Abstract
In many parts of India, because of depletion in high grade iron ore, efforts are focused on the use of low-grade iron ore for steel production. As an alternative iron source, low-grade banded iron ores have attracted attention. The beneficiation and utilization of banded iron ore is closely connected to their mineralogical characteristics, nature of impurity inclusion and liberation behavior. Characterization studies indicated that ore was low in iron value (~ 36.5 wt%), and contained high amount of silica (~ 46.3 wt%), which made it difficult to process by conventional beneficiation method. Therefore, we used reduction roasting method on the ore to produce the pellet feed material. Different process parameters such as roasting time, temperature and coal-ore ratio were optimized to achieve the maximum recovery and grade of magnetic product. Using coal as reducing agent, the optimum condition was found to be as, reduction temperature: 900 °C, time: 90 min, and coal-ore ratio: 15%. Under the optimum condition, maximum recovery and grade of magnetic product were found to be 72% and 65%, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
India’s 2030 steel vision aims to produce 300 million ton steel per year which needs 550 million tons of high quality iron ore per year (approx. 60% Fe). India does not have sufficient rich quality iron ore reserves but carries plentiful resources of banded iron ore. A major amount of banded iron ore is generated during the traditional mining of hematite ore, which is stockpiled as waste resources around the mines site [1]. These ores contain 30–40 wt% iron. It is anticipated that effective utilization of such resources can completely eliminate the crises of desirable rich quality iron ore. Research studies on the processing of banded iron ore are limited and beneficiation methods such as hydrocyclone, WHIMS and flotation are described. These traditional processing techniques give poor response due to complex mineralogical nature of ore. In conventional method, fine grinding of ore is required to perform the various beneficiation operations. In some cases, using the conventional method, iron grade can be improved up to the required pellet feed (> 60% Fe), but due to low value of recovery and yield, this method is not economically feasible [2,3,4]. Therefore, to overcome all these problems, an alternative method such as reduction roasting with low-intensity magnetic separation needs to be implemented. Rath et al. utilized cow dung cakes for the reduction studies of iron ore slimes of Barsua area. The result showed that a product of 64% Fe with 66% recovery could be attained from a feed of 56.2% Fe [5]. Ray et al. used reduction process to improve the grade of BHJ ore of Barbil region. The best result was found at a temperature of 900 °C, coal-ore ratio 0.15 and 90 min. A magnetic product of 66% Fe with 72% recovery was found from an ore of 47% Fe. Smelting of non-magnetic product produced the ferrosilicon alloy with 20% silica [6]. Das et al. observed that finer grains contained less iron as compared to coarser grains. Reduction roasting was best achieved at a temperature 600 °C, time 60 min and 13 mm particle size [7]. Rath et al. concluded that an iron ore comprising 56.2% Fe could be improved to 64% Fe with 71% recovery by reduction roasting technique using dolochar as a reductant [8]. Shankar et al. made an effort to increase the grade of iron ore of Gua area using reduction roasting with LIMS. They found a concentrate of 66.6% Fe with 90.4% recovery at 800 °C for 30 min with 10% coal [9]. Rath et al. found that an iron ore comprising 51.6% iron could be enhanced to 63.34% Fe with 79% recovery at a temperature of 950 °C for a roasting period of 53 min [10]. Some literature has also been observed on the direct reduction and microwave carbothermal reduction in the BHJ ores. Rayapudi et al. carried out microwave carbothermal reduction on banded hematite jasper ore of southern part of India ~ 37% Fe. Fayalite phase easily formed due to presence of banded silica in the BHJ ore. The iron grade could be increased to 61.6% Fe, 73.4% recovery with a yield of 44% at the optimum condition of 720 W power for 8 min with 9% wt charcoal [11]. Das et al. studied the reaction kinetics during reduction in BHJ with coal at temperature range of 1273–1473 K. They observed that at 1273–1373 K, SiC phase was formed which hindered the reduction rate, but this phase was not stable at temperature above 1473 K. So, reduction in iron oxide accelerated at higher temperature. At higher temperature, reduction was controlled by diffusion while at lower temperature, it was controlled by chemical reaction kinetics [12]. Rayapudi et al. executed microwave reduction on the three-low-grade BHQ and BHJ ores using charcoal as a reductant. The result indicated that ferrite phase was appeared at 900 W, ≤ 8% wt charcoal within 10 min. They concluded that considerable quantity of ferrite balls were obtained at 900 MW power for 10 min with 12% wt charcoal [13]. This investigation is performed to upgrade the banded hematite jasper ore with maximum iron recovery.
Materials and Method
Materials
Approx. 400 kg of BHJ sample was collected from the Joda mine ensuring that the sample was representative of the mines ore body. This area is located in the survey of India toposheet no. 73G/5 in between 22°01′04″ N:85°28′52″ E in Orissa state. The collected samples were blended properly to provide the compositional homogeneity.
Reactions Occurred During Reduction in Iron Ore
Das et al. [3] observed that conventional magnetic separation method was not effective for BHJ ore. Hence, reduction roasting with magnetic separation was used to increase the grade of banded hematite jasper. For hematitic ore, the basic aim of reduction roasting is to reduce the hematite to magnetite using some reductant such as C, CO and H2. The equations involved in the reaction theory are expressed below:
The formation and transfer of CO to the iron particles interface play a very significant role in the entire reaction series. In this study, only reaction (3) is desired for the formation of magnetite by direct contact of iron particles with CO. Reduction parameters are optimized to confirm the formation of magnetite so that it can be recovered by using the low-intensity magnetic separator.
Characterization Studies
Chemical analysis of ore was performed using X-ray fluorescence spectroscopy (WDXRF), Model BRUKER S8 TIGER. The BHJ sample of below the size of 75 micron was used for this study. A BSS standard sieve set of different aperture size was used for the size analysis of BHJ ore. This analysis was carried out by using Model LM-17596 (LAWRENCE AND MAYO). X-ray diffractometer test was carried out on BHJ sample to determine the different mineral phases present in the sample. Bruker-D8™ X-ray diffractometer model with Co-Kα target (40 kV, 30 mA) was used for this study. Microscopic study was performed to observe the various mineral phases and their liberation behavior. Polished and thin section of BHJ ore were observed through DM 2700 model for both reflected and transmitted light.
Reduction Roasting of BHJ Ore
Reduction roasting of BHJ with coal was performed in a laboratory muffle furnace. The proximate analysis of coal was found to be 51.48% fixed carbon, 13.17% volatile matter, 26.3% ash and 8.16% moisture. About 200 gm of banded hematite jasper ore (below the size of 10 mm) was properly mixed with powdered coal for reduction experiment. After reduction, roasted samples were ground below 75 micron size and further treated in the low-intensity magnetic separator of ~ 0.18 T magnetic intensity. Magnetic fraction was considered as a concentrate and analyzed for grade and recovery of iron, while non-magnetic fraction was considered as a tailing.
Result and Discussion
Elemental Analysis of Ore and Coal
XRF study has been performed on the powder sample of BHJ ore (< 75 micron). The result shows that the raw ore mainly contains silica (46.34% SiO2) with a total iron (36.51% Fe). Silica is present as Jasper, a microcrystalline form of quartzite. Elemental analysis of ore is presented in Table 1. Table 2 displays the proximate coal analysis, which is used as a reduction agent for mineral transformation from one phase (hematite) to another phase (magnetite).
Particle Size Analysis
The sample is crushed in laboratory using jaw crusher and roll crusher. The rolled crushed product is further ground in the laboratory ball mill. The particle size distribution of rolled crushed product is shown in Fig. 1. From the size distribution curve, 80% passing size of the particle is obtained to be 2.46 mm. After 20 min of grinding of rolled crushed product into ball mill, 80% passing size of the particle is reduced to be 75 µm. The ground product is further used for the characterization studies.
XRD Analysis
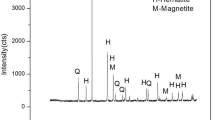
XRD graph of the feed sample (Fig. 2) represents that the major iron bearing phase is hematite, and the main gauge bearing phase is silica. The BHJ sample is ground below 75 micron size before XRD analysis. Alumina phase can also be seen from the XRD graph.
Microscopic Studies
Microscopic images of BHJ ore are shown in Fig. 3. The overview of BHJ polished section is presented in Fig. 3a. Figure 3b shows that hematite grains are finely disseminated around a thick layer of silicate. It can be concluded from the microscopic images that BHJ ore needs to be finely ground for the separation of valuable minerals.
Reduction Roasting of BHJ Ore
In reduction roasting method, three principal factors which mainly affect the roasted products are roasting temperature, roasting time and reducing agent. The influence of roasting temperature, time and coal-ore ratio on grade and recovery are studied to determine the effective reduction parameter.
Effect of Varying Roasting Temperature on Recovery and Grade
As presented in Fig. 4, the iron recovery of the magnetic product increases with the increase in roasting temperature until it reaches the extreme value at a temperature of 900 °C, afterward, it slightly decreases as the roasting temperature increases further. The maximum recovery of iron is about 72% with 65.12% Fe. In the meantime, the iron grade decreases when the temperature further increases. This result may be found because the reduction in hematite to magnetite at a lower temperature is incomplete due to the slow reaction rate [14]. Due to solid carbon gasification, the conversion of hematite into magnetite is accelerated at a high temperature, and at too high temperature, weakly magnetic wustite is formed from iron oxide, which decreases the recovery of iron.
Effect of Varying Roasting Time on Recovery and Grade
As presented in Fig. 5, with the increase in roasting time, grade and iron recovery of magnetic concentrate increase up to 90 min of roasting time and then both grade and recovery decrease as the roasting time increases further. This result may be found because the reduction in hematite to magnetite is incomplete at low roasting time and at an excessively high roasting time, the formed and originally present magnetite may be re-oxidized which decreases recovery and grade [15].
Effect of Varying Coal-Ore Ratio on Recovery and Grade
As presented in Fig. 6, with the increase of coal-ore ratio, iron recovery of magnetic concentrate increases until it attains the extreme value at a coal to ore ratio of 15% and then it slightly decreases when the coal-ore ratio further increases. In the meantime, the grade of iron increases gradually by increasing the coal-ore ratio. This result may be observed because complete reduction of hematite to magnetite is not possible when the coal quantity is insufficient and at an excessively high amount of coal, formed and original magnetite may be converted into the ferrous oxide which is less magnetic and undesirable in low intensity magnetic separation. The maximum iron recovery is about 72.01% with a grade of 65.07% Fe. A schematic of the experimental setup is shown in Fig. 7.
Conclusion
XRD study of banded hematite jasper ore indicates that the main iron bearing phase is hematite while the main gangue bearing phase is quartz. Microscopic studies reveal that silicate minerals are attached with hematite grains in the form of band. In the reduction roasting method, the effect of roasting temperature, roasting time and reductant-ore ratio on the recovery and grade of magnetic concentrate are investigated. The optimized condition for reduction roasting of BHJ ore is obtained as: temp 900 °C temp, time 90 min and coal/ore ratio 15%. At the optimum condition, a concentrate of 72% recovery with 65% Fe is obtained. The product of carbothermic reduction can be investigated using the XRD and SEM analysis to observe metallization and phase transformation along with the reduction process. The results are showing the feasibility of this process to upgrade the banded hematite jasper ore with high recovery rate. This technique may be quite effective to produce the pellet feed material from banded hematite jasper ore.
References
IBM. Indian Minerals Yearbook (Part- III: Mineral Reviews) 57th edition: IRON ORE (2018)
B. Das, B.K. Mishra, S. Prakash, S.K. Das, P.S.R. Reddy, S.I. Angadi, Magnetic and flotation studies of banded hematite quartzite (BHQ) ore for the production of pellet grade concentrate. Int. J. Miner. Metal. Mater. 17(6), 675–682 (2010)
R. Srivastava, R. Prasad, Studies on characterization and beneficiation of banded hematite Jasper of Joda Area, Eastern India. Trans. Indian Inst. Met. 73, 215–221 (2020)
S.N. Sahu, K. Sharma, S.D. Barma, P. Pradhan, B.K. Nayak, S.K. Biswal, Utilization of low-grade BHQ iron ore by reduction roasting followed by magnetic separation for the production of magnetite-based pellet feed. Metall. Res. Technol. 116, 611–621 (2019)
S.S. Rath, D.S. Rao, B.K. Mishra, A novel approach for reduction roasting of iron ore slime using cow dung. Int. J. Miner. Process. (2016). https://doi.org/10.1016/j.minpro.2016.11.015
N. Ray, D. Nayak, N. Dash, S.S. Rath, Utilization of low-grade banded hematite jasper ores: recovery of iron values and production of ferrosilicon. Clean Technol. Environ. 20(8), 1761–1771 (2018)
S.K. Das, R. Prasad, R.P. Singh, Characterisation-assisted reduction roasting of BHJ, West Singhbhum, Jharkhand, India. Trans. Indian Inst. Met. 71(6), 1357–1362 (2018)
S.S. Rath, D.S. Rao, Dolochar as a reductant in the reduction roasting of iron ore slimes. Int. J. Miner. Met. Mater. 24(12), 1341–1351 (2017)
V. Ravisankar, R. Venugopal, H. Bhat, Investigation on beneficiation of goethite-rich iron ores using reduction roasting followed by magnetic separation. Miner. Process. Extr. Metall. (Trans. Inst. Min. Metall. C) 128(3), 175–182 (2019)
S.S. Rath, H. Sahoo, N. Dhawan, D.S. Rao, B. Das, B.K. Mishra, Optimal recovery of iron values from a low grade iron ore using reduction roasting and magnetic separation. Sep. Sci. Technol. 49(12), 1927–1936 (2014)
V. Rayapudi, S. Agarwal, N. Dhawan, Optimization of microwave carbothermal reduction for processing of banded hematite jasper ore. Miner. Eng. 138, 204–214 (2019)
S.K. Das, R. Prasad, R.P. Singh, S. Ranganathan, Kinetics of reduction of Banded Haematite Jasper ore with coal. Miner. Process Extr. Metall. (2018). https://doi.org/10.1080/25726641.2018.1538194
V. Rayapudi, N. Dhawan, Investigation of microwave reduction of low-grade banded iron ores. Miner. Process Extr. Metall. (2019). https://doi.org/10.1080/25726641.2019.1668662
Y. Zhang, H. Li, X. Yu, Recovery of iron from cyanide tailings with reduction roasting–water leaching followed by magnetic separation. J. Hazard. Mater. 213, 167–174 (2012)
Z. Cui, Q. Liu, T.H. Etsell, Magnetic properties of ilmenite, hematite and oilsand minerals after roasting. Miner. Eng. 15(12), 1121–1129 (2002)
Acknowledgements
The authors are thankful to the metallurgical and materials engineering department, NIT Jamshedpur. The authors would like to acknowledge the authorities of the Joda mines to supply the banded hematite jasper ore and also extended their acknowledgment to CSIR-NML, Jamshedpur, for their help throughout the characterization studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Srivastava, R., Prasad, R. Mineralogical Characterization-Assisted Reduction Roasting of Banded Hematite Jasper Ore. J. Inst. Eng. India Ser. D 101, 247–252 (2020). https://doi.org/10.1007/s40033-020-00234-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40033-020-00234-1