Abstract
Usage of cephalosporin and quinolone antibiotics has aggravated the development of extended-spectrum beta-lactamase (ESBL)–producing quinolone-resistant (QR) pathogenic Enterobacteriaceae. The present study aims to determine antimicrobial activity of cinnamaldehyde alone or in combination with cefotaxime/ciprofloxacin to reverse the drug resistance and evaluations of efficacy, and possible molecular mechanism of action of the combination was also evaluated using in vitro assays. Broth microdilution assay was used to determine minimum inhibitory concentrations (MICs) of cinnamaldehyde and antibiotics against ESBL-QR Enterobacteriaceae. Synergistic effect and dynamic interaction with antibiotics were further examined by checkerboard assay, isobologram analysis, and time-kill assay, respectively. Cellular morphology of bacteria was viewed with scanning electron microscopy (SEM). Effects of cinnamaldehyde and its combination on the expression of gene encoding—porins (ompC, ompF, ompK35, and ompK36), efflux pump genes (acrB–E. coli, acrB–K. pneumoniae), and antibiotic-resistant genes (blaTEM, blaSHV, blaCTXM, and QnrB) were evaluated using real-time quantitative PCR (RT-qPCR). Majority of the E. coli (32.1%) and K. pneumoniae (24.2%) isolates demonstrated MIC of cinnamaldehyde at 7.34 μg/mL and 0.91 g/mL, respectively. Synergism between cinnamaldehyde and cefotaxime was noted among 75% E. coli and 60.6% K. pneumoniae. Similarly, synergism with ciprofloxacin was observed among 39.6% and 42.4% of the bacteria, respectively. Thus, cinnamaldehyde reduced MIC of cefotaxime and ciprofloxacin 2–1024-fold with bactericidal and/synergistic effect after 24 h. Cinnamaldehyde and its combination altered gene expression by ~ 1.6 to ~ 400-fold. Distorted bacterial cell structures were visible after treatment with cinnamaldehyde and/or with cefotaxime/ciprofloxacin. The results indicated the potential efficacy and mode of action of cinnamaldehyde alone and in combination with antibiotics against pathogenic ESBL-QR bacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Indiscriminate and irrational usage of cephalosporin and quinolone antibiotics has aggravated the development of extended-spectrum beta-lactamase (ESBL)–producing quinolone-resistant (QR) pathogenic Enterobacteriaceae, thereby reducing efficacy of cephalosporins and quinolone drugs against these bacteria [1,2,3]. ESBL production with quinolone resistance development among Enterobacteriaceae results from production of beta-lactamases (TEM, SHV, and CTX-M), expression of QNR genes, enhanced efflux pump expression (AcrAB-TolC), and alteration of outer membrane permeability (OmpF/OmpC: Escherichia coli and Ompk35/Ompk36: Klebsiella pneumoniae, respectively) [4, 5]. Thus, development of an alternative drug line to treat and control ESBL-QR pathogenic bacteria is urgently needed. Plant bioactive compounds with intrinsic antimicrobial properties may offer a plethora of interesting possibilities to combat antibiotic resistance [6]. The use of plant bioactive compounds in combination with conventional antibiotics has been proposed to be an effective method to control multidrug resistance as this combination targets multiple facets of infectious agents. Cinnamaldehyde demonstrated synergistic interaction with various antibiotics against Gram-positive and Gram-negative bacteria [7,8,9,10,11].
However, antibacterial activity of cinnamaldehyde along with its combinatorial effect with traditional antibiotics has not yet been tested against ESBL-QR Enterobacteriaceae. The present study aims to analyze antibacterial and synergistic activity of cinnamaldehyde against pathogenic ESBL-QR Enterobacteriaceae and evaluate their effect through several antibacterial, microscopic, and gene-expressional analysis to find out its therapeutic potential in antibacterial applications.
Materials and method
Bacterial sample collection
After obtaining institutional ethical committee approval, sixty-one ESBL-QR clinical isolates (E. coli (n = 28); K. pneumoniae (n = 33)) were collected from unrelated patient’s visiting Calcutta School of Tropical Medicine (ref. no. CREC-STM/53 dated 23/09/2011). All ESBL-QR bacteria demonstrated ESBL property against ceftazidime, cefotaxime, their inhibitor combinations, and quinolone resistance against any three of the following quinolone drugs: nalidixic acid, ciprofloxacin, and levofloxacin prulifloxacin (Hi-Media Lab Ltd., India).
Determination of minimum inhibitory concentrations
Minimum inhibitory concentrations (MICs) of cinnamaldehyde (CIN) (HiMedia Lab Ltd., India), cefotaxime (CTX), and ciprofloxacin (CIP) against ESBL-QR bacteria were determined by microbroth dilution method according to the guidelines of the Clinical and Laboratory Standards Institute [12]. MIC of each compound against a maximum number of ESBL-QR Enterobacteriaceae was considered as MIC (mode-MIC value) of that compound, and those concentrations (MIC-CIN, MIC-CTX, and MIC-CIP) were selected for downstream studies.
Determination of synergism between cinnamaldehyde and antibiotics
Combinatorial effects of CIN with antibiotics were determined by the checkerboard method [13]. Twofold serial dilutions of each compound were prepared to achieve final concentrations of CIN and antibiotics from 0.22 to 7.28 μg/mL and 0.5 to 512 μg/mL, respectively. Synergistic interactions were validated using the CompuSyn software version1.0 to generate isobologram, combination index (CI), and drug-reduction index (DRI) [14].
Time-kill kinetics assay
To determine the dynamic interaction of CIN and antimicrobial agent(s) against ESBL-QR isolates, the time-kill test was performed according to the CLSI guidelines and Zhou et al. [12, 15].
Bacterial membrane integrity assay
Intactness of bacterial cell membrane was measured by fluorescence using Live/Dead BacLight assay kit (Thermo Fisher Scientific, USA) according to manufactures’ protocol [16]. Bacterial cells were incubated in the presence of MIC-CIN/CTX/CIP alone and their combinations and visualized under a fluorescence microscope (LEICA DM2000, India).
Bacterial morphology study
Morphological changes of bacterial cells grown for 6 h at 37 °C in MHB supplemented with or without 1/2-MIC-CIN/CTX/CIP alone and their combinations were visualized using scanning electron microscope (SEM) (ZEISS EVO-MA 10, Denmark) following standard protocol [17].
Transcriptional expression profiles of antibiotic-resistant genes, efflux pump gene, and porins
Quantitative RT-PCR was performed to determine changes in expression of antibiotic-resistant genes (blaTEM, blaSHV, blaCTXM, and QnrB), efflux pump (acrB–E. coli, acrB–K. pneumoniae), and porins (ompC, ompF, ompK35, and ompK36) in the presence and absence (untreated control) of 1/2-MIC CIN alone and combined with CTX/CIP [15]. Quantification of target genes was performed on ABI Prism 7500 using Power SYBR Green PCR MasterMix (Applied Biosystems, USA). Oligonucleotides used in this study were mentioned in online resource 1. Relative level of target gene expression compared with 16S ribosomal RNA (internal control) was determined by calculating 2-∆∆CT method.
Statistical analysis
Time-kill curves and RT-PCR data were expressed as mean ± standard error. Values of the treated groups were statistically compared with those of untreated control group by Student’s t test and one-way ANOVA performed with Dunnett’s HSD post hoc comparison. For CIN- and CTX/CIP-treated group comparison, one-way ANOVA with Tukey’s HSD post hoc was performed. P < 0.01 and P < 0.001 were considered statistically significant and highly significant. Statistical analysis was carried out using GraphPad prism.
Results and discussion
Indiscriminate use of beta-lactam and quinolone antibiotics often results in the development of resistance against these antibiotics. Therefore, investigations of a novel antibacterial candidate to overcome such resistance would be intriguing. In the present study, efficacy of cinnamaldehyde as candidate molecule for the treatment of ESBL-producing and quinolone-resistant (ESBL-QR) pathogenic bacteria was evaluated.
Microdilution assay demonstrated MIC of CTX and CIP at 512 μg/mL among 67.9% and 42.9% of ESBL-QR E. coli, whereas CIN exhibited MIC at 7.34 μg/mL among 32.1% isolates. Likewise, traditional antibiotics and CIN showed MIC at 512 μg/mL and 0.91 μg/mL among 54.54%, 57.6%, and 24.2% ESBL-QR K. pneumoniae, respectively—indicating greater efficacy of cinnamaldehyde compared with traditional antibiotics against both organisms (Fig. 1a).
Antibacterial activity and synergy determination of cinnamaldehyde with antibiotics. a Distribution of MICs of cefotaxime (CTX), ciprofloxacin (CIP), and cinnamaldehyde (CIN). b, c Combined effect of cinnamaldehyde and CTX and CIP against ESBL-QR E. coli. d, e Combined effect of cinnamaldehyde and CTX and CIP against ESBL-QR K. pneumoniae. f, g Isobologram of cinnamaldehyde and CTX and CIP against ESBL-QR E. coli. h, i Isobologram of cinnamaldehyde and CTX and CIP against ESBL-QR K. pneumoniae
Synergistic interaction between CIN and CTX was noticed among 75% (21/28) E. coli and 60.6% (20/33) K. pneumoniae with FIC-index (E. coli, 0.07–0.3; K. pneumoniae, 0.07–0.5) (Fig. 1b, d and online resource 2). In the presence of MIC-CIN, MIC-CTX was reduced 4–1024-fold among E. coli and 2–1024-fold among K. pneumoniae. However, synergism between CIN and CIP (FIC-index E. coli, 0.07–0.5; K. pneumoniae, 0.1–0.5) was observed among 39.6% (12/28) E. coli and 42.4% (14/33) K. pneumoniae (Fig. 1c, e). MIC-CIP was reduced 2–512-fold and 2–1024-fold for E. coli and K. pneumoniae, respectively, in the presence of MIC-CIN. Further, the presence of combination data points below additive lines, CI between 0.01 and 0.23 and DRI > 98-fold confirmed clear synergistic activity of traditional antibiotics with cinnamaldehyde (Fig. 1f–i and online resource 3).
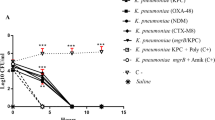
Time-dependent bactericidal effect of MIC-CIN alone and in combination with antibiotics against ESBL-QR strains indicated a decrease in viable bacterial cell count by > 2log10 CFU/mL within 2–3 h (Fig. 2a–d). In contrast to CIN and antibiotic-alone treatments, combination regimens displayed better bactericidal activities that persist for 24 h.
Time-kill kinetics of cinnamaldehyde with antibiotics alone and its combination on bacterial membrane integrity. Effect of cinnamaldehyde alone and its combination with cefotaxime (CTX) (a) and ciprofloxacin (CIP) (b) on growth of E. coli. Effect of cinnamaldehyde alone and its combination with cefotaxime (CTX) (c) and ciprofloxacin (CIP) (d) on growth of K. pneumoniae. e Representative photomicrographs showing cell viability of ESBL-QR E. coli (i–viii) and K. pneumoniae (ix–xvi). Bar = 50 휇m
After 8 h of drug treatment, MIC-CIN in combination with MIC-CTX/MIC-CIP had reduced E. coli colony count by 0.7- and 0.9-fold compared with MIC-CTX/MIC-CIP alone. At sub-MIC synergistic combination of CIN with CTX/CIP, viable bacterial cell count decreased by 0.5- and 0.4-fold, respectively (Fig. 2a, b).
Similarly, after 8 h of drug treatment, MIC-CIN in combination with MIC-CTX/MIC-CIP reduced K. pneumoniae bactericidal activity by 0.6- and 0.8-fold compared with MIC-CTX/MIC-CIP alone (Fig. 2b, d). At sub-MIC synergistic combination of CIN with CTX/CIP, viable bacterial cell count decreased by 0.3- and 0.4-fold.
Fluorescence microscopic analysis of ESBL-QR E. coli and K. pneumoniae with MIC-CIN alone and in combination with CTX/CIP demonstrated the presence of dead cells (red fluorescence) (Fig. 2e). E. coli and K. pneumoniae treated with CTX/CIP alone demonstrated the presence of live cells at reduced number compared with untreated control (green fluorescence) (Fig. 2e; iii, iv and xi, xii).
Untreated ESBL-QR E. coli and K. pneumoniae were visualized as oval- and spherical-shaped cells with very smooth surfaces (Figs. 3a and 4a). However, after treatment with MIC-CTX/CIP alone, E. coli exhibited elongated structure with some particulate around their surfaces and K. pneumoniae became filamentous with rough surfaces (Figs. 3b, c and 4b, c). This phenomenon has been previously documented as bacterial filamentation as a survival strategy during stressful condition to maintain their morphological plasticity [18]. Some earlier studies reported cefotaxime/ciprofloxacin induced SOS response among ESBL strain, which triggered filamentation [15, 19].
Effect of cinnamaldehyde with antibiotics alone and its combination on ESBL-QR E. coli cellular morphology. Scanning electron micrograph of ESBL-QR E. coli cells treated with cinnamaldehyde (CIN), cefotaxime (CTX), and ciprofloxacin (CIP) alone and its combination. a Untreated E. coli cells showing an intact, regular oval shape with smooth cell membrane. b, cE. coli cells grown in the presence of MIC CTX/CIP. 1, elongated; 2, receding of cytoplasmic membrane; 3, rough cell membrane with some particulate material. d, eE. coli cells were grown in the presence of MIC CIN. 4, pore and crumpled on cell surface; 5, diminishing of cytoplasmic membrane and empty cells. f–hE. coli cells grown in the presence of combination of MIC CIN with CTX. 6, leakage and cell debris depositon on cell surfaces due to the bursting of cells were visible; 7–10, deep pore with rough irregular cell membrane. i, jE. coli cells grown in the presence of combination of MIC CIN with CIP. 11, receding of cytoplasmic content from cell membrane and crumbling of cellular content; 12, deposition of lytic material in a form of vesicles; 13, large groove
Effect of cinnamaldehyde with antibiotics alone and its combination on ESBL-QR K. pneumoniae cellular morphology. Scanning electron micrograph of ESBL-QR K. pneumoniae cells treated with cinnamaldehyde (CIN), cefotaxime (CTX), and ciprofloxacin (CIP) alone and its combination. a Untreated K. pneumoniae cells showing an intact, regular oval shape with smooth cell membrane. b, cK. pneumoniae cells grown in the presence of MIC CTX/CIP. 1–4, elongated; 2–3, rough cell membrane with some particulate material. d–fK. pneumoniae cells grown in the presence of MIC CIN. 5, convoluted surfaces with loosened cell membrane; 6, shrinkage cell membrane
After CIN treatment, while ESBL-QR E. coli cell surface became crumpled or grooves appeared on the cell surface with abnormal division of cells, K. pneumoniae exhibited cell membrane shrinkage with convoluted surfaces and loosened cell wall indicating initial damage (Figs. 3d and 4d). Such finding was in accordance with earlier studies that demonstrated damage to cell permeability and membrane integrity in pathogenic Porphyromonas gingivalis by Cinnamon bark essential oil and cinnamaldehyde [20].
In this study, altered cell surface morphology, shrinkage of cell surfaces, and diminished cytoplasm among ESBL-QR E. coli and K. pneumoniae were noticed on the treatment with CIN alone, indicating permeability changes that created osmotic inequity leading to cell lysis. Similar observations have also been reported in previous works on E. coli–ATCC8735 and S17 treated with cinnamaldehyde [21, 22].
Treatment with CIN and CTX/CIP revealed deep pore, disruption of cytoplasmic membrane, and decomposition of inner organelles on cell surfaces indicating autolysis leading to cell death, validating their synergistic relationship (Fig. 3f–k). These effects have been previously documented as converging effect of exogenous and endogenous oxidative stress generated by bioactive compound and cefotaxime which instigated membrane degradation with shrunken cell wall and disruption of cellular proteins [17, 23].
A significant change in expression level of outer membrane porins and efflux pump was noticed between CIN-treated and untreated control cell (Fig. 5a, b and Table 1). However, the change in beta-lactamase genes’ expression level due to CIN exposure was gene-specific (Fig. 5c and Table 1). OmpC was upregulated in the presence of CIN/CTX alone and in combination with CTX-CIP. Overexpression of OmpF was noticed after CIN/CTX/CIP-alone treatment and with CTX-CIP combination, respectively.
Effect of cinnamaldehyde with antibiotics alone and its combination on gene expression profile. Effect of cinnamaldehyde (CIN), cefotaxime (CTX), and ciprofloxacin (CIP) alone and its combination on gene expression profile. a Analysis of the expression of ompF, ompC, ompK35, and ompK3. b Analysis of the expression of acrB (E. coli) and acrB (K. pneumoniae). c Analysis of the expression of blaTEM, blaSHV, blaCTX-M, and QNRB gene. Asterisks indicate level of significance of a two-tailed Student’s t test (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001). Daggers indicate level of significance of ANOVA (†P ≤ 0.05, ††P ≤ 0.01, †††P ≤ 0.001)
OmpK35 was upregulated after treatment with CIN/CIP alone and using CTX/CIP and CIN combination. OmpK36 was upregulated in the presence of CIN, CTX, CIP alone, and after treatment with combination of CIN with CIP. Thus, upregulation of Omp expressions results in alteration of porin channels that might have decreased antibiotic resistance profile. Similar phenomenon was demonstrated with the upregulation of ompF in the presence of aqueous extract of Aegle marmelos fruit which allowed greater permeability of beta-lactam antibiotics among Enteropathogenic E. coli and multidrug-resistant Shigella dysenteriae and S. flexneri [24, 25].
Transcription levels of acrB were downregulated in the presence of CIN/CIP alone and CTX/CIP and CIN combination (Fig. 5b). Previous studies also documented downregulation of adeAB and AcrAB-TolC efflux pumps’ expressions among Acinetobacter baumannii and E. coli by cinnamaldehyde and total alkaloids [26, 27].
BlaTEM expression was noticeably inhibited after treatment with CIN and CTX respectively and with their combination. CIN also inhibited blaSHV and blaCTX-M expression (Fig. 5c). In contrast, no significant change was noticed in QNRB expression level after treatment with CIN and CIP. A similar reduction in the expression of these genes was demonstrated by baicalein against ESBL–K. pneumoniae [28]. All these findings suggested that efflux pump downregulation, porin overexpression, and beta-lactamase gene inhibition of ESBL-QR bacteria by cinnamaldehyde alone or in combination with traditional antibiotics might be attributed to overcoming bacterial drug resistance. Moreover, cinnamaldehyde was found to be nontoxic among intravenously treated mice previously [29]. Trans-cinnamaldehyde failed to exhibit detectable hepatocarcinogenic activity in mice. All these observations along with data obtained from the present study suggested efficient therapeutic potential of cinnamaldehyde. Thus, cinnamaldehyde seemed to enhance activities of traditional antibiotics also, thereby reducing their usage and toxicity, and combination therapy with cinnamaldehyde might eventually help to deter development of antibiotic resistance property of pathogenic bacteria.
Conclusion
Combination of cinnamaldehyde and cefotaxime/ciprofloxacin exhibited antibacterial as well as synergistic effects against ESBL-QR E. coli and K .pneumoniae. Thus, this work confirmed the therapeutic value of cinnamaldehyde against both ESBL-producing and quinolone-resistant pathogenic bacteria.
References
Chandra H, Bishnoi P, Yadav A, Patni B, Mishra AP, Nautiyal AR (2017) Antimicrobial resistance and he alternative resources with special emphasis on plant-based antimicrobials-a review. Plants (Basel) 10(2):6
Frank T, Mbecko JR, Misatou P, Monchy D (2011) Emergence of quinolone resistance among extended-spectrum beta-lactamase-producing Enterobacteriaceae in the Central African Republic: genetic characterization. BMC Res Notes 25:309
Laxminarayan R, Chaudhury RR (2016) Antibiotic resistance in India: drivers and opportunities for action. PLoS Med 13:e1001974
Alekshun MN, Stuart B (2007) Molecular mechanisms of antibacterial multidrug resistance. Cell 128:1037–1050
van Hoek Angela HAM, Mevius D, Guerra B, Mullany P, Roberts Paul A, Aarts Henk JM (2011) Acquired antibiotic resistance genes: an overview. Front Microbiol 2:1–27
Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P et al (2015) Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv 33:1582–1614
Jia P, Xue YJ, Duan XJ, Shao SH (2011) Effect of cinnamaldehyde on biofilm formation and sarA expression by methicillin-resistant Staphylococcus aureus. Lett Appl Microbiol 53:409–416
López P, Sanchez C, Batlle R, Nerín C (2007) Vapor-phase activities of cinnamon, thyme, and oregano essential oils and key constituents against foodborne microorganisms. J Agric Food Chem 55:4348–4356
Liu Q, Niu H, Zhang W, Mu H, Sun C, Duan J (2015) Synergy among thymol, eugenol, berberine, cinnamaldehyde and streptomycin against planktonic and biofilm-associated food-borne pathogens. Lett Appl Microbiol 60:421–430
Palaniappan K, Holley RA (2010) Use of natural antimicrobials to increase antibiotic susceptibility of drug resistant bacteria. Int J Food Microbiol 140:64–68
Hossan MS, Jindal H, Maisha S, Samudi Raju C, Devi Sekaran S, Nissapatorn V, Kaharudin F, Su Yi L, Khoo TJ, Rahmatullah M, Wiart C (2018) Antibacterial effects of 18 medicinal plants used by the Khyang tribe in Bangladesh. Pharm Biol 56:201–208
Clinical and Laboratory Standards Institute (2008) Performance standards for antimicrobial susceptibility testing; eighteenth informational supplement, Document M100-S18. CLSI, Wayne, PA
Garcia L (2010) Synergism testing: broth microdilution checkerboard and broth macrodilution methods. In: Garcia L (ed) Clinical microbiology procedures handbook, 3rd edn. ASM Press, Washington, pp 140–162
Chou TC (2006) Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacolo Rev 58:31
Zhou XY, Ye XG, He LT, Zhang SR, Wang RL, Zhou J, He ZS (2016) In vitro characterization and inhibition of the interaction between ciprofloxacin and berberine against multidrug-resistant Klebsiella pneumoniae. J Antibiot (Tokyo) 69:741–746
Salmi C, Loncle C, Vidal N, Letourneux Y, Fantini J, Maresca M, Taïeb N, Pagès JM, Brunel JM (2008) Squalamine: an appropriate strategy against the emergence of multidrug resistant gram-negative bacteria? PLoS One 23:e2765
Cui Y, Kim SH, Kim H, Yeom J, Ko K et al (2012) AFM probing the mechanism of synergistic effects of the green tea polyphenol (2)-epigallocatechin-3-gallate (EGCG) with cefotaxime against extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli. PLoS One 7:e48880
Justice SS, Hunstad DA, Cegelski L, Hultgren SJ (2008) Morphological plasticity as a bacterial survival strategy. Nature 6:162–168
Bos J, Zhang Q, Vyawahare S, Rogers E, Rosenberg SM, Austin RH (2015) Emergence of antibiotic resistance from multinucleated bacterial filaments. Proc Natl Acad Sci U S A 112:178–183
Wang Y, Zhang Y, Shi YQ, Pan XH, Lu YH, Cao P (2018) Antibacterial effects of cinnamon (Cinnamomum zeylanicum) bark essential oil on Porphyromonas gingivalis. Microb Pathog 116:26–32
Helander IM, Alakomi HL, Kyösti KL, Mattila-Sandholm T, Pol I, Smid EJ (1998) Characterization of the action of selected essential oil components on gram-negative bacteria. J Agric Food Chem 46:3590–3595
Shen S, Zhang T, Yuan Y, Lin S, Xu J, Ye H (2015) Effects of cinnamaldehyde on Escherichia coli and Staphylococcus aureus membrane. Food Control 47:196–202
García-Salinas S, Elizondo-Castillo H, Arruebo M, Mendoza G, Irusta S (2018) Evaluation of the antimicrobial activity and cytotoxicity of different components of natural origin present in essential oils. Molecules 23:1399
Raja SB, Murali MR, Devaraj SN (2008) Differential expression of OmpC and OmpF in multidrug-resistant Shigella dysenteriae and Shigella flexneri by aqueous extract of Aeglemarmelos, altering its susceptibility toward beta-lactam antibiotics. Diagn Microbiol Infect Dis 61:321–328
Raja SB, Murali MR, Malathi GK, Anbarasu K, Devaraj SN (2009) Effect of aqueous extract of Aegle marmelos fruit on adherence and β-lactam resistance of Enteropathogenic Escherichia coli by down regulating outer membrane protein C. Am J Infect Dis 5:161–169
Karumathil DP, Nair MS, Gaffney J, Kollanoor-Johny A, Venkitanarayanan K (2018) Trans-cinnamaldehyde and eugenol increase Acinetobacter baumannii sensitivity to beta-lactam antibiotics. Front Microbiol 9:1011
Zhou X, Jia F, Liu X, Wang Y (2012) Total alkaloids of Sophorea alopecuroides-induced down-regulation of AcrAB-TolC efflux pump reverses susceptibility to ciprofloxacin in clinical multidrug resistant Escherichia coli isolates. Phytother Res 261637–43
Cai W, Fu Y, Zhang W, Chen X, Zhao J, Song W et al (2016) Synergistic effects of baicalein with cefotaxime against Klebsiella pneumoniae through inhibiting CTX-M-1 gene expression. BMC Microbiol 16:181
National Toxicology Program (2004) NTP toxicology and carcinogenesis studies of trans-cinnamaldehyde (CAS no. 14371-10-9) in F344/N rats and B6C3F1 mice (feed studies). Natl Toxicol Program Tech Rep Ser 514:1–281
Acknowledgments
The authors are extremely grateful to the Director, Calcutta School of Tropical Medicine, Kolkata, India, for providing necessary facilities for this study.
Funding
This study is funded by the Indian Council of Medical Research (Grant No. -58/67/BMS-2012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement and informed consent
The study was approved by the ethical research committee (reference number: CREC-STM/53 dated 23/09/2011). Informed consent was obtained from the patients for participating in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dhara, L., Tripathi, A. Cinnamaldehyde: a compound with antimicrobial and synergistic activity against ESBL-producing quinolone-resistant pathogenic Enterobacteriaceae. Eur J Clin Microbiol Infect Dis 39, 65–73 (2020). https://doi.org/10.1007/s10096-019-03692-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-019-03692-y