showing the formation of wustite and fayalite
XRD pattern of the roasted BHJ sample as obtained from test no. 4 (Table 4)
Ferrosilicon production
In general practice, the non-magnetic fractions are treated as waste. However, in this work, owing to the presence of sufficient amounts of silica and iron in it, further attempts were made to prepare ferrosilicon from the non-magnetic fraction obtained from the experiment conducted at optimum conditions. The product obtained from the smelting was subjected to SEM–EDS studies in order to observe the morphology of the synthesized ferrosilicon alloy, and the results are depicted in Figs. 8 and 9.
The backscattered image (Fig. 8) of the alloy shows two prominent phases: one bright and the other dark. The bright phase represents the presence of higher Fe and lower Si concentration in comparison with the dark phase. The in situ EDS analyses of these two phases are shown in Fig. 9. The average weight percentages of Si in bright and dark phases are found to be 17.5 and 30.1%, respectively. The overall Si weight percentage in the synthesized alloy is 20%. These results confirm the product as a low-grade ferrosilicon alloy. According to previous studies, the formation of ferrosilicon alloy would involve the mechanism as described below (Farzana et al. 2014; Maroufi et al. 2016; Farzana et al. 2017).
The reduction of SiO2 by carbon begins at 1500 °C, and the reaction completes practically at 1800 °C. In the presence of iron, the reduction of silica may follow Eq. (4) to produce ferrosilicon.
In the process of the reduction of SiO2 with carbon, gaseous SiO is formed by Eqs. (5) and (7).
The SiO gas rises up in the burden and reacts with carbon in the low-temperature region of the furnace, to yield silicon carbide and carbon monoxide as products (Eq. 6) or be dissolved in metal (Eq. 8) or partially be removed from the system with the flowing gas. The recovery of silicon will drastically decrease if the reactivity of reductant toward silicon monoxide gas is not high enough. The high reactivity of charcoal with SiO thus makes it attractive for the production of ferrosilicon alloy. The SiO gas removal decreases when the operating temperature is more than 1600 °C (Maroufi et al. 2016).
In the present work, when the non-magnetic part is subjected to carbothermal reduction, the iron oxides would be reduced initially. It will be followed by the reduction of silica. Considering the presence of the silica in the close vicinity of iron, the formation of ferrosilicon is likely to be more favorable compared to the other reactions of SiO2 as described in Eqs. (5)–(7). This explains the suitability of the formation of ferrosilicon from the tailings of BHJ ore.
Conclusion
A comprehensive utilization of BHJ ore was investigated using reduction roasting and smelting method. The influence of temperature, time, coal size, ore size and coal-to-feed ratio on reduction roasting of BHJ ore was established. An innovative approach towards the substitution of silica resource in ferrosilicon production was also proposed. It was observed that a magnetic concentrate assaying ~ 66% Fe at a recovery of ~ 72% is achievable from a feed of ~ 47% Fe. On the other hand, it was possible to produce a ferrosilicon alloy with ~ 20% Si by smelting the non-magnetic fraction in an arc furnace. This study could lead to future pathways for creating a zero-waste technology by using both the magnetic and non-magnetic fractions as iron-rich pellet grade concentrate and raw material for ferrosilicon alloy, respectively. However, the composition of the resulting ferrosilicon alloy, which is ultimately responsible for its commercial value, is a function of the composition of the non-magnetic product and the smelting conditions. Therefore, a detailed systematic investigation is required to understand the making of ferrosilicon from the non-magnetic part vis-á-vis a study on the physical and chemical characteristics of the same.
References
Bai S-J, Li C-L, Fu X-Y et al (2018) Novel method for iron recovery from hazardous iron ore tailing with induced carbothermic reduction-magnetic flocculation separation. Clean Technol Environ Policy. https://doi.org/10.1007/s10098-018-1501-y
Commission USIT, Lukes JJ (1984) Ferrosilicon from the Union of Soviet Socialist Republics: report to the President on investigation no. TA-406-10 under section 406 of the Trade Act of 1974. U.S. International Trade Commission
Das B, Mishra BK (2013) Investigations on recovery of hematite from two different banded iron ores by flotation. In: Proceedings of 2013 SME Annual Meeting & Exhibit and CMA 115th National Western Mining Conference. Denver, Colorado, USA
Das B, Mishra BK, Prakash S et al (2010) Magnetic and flotation studies of banded hematite quartzite (BHQ) ore for the production of pellet grade concentrate. Int J Miner Metall Mater 17:675–682. https://doi.org/10.1007/s12613-010-0373-x
Das B, Rath SS, Reddy PSR et al (2012) Flotation response of banded iron ore of Karnataka region, India. In: Proceedings of the XXVI International Mineral Processing Congress (IMPC). New Delhi, India, pp 1030–1040
Das SK, Prasad R, Singh RP (2018) Characterisation-Assisted Reduction Roasting of BHJ, West Singhbhum, Jharkhand, India. Trans Indian Inst Met. https://doi.org/10.1007/s12666-017-1270-z
Farzana R, Rajarao R, Sahajwalla V (2014) Transforming waste plastic into reductants for synthesis of ferrosilicon alloy. Ind Eng Chem Res 53:19870–19877. https://doi.org/10.1021/ie5041513
Farzana R, Rajarao R, Sahajwalla V (2016) Characteristics of waste automotive glasses as silica resource in ferrosilicon synthesis. Waste Manag Res J Int Solid Wastes Public Clean Assoc ISWA 34:113–121. https://doi.org/10.1177/0734242X15617010
Farzana R, Rajarao R, Sahajwalla V (2017) Reaction mechanism of ferrosilicon synthesis using waste plastic as a reductant. ISIJ Int 57:1780–1787. https://doi.org/10.2355/isijinternational.ISIJINT-2017-199
Ghosh TK, Biswas N, Rao SM (2012) Characterization and assessment of low grade iron ores for their beneficiation from Jharkhand-Orissa region, India. In: Proceedings of the XXVI International Mineral Processing Congress (IMPC). New Delhi, India, pp 1593–1604
Gurulaxmi N, Thella J, Dixit P (2012) Flotation studies of banded hematite jasper iron ores. In: Proceedings of the XXVI International Mineral Processing Congress (IMPC). Indian Institute of Metals, New Delhi, India, pp 5181–5193
Kar B, Sahoo H, Rath SS, Das B (2013) Investigations on different starches as depressants for iron ore flotation. Miner Eng 49:1–6. https://doi.org/10.1016/j.mineng.2013.05.004
Littmann MF (1982) Properties of grain oriented 3% silicon steel for transformers with minimum cost of ownership. J Appl Phys 53:2416–2418. https://doi.org/10.1063/1.330830
Makhija D, Mukherjee AK, Ghosh TK (2013) Preconcentration feasibility of gravity and magnetic techniques for banded hematite jasper. Int J Min Eng Miner Process 2:8–15
Maroufi S, Ciezki G, Jahanshahi S, Ostrovski O (2016) Carbothermal reduction of iron and silicon oxides in ironstone ore. Miner Process Extr Metall 125:86–94. https://doi.org/10.1080/03719553.2016.1156800
Mazumdar R (2017) National Steel Policy 2017 to focus spending on infrastructure, construction. In: Economic Times. Accessed 17 Apr 2018
Mia M, Khan MA, Rahman SS, Dhar NR (2017) Mono-objective and multi-objective optimization of performance parameters in high pressure coolant assisted turning of Ti–6Al–4V. Int J Adv Manuf Technol 90:109–118. https://doi.org/10.1007/s00170-016-9372-z
Rath SS, Rao DS (2017) Dolochar as a reductant in the reduction roasting of iron ore slimes. Int J Miner Metall Mater 24:1341–1351. https://doi.org/10.1007/s12613-017-1526-y
Rath SS, Sahoo H, Das B (2013) Optimization of flotation variables for the recovery of hematite particles from BHQ ore. Int J Miner Metall Mater 20:605–611. https://doi.org/10.1007/s12613-013-0773-9
Roy S, Venkatesh AS (2009) Mineralogy and geochemistry of banded iron formation and iron ores from eastern India with implications on their genesis. J Earth Syst Sci 118:619. https://doi.org/10.1007/s12040-009-0056-z
Sahoo H, Kar B, Rath SS et al (2014) Processing of banded magnetite quartzite (BMQ) ore using flotation techniques. Powder Technol 256:285–292. https://doi.org/10.1016/j.powtec.2014.02.034
Sahoo H, Rath SS, Rao DS et al (2016) Role of silica and alumina content in the flotation of iron ores. Int J Miner Process 148:83–91. https://doi.org/10.1016/j.minpro.2016.01.021
Sales M (1977) Process for the passivation of ferrosilicon
Singh S, Sahoo H, Rath SS et al (2015) Separation of hematite from banded hematite jasper (BHJ) by magnetic coating. J Cent South Univ 22:437–444. https://doi.org/10.1007/s11771-015-2540-8
Skaland T (2004) Method for production of ductile iron
Tangstad M (2013) Chapter 6—Ferrosilicon and silicon technology. In: Gasik M (ed) Handbook of ferroalloys. Butterworth-Heinemann, Oxford, pp 179–220
van den Berg JC, Dippenaar RJ (1989) Fluidized-bed reduction of fine iron ore by the in situ combustion of coal. J S Afr Inst Min Metall 89:89–98
Waanders FB, Rabatho JP (2005) Recovery of heavy minerals by means of ferrosilicon dense medium separation material. In: LACAME 2004. Springer, Berlin, pp 55–60
Zhukov AA, Dybenko IV, Abdullaev ÉV, Afonaskin AV (1989) Novelties in the theory of graphitization. Modification of cast iron by inoculation. Met Sci Heat Treat 31:99–107. https://doi.org/10.1007/BF00738143
Acknowledgements
The authors are thankful to the Director, CSIR-IMMT, Bhubaneswar, for his kind permission to publish this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ray, N., Nayak, D., Dash, N. et al. Utilization of low-grade banded hematite jasper ores: recovery of iron values and production of ferrosilicon. Clean Techn Environ Policy 20, 1761–1771 (2018). https://doi.org/10.1007/s10098-018-1566-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-018-1566-7