Abstract
An enrichment culture technique was used to isolate soil bacteria capable of growing in the presence of two different concentrations of linear alkylbenzene sulfonates (LAS) (10 and 500 μg ml−1). Nine bacterial strains, representatives of the major colony types of aerobic heterotrophic cultivable bacteria in the enriched samples, were isolated and subsequently identified by PCR-amplification and partial sequencing of the 16S rRNA gene. Amongst the isolates, strains LAS05 (Pseudomonas syringae), LAS06 (Staphylococcus epidermidis), LAS07 (Delftia tsuruhatensis), LAS08 (Staphylococcus epidermidis) and LAS09 (Enterobacter aerogenes), were able to grow in pure culture in dialysed soil media amended with LAS (50 μg ml−1). The three Gram-negative strains grew to higher cell numbers in the presence of 50 μg ml−1 of LAS, compared to LAS-unamended dialysed soil medium, and were selected for further testing of their ability to use LAS as carbon source. However, HPLC analysis of culture supernatants showed that the three strains can tolerate but not degrade LAS when grown in pure cultures. A higher concentration of soluble phosphates was recorded in dialysed soil media amended with LAS (50 μg ml−1) compared to unamended control media, suggesting an effect of the surfactant that enhanced the bioavailability of P from soil. The presence of LAS at a concentration of 50 μg ml−1 had an important impact on growth of selected aerobic heterotrophic soil bacteria, a deleterious effect which may be relevant for the normal function and evolution of agricultural soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Linear Alkylbenzene Sulfonates (LAS) are anionic surfactants widely used for the production of detergents and household cleaners (Berth and Jeschike 1989). The world-wide consumption of LAS has been estimated to be 1.5–2.0 million tons per year (De Wolf and Feijtel 1998). LAS is introduced into agricultural soils by several routes: (a) application of sewage sludge as agriculture fertilizer, (b) application of wastewater as irrigation water, (c) soil infiltration of wastewater or polluted river water, and (d) application of pesticide formulations containing LAS as emulsifiers or dispersion agents (Hislop et al. 1977; Jensen and Jepsen 2005). The application of sewage sludge as agricultural fertilizer is the major cause of LAS occurrence in soils (Painter 1992; Ying 2006). Several physical and chemical factors such as temperature, oxygen supply, water content, the amount of LAS applied, and its molecular characteristics (length of chain and position of benzene ring) may condition the toxicity and levels of biodegradation of LAS in a soil ecosystem (Knaebel et al. 1990; Nielsen et al. 2004). Currently, the EU legislation does not mark a cut-off value for the amount of LAS permitted in sludge used for agricultural fertilization. Only the administrative authorities of Denmark have established a maximum value of 1300 mg kg−1 dry weight of LAS in the sludge used as fertilizer (Jacobsen et al. 2004).
Several studies (Berna et al. 1989; Knaebel et al. 1990; Brandt et al. 2003; Ying 2006) have reported LAS to have a half-life ranging from 1 to 33 days in agricultural soils, mainly due to its aerobic degradation by soil microbiota. However, Marcomini et al. (1989) demonstrated that LAS homologues with long chains can be strongly adsorbed to soil particles, thereby becoming unavailable for microbial degradation, and consequently accumulating in soils. In the soil environment, LAS can be inhibitory to biological activity, which is the most important basis for soil fertility and nutrient cycling (Jensen et al. 2001). Data reported by Jensen (1999) indicated that adverse effects for microorganisms in soil start at LAS concentrations of 10–50 mg kg−1. These LAS concentrations are in the range of those that may be discharged in agricultural soils after the addition of sludge as a fertilizer (Jacobsen et al. 2004). Nevertheless, some functional groups of bacteria, such as the ammonia-oxidizers, show a higher sensitivity to the presence of the surfactant, and concentrations ranging between 8 and 10 mg kg−1 LAS are enough to induce a negative impact on Nitrosomonas and Nitrosospira strains (Elsgaard et al. 2001a, b; Brandt et al. 2001, 2002, 2003). The main biological effect of LAS is the disruption of bio-membranes and the denaturation of proteins. In microorganisms, LAS adsorption produces a depolarization of cell membranes, and consequently decreases the absorption of nutrients and modifies the release of substances from cell metabolism (Jensen 1999). Although a vast literature is available on the effects of LAS on the ecology and physiology of the soil microbial communities (Jensen 1999; Schwuger and Bartnik 1980; Elsgaard et al. 2001a, b; Brandt et al. 2002, 2003), few studies have isolated bacteria species or bacteria consortia capable to biodegrade the surfactant, and little is known about the degradation processes and the microbial community involved (Khleifat 2006).
The most commonly used tests to evaluate toxicity on soil microorganisms involve the overall evaluation of enzymatic activities and carbon (C) and nitrogen (N) transformations, while single species tests, needed to elucidate specific responses towards a specific pollutant, are less explored (Elsgaard et al. 2001b). This study was aimed to isolate and characterize aerobic heterotrophic bacterial strains from an agricultural soil, after enrichment in the presence of low (10 μg ml−1) and high (500 μg ml−1) concentrations of LAS. Strains with tolerance for LAS were studied in relation to their growth response in the presence of LAS when cultured in dialysed soil media, which adequately reproduces nutritional conditions of bacteria in soil (González-López and Vela 1981). In order to clarify a positive growth response to LAS of three selected strains, their potential to degrade LAS in pure culture, and the effect of LAS on the availability of soil nutrients (total N and soluble phosphates), were also evaluated.
Materials and methods
Soil samples
Soil samples were collected from 0 to 15 cm of an agricultural field in Belicena, near the city of Granada, in the Southeast of Spain. The field has not been previously in contact with LAS. The soil samples were collected in sterile plastic bags, air dried at room temperature, and sieved (2 mm mesh). The soil used was a Typic Xerofluvent with silt loam texture, containing 20% sand, 14% clay and 65% silt. The chemical composition of the samples was as follows: organic matter, 1.39% (w/w); pH (water) 7.8; N total, 0.14%; phosphorous, 25 mg kg−1, and potassium, 240 mg kg−1. Physical and chemical characteristics of soil samples were analyzed by the techniques described by Pratt (1954), Bremmer (1965), Olsen and Dean (1965), and the Natural Resources Conservation Service (1999).
Enrichment of aerobic heterotrophic cultivable bacteria in the presence of LAS
Linear Alkylbenzene Sulfonates (LAS) (technical purity grade) was purchased from Petresa (San Roque, Cádiz, Spain), dissolved in distilled water, and sterilized by autoclaving prior to being added to the soil samples. The LAS mixture used in this work contains 31% of active matter with the following distribution of the linear alkyl chain homologues: 5-phenyl C10 0.8%; phenyl C10 9.8%; phenyl C11 33.9%; phenyl C12 32.5%; phenyl C13 22.6%; phenyl C14 0.3%; tetra-indol 0.10%, parafine 0.10%, and 69% of water. The product was free of impurities.
The experiments were initiated dispersing 1 g of soil in Erlenmeyer flasks (250 ml) containing 50 ml of sterile modified Bushnell–Haas (BH) salts medium (Toledo et al. 2006), which were then amended with appropriate concentrations of the sterile LAS stock solution, to give final concentrations of either 10 or 500 μg ml−1. The LAS stock solution was made with sterile water (5 ml) where the detergent was dissolved. Appropriate volumes were added to the flasks of BH medium to reach the desired concentrations. Control soil samples without LAS and containing just BH medium (50 ml) and sterile water (5 ml) were also analysed.
All flasks were incubated at 30°C with continuous agitation (100 rpm) for 72 h. About 5 ml of these cultures were subsequently transferred twice to fresh BH medium (50 ml) amended with the corresponding concentration of LAS, and incubated under the same conditions for further 48 h. Then aliquots (0.1 ml) of the enrichment cultures were serially diluted and spread on plates of 1/10 diluted triptycase soy agar (TSA), made by preparing 1/10 diluted TSB (Difco) and adding 1.5% agar. Plates were incubated aerobically at 30°C for 48 h and then checked visually. Isolates representative of the dominant colony morphologies for each of the tested concentrations of LAS were selected and purified, by restreaking them twice on 1/10 TSA plates. All the experiments were made in triplicate.
Genetic identification and phylogenetic affiliation of the isolated bacterial strains
All strains isolated in this study (both from the samples treated with LAS and from the control flasks lacking LAS) were identified by analysis of the partial sequence of the gene encoding 16S rRNA. Primers fD1 and rD1 (Weisburg et al. 1991) were synthesised by Sigma Genosis (UK) and used to amplify almost the full length of the 16S rRNA gene. Fresh cultured colonies of each strain were lysed by the addition of 20 μl of a mixture of NaOH (0.05 M)-SDS (0.25%, w/v) which was then boiled for 15 min. The lysates were adjusted to 200 μl with sterile water and centrifuged at 2500g for 5 min in a table-top centrifuge. The cleared lysates (4 μl) were used as template for amplification. PCR was done adding to the lysates 1×PCR Gold buffer (Applied Biosyetms, Germany), 1.5 mM MgCl2 (Applied Biosystems, Germany), 200 μM dNTPs (Roche Molecular Biochemicals, Germany), 20 pmol of each primer, and 1 U of Ampli-Taq Gold polymerase (Applied Biosystems, Germany). Final volume of the reaction tubes was adjusted to 50 μl. Reactions were run in a Perkin Elmer GeneAmp PCR system 2400 (Perkin Elmer, Norwalk, USA). The temperature profile was the one previously described by Vinuesa et al. (1998), except for the extension of the initial denaturation step to 7 minutes, as required by Ampli-Taq Gold polymerase. Amplification products were run on 1% agarose gels and the bands were purified using the Quiaex II kit (Quiagen, Germany). The nucleotide sequence of the purified bands was determined by the dideoxy chain terminator method, using the ABI-PRISM Big Dye Terminator Cycle Sequencing Ready Reaction kit (Perkin Elmer, USA) and automated sequencer ABI PRISM 3100 Avant Genetic Analyzer (Applied Biosystems, Germany). The sequenced fragment analysed correspond to the first 650 bp of the 16S-rRNA gene, comprising hypervariable regions V1, V2 and V3 (Neefs et al. 1990).
DNA sequences were analysed using the biocomputing tools provided on-line by the European Bioinformatics Institute (http://www.ebi.ac.uk). The BLASTn (Altschul et al. 1997) program was used for preliminary sequence similarity analysis. The ClustalX v.1.8 software (Jeanmougin et al. 1998) was used for sequence alignment, and generation of the distance matrix. Phylogenetic and molecular evolutionary analyses were conducted with MEGA version 2.1 (Kumar et al. 2001), using the Neighbour-Joining method and the bootstrap test (Felsenstein 1985; Jeanmougin et al. 1998).
Phenotypic characterization of bacterial isolates
In addition to genetic characterization, strain LAS09 was further identified biochemically following the criteria outlined in Bergey´s Manual of Systematic Bacteriology (2005). The following morphological and biochemical tests were used: cell morphology, motility, spore formation, Gram-stain, aerobic/anaerobic growth, catalase, oxidase, NO −3 reduction, denitrification activity, glucose fermentation, utilization of carbon substrates (citrate, mannitol, inositol, sorbitol, ramnose, sucrose, melibiose, amigdaline, arabinose), O/F test, ONPG, arginine dihydrolase, lysine decarboxylase, ornithine decarboxylase, H2S production, urease activity, tryptophan deaminase, indol, Voges–Proskauer test, and gelatinase. Most of the tests were performed using the API 20E identification system (BioMerieux, SA, France).
Growth of bacterial strains in dialysed soil media amended with LAS
All the nine strains isolated from soil enrichment cultures amended with LAS in the first experiment were subsequently tested for growth in dialysed soil medium in the presence of 50 μg ml−1 of LAS. The dialysed soil medium was prepared according to González-López and Vela (1981) and it was characteristically oligotrophic. Agricultural soil samples were air-dried and sieved through a 2 mm mesh screen, and placed in 15-g amounts into dialysing tubing (14 kDa molecular weight cut off). The tubes were tied at both ends, placed in 250-ml Erlenmeyer flasks (amended with 50 ml of distilled water) and autoclaved at 121°C for 30 min. The dialysis bags filled with soil were left in the flask cultures as continuous source of nutrients. This growth medium remained clear, soil particles did not spill out of the dialysis bags, and when bacteria grew they did not penetrate the bag. The sterile, equilibrated system, had a final pH of 7.0. Sterile LAS solution was added after autoclaving, to reach the desired concentration. All strains were cultured in 1/10 TSB medium for 24 h, cell were spun down and washed with sterile saline (0.9% NaCl), and 0.5 ml were used to inoculate each dialysed soil media flask. Then the cultures were incubated at 30°C under aerobic conditions (orbital shaker, 100 rpm). Platable cell counts were estimated in diluted (1/10) TSA medium (Difco), as previously described. The inoculated agar plates were incubated at 30°C for 48 h before colonies were counted. All the experiments were done in triplicate.
Biodegradation studies
The three strains growing better in the presence of LAS were selected to study their capacity for LAS biodegradation in dialysed soil medium in the presence of 50 μg ml−1 of LAS and along a 10 days study period. Inoculated and control (lacking bacteria) dialysed-soil media amended with 50 μg ml−1 of LAS were cleared by centrifugation at 10000g for 10 min. Samples (30 ml) were cleaned and concentrated in a C18 solid-phase extraction cartridge. LAS was eluted with a mixture of methanol and water. The filtered samples were analysed by high performance liquid chromatography (HPLC) according to Nimer et al. (2007). Analysis was done with a Hewlett Packard HP 1050 series high-performance liquid chromatograph, equipped with a quaternary pump, an online degasser, an autosampler, an automatic injector with a loop of up to 100 μl, a thermostated column compartment, and a fluorescence detector (flow-cell volume 8 μl, HP 1049 A) connected on-line. Compounds were separated on a 125 × 4 mm I.D., 5 μm particle size, LiChrospher-100 RP-8 column with a 15 mm LiChrospher-100 RP-8 safeguard column and on two 250 × 4 mm I.D., 5 μm particle size, LiChrospher-100 RP-18 columns, purchased from Merck. The mobile-phase gradient was prepared from methanol and 30 mmol L-1SDS. The initial conditions were 55% for methanol and 45% for SDS and the percentage of methanol was then increased linearly to 70% in 15 min and then returned to 55% in 1 min. The amount of SDS was then set at 45% for 5 min to restore the initial conditions. With this method, LAS homologues C10, C11, C12 and C13 were detected, but no sulfophenyl carboxilates (SPCs) were determined.
Linear Alkylbenzene Sulfonates (LAS) was identified according to its retention time, by comparison of the fingerprint with a qualitative standard. The quantitative calculation was carried out on the basis of peak areas. The detection limit was 0.05 μg ml−1. The recovery rate was 89.3 ± 3.0%.
Evolution of the availability of total N and soluble phosphates in dialysed culture media amended with LAS
Uninoculated flasks of dialysed soil media, prepared as described in section “Growth of bacterial strains in dialysed soil media amended with LAS” and containing 0 or 50 μg ml−1 LAS, were incubated for 0, 24, 48 and 72 h and analysed for the concentration of total N and phosphates in the solution. Total N was measured by the Kjeldahl method as previously described (APHA 2001), and phosphates were measured according to Rodier (1989). This method was previously validated regarding the possible effects of surfactant LAS on phosphate quantification.
Statistical analysis
Two-way analysis of variance (ANOVA) using the software package Statgraphics 3.0 Plus version (STSC Inc., Rockville, MD, USA) was done to identify significant differences between the treatments. A significance level of 95% (P < 0.05) was selected.
Results
Nine major colony types were detected as the dominant heterotrophic platable bacteria growing aerobically in diluted (1/10) TSA, from the enrichment cultures produced from suspensions of soil in LAS-amended BH medium. The number of major colony types isolated from soil samples unamended with LAS (controls) was equal to the number of major colony types isolated from the culture amended with 10 μg ml−1 LAS. Strains LAS01 to LAS08, representatives of the dominating colony types, were purified from plates coming from the enrichment culture amended with 10 μg ml−1 LAS. In contrast, a single colony morphology type was detected in plates coming from the enrichment cultures amended with 500 μg ml−1. The reduction of colony types compared to the controls and to the soil treated with 10 μg ml−1 demonstrated a notable effect of this concentration of LAS on the diversity of aerobic heterotrophic platable soil microorganisms, under the conditions tested in the study. Ten different colonies of identical morphology were randomly selected from plates, reisolated and tested for phenotypical characters, yielding identical results. Only one strain (LAS09), randomly chosen from these 10 colonies, was further identified by 16S rRNA sequencing and selected for further analyses.
The phylogenetic affiliations of the 9 strains, based in partial sequencing of the 16S-rRNA gene (V1 to V3 hypervariable regions, ca. 650 nt), are shown in Fig. 1. The strains fell into six different genera of Gram-negative and Gram-positive bacteria. Sequence comparison with databases demonstrated the affiliation of strain LAS01 to Pseudomonas aeruginosa (99.8% identity), LAS02 and LAS03 strains to Bacillus cereus (99.7 and 98.8% identity, respectively), LAS04 strain to Acinetobacter sp. (99.4%, identity), LAS05 to Pseudomonas syryngae (99.4% identity), LAS06 and LAS08 strains to Staphylococcus epidermidis (99.4 and 100% identity, respectively), LAS07 to Delftia tsuruhatensis (99.9% identity), and finally LAS09 to Enterobacter sp. (99.5% identity). As previously mentioned, strain LAS09 was further characterized by biochemical tests, allowing its classification as Enterobacter aerogenes. The bacterial colonies isolated from the controls without LAS were morphologically similar to those obtained from the enrichment experiments with 10 μg ml−1 LAS, meaning that the application of LAS at this concentration had no effect on the initial composition of the soil microbiota.
The nine strains were subsequently cultured in dialysed soil media either amended with 50 μg ml−1 of LAS or lacking surfactant, and viable bacterial counts were done after 24 and 48 h of culture. Positive results were considered when the viable counts demonstrated an increase of cell numbers of at least one logarithm unit in the presence of LAS. Only strains LAS05 (P. syringae), LAS06 (S. epidermidis), LAS07 (D. tsuruhatensis), LAS08 (S. epidermidis) and LAS09 (E. aerogenes), were able to grow in dialysed soil medium amended with 50 μg ml−1 of surfactant (Table 1). The two Bacillus strains were particularly sensitive to LAS addition and their cell numbers sharply decreased after 48 h of incubation. Strains LAS05, LAS07 and LAS09 grew to higher cell numbers in media amended with LAS, compared to controls. These results suggested that the selected strains could be degrading LAS to use it as a C source.
The ability of strains LAS05, LAS07 and LAS09 to remove LAS was further checked by culturing the strains for 10 days in dialysed soil media amended with 50 μg ml−1 LAS, and performing an HPLC analysis of the supernatants of the cultures. Again, the presence of LAS in the culture media significantly enhanced the growth of the 3 strains assayed, compared to curves in LAS-free dialysed soil medium (Fig. 2). Figure 3 shows the evolution of LAS concentration in the supernatants, compared to uninoculated culture media. No significant differences were found, suggesting that LAS was being removed from the liquid phase by non-biological processes.
Growth curves (as log CFU ml−1) of Pseudomonas syringae strain LAS05, Delftia tsuruhatensis strain LAS07, and Enterobacter aerogenes strain LAS09, in dialysed soil media amended with 50 μg ml−1 of LAS (■). Control growth curves in media without LAS (▲) are included for comparison. Values are an average of three experiments (P ≤ 0.05)
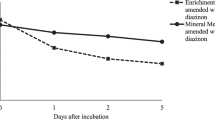
Evolution of the concentration of LAS (mg l−1) available in dialysed soil media amended with 50 μg ml−1 of LAS and inoculated with Pseudomonas syringae strain LAS05, Delftia tsuruhatensis strain LAS07, and Enterobacter aerogenes strain LAS09. Striped bars: inoculated media. Dotted bars: uninoculated media. Values are an average of three experiments (P ≤ 0.05)
In order to explain the enhancement of the growth of bacterial strains when LAS was present in dialysed culture media, in spite of their inability to use it as C source, the effect of the addition of LAS on the availability of inorganic nutrients (N and P) was evaluated. For this purpose, the concentration of total N and soluble phosphate were measured in uninoculated oligotrophic media after being incubated for the same period of time than the bacterial strains tested. As shown in Table 2, dialysed culture media amended with 50 μg ml−1 had significantly higher concentrations of soluble phosphates compared to unamended control media, while changes on the concentration of total N were not significant.
Discussion
The fertility of an agricultural soil is intimately linked to its particular microbiota and the relationships that exist between the microbial groups involved in nutritional cycles, which are essential for the normal function and evolution of the soil. Previous studies on the effect of LAS on soil microbiota generally agree that concentrations of LAS in the range derived from sludge application to soil as fertilizer had no significant influence in functional diversity on the activity of the heterotrophic bacterial community (Elsgaard et al. 2001a; Brandt et al. 2003; Vinther et al. 2003). These studies were based on evaluation of basal and substrate-induced respiration, selected enzymatic activities, and community level physiological profiles. However, studies focused on the specific composition of the heterotrophic bacterial community in soils exposed to LAS are scarce.
According to our experiments (“in vitro” studies), the addition of LAS to an agricultural soil exerted a selective pressure on the diversity of the heterotrophic platable bacteria, incubated under aerobic conditions. Our data show that selected groups of Gram-positive bacteria such as Bacillus-related strains were particularly sensitive to the application of LAS in pure cultures at a dose of 50 μg ml−1 (Table 1). Thus, LAS doses of 50 μg ml−1 or higher are deleterious against particular strains of soil microorganisms, in agreement with previous studies (Wilke 1997). There is a long-time controversy regarding whether LAS may be more toxic for Gram-positive or Gram-negative bacteria. Early studies by Hartmann (1966) described the higher sensitivity of Gram-positive bacteria to anionic detergents, while Lee (1970) reported that Gram-positive bacteria were more resistant to LAS than Gram-negative strains. More recently, Elsgaard et al. (2001b) concluded that LAS affects both Gram-negative and Gram-positive bacteria. The evaluation of the results of our study suggest that Gram-negative bacteria are more resistant to higher concentrations of LAS than Gram-positive bacteria.
Several studies (Berna et al. 1989; Knaebel et al. 1990; Brandt et al. 2003; Pozo et al. 2003) have repeatedly reported that LAS have a short half-life in agricultural soils, due to its degradation by soil microbiota. According to our experiments, the growth of three bacterial isolates classified as Enterobacter aerogenes, Pseudomonas syryngae and Delftia tsuruhatensis was improved in dialysed soil media amended with 50 μg ml−1 of LAS. Bacterial strains affiliated to these three genera have been often described in the literature as showing ability to biodegrade aromatic compounds (López et al. 2005). However, although no studies with radiolabeled LAS have been performed here, our results suggest that the strains mentioned above can tolerate high concentrations of LAS (particularly Enterobacter aerogenes LAS09) but are unable to remove it from the culture media. In this context, although microorganisms are ultimately responsible for the degradation of LAS, the limited metabolic abilities of individual bacterial isolates suggest that bacterial consortia are necessary for LAS biodegradation (Van Ginkel 1996). However, a final conclusion on this point has not been yet determined.
Brandt et al. (2003) reported a significant disturbance of the heterotrophic soil microbial community after exposition to sludge amended with high concentration of LAS, which overlapped with the degradation of 90% of the surfactant. In the same study, stimulation of microbial activity in the soil surrounding the sludge was also reported. Addition of LAS (174 mg kg−1) to soil increases the total number of soil bacteria over twofold, compared to LAS-free soil (Vinther et al. 2003). These increase was assumed as due to the use of LAS as carbon source and the surfactant effect of LAS on the release of bacteria attached to soil particles. Our studies show that the increase of cell numbers of specific strains in the presence of LAS in liquid culture media can be unrelated to their ability to use it as C source. Dialysed soil media provide an oligotrophic milieu where microbial growth is not only limited by C availability but also by P availability (González-López and Vela 1981). Our results have demonstrated that phosphate concentrations in uninoculated dialysed soil media amended with LAS were significantly higher than the phosphate concentrations in the same dialysed soil media without LAS, suggesting an effect of the surfactant enhancing the phosphate mobilization from the soil and making it available for bacteria. P is well known as a limiting factor for microbial growth in the soil environment, and thus the effect of LAS on the concentration of soluble phosphates released from soil is an explanation for the stimulation of growth of the strains studied in this work. A possible consequence of the surfactant properties of LAS on the mobilization of nutrients from soil was also proposed by Elsgaard et al. (2001b), but not actually investigated.
Conclusions
The application of high concentrations of LAS (500 μg ml−1) to an agricultural soil produces a significant reduction of the biological diversity of the heterotrophic platable microorganisms incubated under aerobic conditions. An isolate identified as Enterobacter aerogenes, was highly resistant to the addition of this anionic surfactant to concentrations up to 500 μg ml−1. Any of the assayed strains was able to utilize LAS as C source when grown in pure culture, in spite of their growth being enhanced in dialysed soil media amended with 50 μg ml−1 of LAS, possibly due to the mobilization of phosphates from soil into the media induced by LAS. Our data indicate that the application of sewage sludge, wastewater or pesticides formulations containing LAS to an agricultural soil could be considered a potential risk for selected species of the soil microbiota and their microbial activities. Experiments are in progress to further explore the effect of LAS on particular functional groups of bacteria in soil.
References
Altschul SF, Madden TL, Schaeffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acid Res 25:3389–3402
APHA (2001) Standard methods for the examination of water and wastewater, 20th edn. Clesceri LS, Greenberg AE, Eaton AD (eds) American Public Health Association, Washington DC, USA
Bergey´s Manual of Systematic Bacteriology (2005) 2nd edn. Garrity GM (ed) Springer, NY, USA
Berna JL, Ferrer J, Moreno A, Prats D, Ruiz F (1989) The fate of LAS in the environment. Tenside Surf Det 26:101–107
Berth P, Jeschike P (1989) Consumption and fields of application of LAS. Tenside Surf Det 26:75–79
Brandt KK, Hesseløe M, Roslev P, Henriksen K, Sørensen J (2001) Toxic effects of linear alkylbencene sulfonate on metabolic activity, growth rate and microcolony formation of Nitrosomonas and Nitrosospira strains. Appl Environ Microbiol 67:2489–2498
Brandt KK, Pedersen A, Sorensen J (2002) Solid-phase contact assay that uses a luxmarked nitrosomonas europea reported strain to estimate toxicity of bioavailale linear alkylbenzene sulfonate in soil. Appl Environ Microbiol 68:3502–3508
Brandt KK, Krogh PH, Sørensen J (2003) Activity and population dynamics of heterotrophic and ammonia-oxidizing microorganisms in soil surrounding sludge bands spiked with linear alkylbenzene sulfonate: a field study. Environ. Toxicol Chem 22:821–829
Bremmer JM (1965) Inorganic forms of nitrogen. Agronomy 9:1179–1237
De Wolf W, Feijtel T (1998) Terrestrial risk assessment for linear alkylbenzene sulphonate (LAS) in sludge amended soils. Chemosphere 36:1319–1343
Elsgaard L, Petersen S, Debozs K (2001a) Effects and risk assessment of linear alkylbenzene sulfonates in agricultural soil. 1. Short-term effects on soil microbiology. Environ Toxicol Chem 20:1656–1663
Elsgaard L, Petersen S, Debozs K, Kristiansen IB (2001b) Effects of linear alkylbenzene sulfonates (LAS) on soil microbial ecology. Tenside Surf Det 38:94–97
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
González-López J, Vela GR (1981) True morphology of the Azotobacteracea-filterable bacteria. Nature 289:588–590
Hartmann L (1966) Effects of surfactants on soil bacteria. Bull Environ Cont Toxicol 1:219–224
Hislop EC, Barnaby VM, Burchill RT (1977) Aspects of the biological activity of surfactants that are potential eradicants of apple mildew. Ann Appl Biol 87:29–39
Jacobsen AM, Mortensen GK, Hansen HCB (2004) Degradation and mobility of linear alkylbenzene sulfonate and nonylphenol in sludge-amended soil. J Environ Qual 33:232–240
Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ (1998) Multiple sequence alignment with Clustal X. Trends Biochem Sci 23:403–405
Jensen J (1999) Fate and effects of linear alkylbenzene sulphonates (LAS) in the terrestrial environment. Sci Total Environ 226:93–111
Jensen J, Jepsen SE (2005) The production, use and quality of sewage sludge in Denmark. Waste Manag 25:239–247
Jensen J, Lokke H, Holmstrup M, Krog PH, Elsgaard L (2001) Effects and risks assessment of linear alkyl-benzene sulfonates in agricultural soils. Probabilistic risk assessment of linear alkylbenzene sulfonates in sludge-amended soils. Environ Toxicol Chem 20:1690–1697
Khleifat KM (2006) Biodegradation of linear alkylbenzene sulfonate by a two-member facultative anaerobic bacterial consortium. Enzyme Microb Tech 39:1030–1035
Knaebel DB, Federle TW, Vestal JR (1990) Mineralization of LAS and LAE in 11 contrasting soils. Environ Toxicol Chem 9:981–988
Kumar S, Tamura K, Jakobsen IB, Nei M (2001) MEGA2: molecular evolutionary genetics analysis software. Arizona State University, Tempe, Arizona, USA
Lee BKH (1970) The effect of anionic and non-anionic detergents on soil microfungi. Can J Bot 48:583–589
López L, Pozo C, Calvo C, Juárez B, Martínez-Toledo MV, González-López J (2005) Identification of bacteria isolated from an oligotrophic lake with pesticides removal capacities. Ecotoxicology 14:299–312
Marcomini A, Capel PD, Lichtensteiger TH, Brunner PH, Giger W (1989) Behaviour of aromatic surfactants and PCBs in sludge-treated soil and landfills. J Environ Qual 18:523–528
Natural Resources Conservation Service (1999) Soil taxonomy, a basic system of soil classification for making and interpreting soil surveys, 2nd edn. U.S. Department of Agriculture, Washington, DC, USA
Neefs J, Van de Peer Y, Hendriks L, De Wachter R (1990) Compilation of small ribosomal subunit RNA sequences. Nucleic Acid Res 18:2237–2242
Nielsen KB, Brandt KK, Jacobsen AM, Mortensen G, Sørensen J (2004) Influence of soil moisture on linear alkylbenzene sulfonate-induced toxicity in ammonia-oxidizing bacteria. Environ Toxicol Chem 23:363–370
Nimer M, Ballesteros O, Navalón G, Crovetto C, Verge C, López I, Berna JL, Vílchez JL (2007) New simple treatment for determination of linear alkylbenzene sulfonate (LAS) in agricultural soil by liquid chromatography with fluorescence detection. Anal Bioanal Chem 387:2175–2184
Olsen SR, Dean LA (1965) Phosphorous. In: Black CA et al (ed) Methods of soil chemical analysis, Part 2. Agronomy, vol 9. American Society of Agronomy, Inc., Madison, Wiscosin, USA pp 1035–1049
Painter HA (1992) Anionic surfactants. Handbook Environ Chem 3:2–88
Pozo C, Rodelas B, Calvo C, Martínez-Toledo MV, González-López J (2003) Linear alkylbenzene sulfonates (LAS) on soil microbial activity. Food Agric Environ 1:348–350
Pratt PF (1954) Potassium release from exchangeable and non-exchangeable forms in soils. Ohio Agric Exp Stn Resour Bull 47:747
Rodier J (1989) Anàlisis de aguas. Aguas naturales, aguas residuales, agua de mar. Ediciones Omega SA. Barcelona, Spain, pp 186–191
Schwuger MJ, Bartnik FG (1980) Interaction of anionic surfactants with proteins, enzymes and membranes. In: Gloxhuber C (ed) Anionic surfactants-biochemistry, toxicology, dermatology. Marcel Dekker, New York, USA, pp 1–49
Toledo FL, Calvo C, Rodelas B, González-López J (2006) Selection and identification of bacteria isolated from waste crude oil with polycyclic aromatic removal capacities. Syst Appl Microbiol 29:244–252
Van Ginkel CG (1996) Complete degradation of xenobiotics surfactants by consortia of aerobic microorganisms. Biodegradation 7:151–164
Vinther FP, Mortensen G, Elsgaard L (2003) Effects of linear alkylbenzane sulfonates on functional diversity of microbial communities in soil. Environ Toxicol Chem 22:35–39
Vinuesa P, Rademaker JLW, De Bruijn FJ, Werner D (1998) Genotypic characterization of Bradyrhizobium strains nodulating endemic woody legumes of the Canary Islands by PCR-restriction fragment length polymorphism analysis of genes encoding 16S rRNA (16S rDNA) and 16S−23S rDNA intergenic spacers, repetitive extragenic palindromic PCR genomic fingerprinting, and partial 16S rDNA sequencing. Appl Environ Microbiol 64:2096–2104
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Wilke BM (1997) Effects of non-pesticide organic pollutants on soil microbial activity. Adv Geoecol 30:117–132
Ying GG (2006) Fate, behaviour and effects of surfactants and their degradation products in the environment. Environ Int 32:417–431
Acknowledgments
This work was funded by the Spanish Ministerio de Educación y Ciencia (MEC), as part of Project Reference PPQ2003-07978-V02-02 (Programa Nacional de I+D). B. Rodelas was supported by Programa Ramón y Cajal (MEC, Spain). C. Pozo was granted by Programa “Retorno de Doctores”, Consejería de Educación y Ciencia, Junta de Andalucía, Spain.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sánchez-Peinado, M., González-López, J., Rodelas, B. et al. Effect of linear alkylbenzene sulfonates on the growth of aerobic heterotrophic cultivable bacteria isolated from an agricultural soil. Ecotoxicology 17, 549–557 (2008). https://doi.org/10.1007/s10646-008-0212-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-008-0212-2