Abstract
The dynamics related to the loss of stx genes from Shiga toxin-producing Escherichia coli remain unclear. Current diagnostic procedures have shortcomings in the detection and identification of STEC. This is partly owing to the fact that stx genes may be lost during an infection or in the laboratory. The aim of the present study was to provide new insight into in vivo and in vitro stx loss in order to improve diagnostic procedures. Results from the study support the theory that loss of stx is a strain-related phenomenon and not induced by patient factors. It was observed that one strain could lose stx both in vivo and in vitro. Whole genome comparison of stx-positive and stx-negative isolates from the same patient revealed that different genomic rearrangements, such as complete or partial loss of the parent prophage, may be factors in the loss of stx. Of diagnostic interest, it was shown that patients can be co-infected with different E. coli pathotypes. Therefore, identification of eae-positive, but stx-negative isolates should not be interpreted as “Shiga toxin-lost” E. coli without further testing. Growth and recovery of STEC were supported by different selective agar media for different strains, arguing for inclusion of several media in STEC diagnostics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Shiga toxin-producing Escherichia coli (STEC) are foodborne pathogens that can cause a wide range of gastrointestinal and systemic diseases. Shiga toxins are considered to be the major virulence factors of STEC, and are necessary for the development of severe conditions including hemorrhagic colitis and hemolytic uremic syndrome (HUS) [1]. The family of Shiga toxins contains two subgroups, Stx1 and Stx2, and several subtypes, Stx1a, c, d and Stx2a-h, of which Stx2h is the most recently described subtype [2]. Stx2 subtypes vary in their pathogentic potential. Most studies describe Stx2a, c and d as the most virulent [3]. However, severe clinical outcomes have also been described for other subtypes [4, 5]. Genes encoding Stx1 and Stx2 are located on different prophages that integrate at specific sites into the E. coli genome, a process known as lysogenic conversion. When the phages are silent, their E. coli hosts survive as lysogenic strains without expressing the stx genes [6]. Environmental stress factors and/or DNA-damaging agents may activate the phage lytic cycle, with concomitant stx expression and release of Stx toxins and phage particles [7, 8]. Alternatively, the Stx encoding phage DNA may spontaneously excise from the STEC genome without subsequent cell lysis. The resulting E. coli, without integrated stx, is termed ‘STEC lost shiga toxin’ (STEC-LST). STEC and STEC-LST may co-exist in vivo and recycle Stx-encoding phages so that they can exist as stx-negative variants and then convert back to stx-positive forms [9,10,11]. The Stx-encoding phage may also be lost during laboratory passages [11, 12].
In clinical diagnostics, the presence of STEC is usually confirmed by PCR-based detection of stx in cultured E. coli or directly in stool samples. In the latter instance, positive results are usually followed up by culturing E. coli from stools and performing stx-PCR on isolates. Excision or loss of the stx prophage in vivo or during laboratory sample processing, complicates STEC diagnostics. STEC with intact prophages may be low in numbers and, therefore, difficult to detect. Furthermore, induction into the lytic cycle with subsequent cell disruption adds to the complexity of STEC diagnostics. The aim of the present study was to provide new insight into in vivo and in vitro stx-loss in order to improve diagnostic procedures. Loss of stx was investigated in patient stool samples at different stages of infection and after inoculation onto agar, using qPCR. Whole genome sequencing of stx-positive and stx-negative bacterial isolates was performed to gain insight into genomic rearrangements that had occurred. Finally, different selective agars were tested for recovery of STEC in the presence of STEC-LST and background flora. Based on our findings, we discuss strategies to improve STEC diagnostics.
Materials and methods
Patient samples and clinical information
The period of investigation was from 2013 to 2014. Patients included in the study were either hospitalized at Akershus University Hospital, Norway (Ahus) or in primary health care. All patients were suffering from infectious diarrhea, which was in some cases bloody. Diagnostic samples were received at the Ahus Department of Clinical Microbiology and Infection Control. When STEC was detected in a sample, the patient, or if a juvenile the parents of the patient, were invited to participate in the study. Written consent was obtained from all participants. The study was approved by the Data protection manager at Ahus (Project number12-042) and by the Regional Committees for Medical and Health Research Ethics (REK), South East, Norway (Project number 2012-102).
Initial diagnostic procedures
Stool samples were investigated for the presence of STEC and other gastrointestinal pathogens using a commercial CE-labeled PCR kit (RIDA®GENE EHEC/EPEC real-time PCR, R-Biopharm AG, Darmstadt, Germany) [13]. Samples that tested positive for stx were cultured to recover the STEC isolate, which was then verified, serotyped, and further characterized at the National Reference Laboratory for Enteropathogenic Bacteria (NRL) at the Norwegian Institute of Public Health. Stx subtype was determined as described by Scheutz et al. [14] with minor modifications. Patients with STEC serotypes known to cause severe infection and subtypes stx2a, stx2c, stx2d, and stx1 were included in the study. STEC subtypes stx2b and stx2e-g were excluded as they have only rarely been associated with clinical infections in Norwegian patients [3]. STEC subtype stx2h was not identified in Norway at the time of the study. Two or three follow-up stool samples were collected from each patient at intervals of approximately 1 week during the period of illness. Stool samples were stored at − 80 °C until processed Table 1.
Recovery of STEC on different culture media
Samples included in the study were cultured onto CHROMagar STEC (CHROMagar Microbiology, Paris, FR) and lactose agar to quantify STEC and putative STEC-LST. Lactose agar, an in-house medium for detection of Enterobacteriaceae, contains tryptose agar base, lactose, sodium chloride, and bromothymol blue. For each sample, 16–48 different colonies growing on the agars were examined for stx, eae, and the corresponding O-serogroup (target genes wzx or wzy), H-serogroup (target gene fliC), or lysozyme P (lysP) using real-time PCR [13, 15,16,17,18,19]. Primers and probes are described in Table 2. QuantiFast Pathogen PCR/IC Kit (Qiagen, Hilden, Germany) was used for TaqMan probe assays, while SYBR Premix Ex Taq (Takara Bio, Inc., Otsu, Japan) was used for the SYBR green assays.
Loss of stx during in vitro culture
To investigate if stx was lost during the in vitro culture step, the amount of stx relative to its corresponding STEC O-serogroup target gene (alternatively H-serogroup/lysP) was determined before and after culture on lactose agar. DNA was extracted from stool samples (100 μl) in Cary-Blair transport medium (Copan Italia S.P.A, Brescia, Italy) or RNAlater™ stabilization reagent (Qiagen, Hilden, Germany) using QIAsymphony (Qiagen) as previously described [13]. Following stool culture (100 μl) on lactose agar, all the colonies on the plate were suspended in 5 ml PBS, and 200 μl of this suspension was used for DNA extraction using the QIAsymphony protocol. qPCR was performed in triplicate (Table 2) and the relative quantity of stx (∆Ct stx-O-serogroup target gene) in the stool sample versus colonies on lactose agar was determined for every patient. Four patients were excluded from these analyses due to the unsuccessful recovery of STEC (patients 3 and 9) or insufficient amounts of sample material (patients 8 and 10).
Loss of stx across stages of infection
Follow-up stool samples from each patient were investigated for stx and the O-sergroup target gene (alternatively H-serogroup or lysP) that was detected in the initial sample. At intervals of approximately 1 week from the onset of infection, the relative quantities of stx (∆Ct stx-O-serogroup target gene) were determined and compared to the primary diagnostic sample (time point 1). Patients 2, 3, 4, 9, and 12 were not included in these analyses as follow-up samples were not available.
Whole genome sequencing of stx-positive and stx-negative isolates
When stx-positive and stx-negative E. coli of the same serogroup were identified in a sample, whole genome sequencing (WGS) was performed for comparison of isolates. DNA was extracted with QIAGEN Genomic-tip 100/G (Qiagen) and a library was prepared using the Pacific Biosciences 20 kb library preparation protocol (Pacific Biosciences, Menlo Park, CA). Size selection of the final library was performed using BluePippin (Sage Science, Beverly, MA, USA) with 10 kb cut-off. The library was sequenced using a Pacific Biosciences RS II instrument employing P6-C4 chemistry with 360 min movie time. One SMRT cell was used for sequencing.
Bioinformatic analysis of whole genome sequence data
Reads were assembled using HGAP v3 (Pacific Biosciences, SMRT Analysis Software v2.3.0). Only subreads longer than 8 kb were used for assembly. Minimus2 software of Amos package was used to circularize contigs which were confirmed by dotplot to contain the same sequence at the beginning and end of the contig. RS_Resequencing 1 software (SMRT Analysis v2.3.0) was used to map reads back to assembled and circularized sequences. Low-coverage contigs were removed from the genome sequence before the mapping. Fasta files were submitted to the Centre for Genomic Epidemiology (CGE) (http://www.genomicepidemiology.org/) [20] for species identification with PathogenFinder [21], serotype- and multilocus sequence type identification with SerotypeFinder and MLST Finder [22, 23] and the detection of plasmids, and virulence genes with PlasmidFinder and VirulenceFinder [24, 25] (Table 3). The assembled chromosome sequences of each pair of stx-positive and stx-negative E. coli of the same serogroup were compared using MAUVE genome comparison tool version 2.4.0 [26]. Core genome MLST (cgMLST) was performed in Ridom SeqSphere+ version 5.1.0 (Ridom GmbH, Germany). Briefly, raw sequence reads were trimmed until an average base quality of 30 was reached in a window of 20 bases, and de novo assembly was performed using Velvet version 1.1.04 with default settings. The integrated Escherichia coli cgMLST scheme v1 from Enterobase (https://enterobase.warwick.ac.uk/species/index/e.coli) was used. The allelic profiles of the isolates were visualized as a minimum spanning tree using the parameter “pairwise ignoring missing values”.
Results
STEC isolates and their recovery on different culture media
Thirteen patients were enrolled in the study and 12 stx-positive isolates were isolated from 11 of these patients. Two different STEC were identified from one of the patients. In the case of two patients, no STEC were cultured from the samples. The 12 stx-positive isolates represented four different O-serogroups. One isolate was an unknown O-serogroup and one isolate was identified as Escherichia albertii [27] (Table 1). CHROMagar STEC was successful in selecting for STEC O157 and suppressed growth of commensal E. coli. STEC O157:H7 from patient 13 was recovered in pure culture on CHROMagar STEC, while it was suppressed by commensal flora on lactose agar in the primary cultivation step (Fig. 1). CHROMagar STEC was also successful in selecting for STEC O103 from two patient samples, although a third STEC O103 was not initially found using this medium. STEC O26:H11 was not able to grow on CHROMagar STEC, while lactose agar supported growth of all STEC in this study (Fig. 1).
Co-existence of STEC, STEC-LST, and other E. coli pathotypes
In samples from three different patients, a co-existence of stx2-positive/eae-negative and stx2-negative/eae-positive Escherichia was found. In one of these patients (p5), 14 of 40 colonies from lactose agar were stx2-negative E. coli O145:H28, while only one of 40 colonies was stx2-positive E. coli O145:H28. Twenty-five colonies from lactose agar were commensal E. coli (Fig. 1). In samples from patient 7, one colony of stx2-positive E. albertii and four colonies of stx2-negative E. albertii were identified out of 34 colonies tested from lactose agar (Fig. 1).
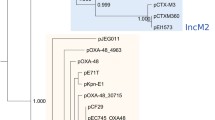
Pairs of stx-positive and stx-negative colonies of the same serogroup were subjected to whole genome sequencing. Bioinformatic analysis identified the pair of O145:H28 isolates as E. coli of the same MLST type. Both isolates contained the same plasmids and virulence genes and differed only in their Stx-encoding genes (Table 3). The pair of E. albertii isolates was also shown to contain identical virulence genes, except for stx (Table 3). Genome analysis using MAUVE showed that the stx-negative O145:H28 isolate was lacking a ~ 9000 base pair partial sequence region of the stx-prophage (Fig. 2a). The stx-negative E. albertii was missing approximately 62,000 base pairs (corresponding to a complete prophage DNA sequence) present in its stx-positive counterpart (Fig. 2b). cgMLST using 2513 targets detected illustrated 0 and 1 allelic differences between the stx-negative and stx-positive E. coli O145:H28 (patient 5) and stx-negative and stx-positive E. albertii (patient 7), respectively (Fig. 3). Whole genome sequences of the isolates have been submitted to the European Nucleotide Archive Study ID PRJEB27634 (ERP109742).
a Graphical presentation of the integrated Stx encoding bacteriophage sequence in stx-positive and stx-negative isolates of the same serotype. The stx-negative E. coli O145:H28 isolate is missing a stretch of approximately 9000 basepairs of the Stx encoding bacteriophage compared to the stx-positive E. coli O145:H28. b The stx-negative E. albertii is missing the complete Stx prohage DNA sequence compared to its stx-positive counterpart (62,000 bp)
Minimum-spanning tree based on cgMLST allelci profiles of two E. coli and two E. albertii isolates (stx-positive and stx-negative pairs). Each circle represents an allelic profile based on sequence analysis of 2513 targets. The numbers of the connecting lines illustrate the numbers of target genes with differing alleles. Both E. coli isolates had 98.4% good cgMLST targets, whereas the E. albertii isolates had 92.8 and 94.6% good cgMLST targets, respectively. Only, one core genome gene showed allelic difference between the E. albertii isolates, whereas none differences were observed between the E. coli isolates
Samples from patient 1 also contained a co-existence of stx2-positive/eae-positive and stx-negative/eae-positive E. coli. In these samples, the isolates were found to be of different serotypes. Of 24 colonies tested from lactose agar, six were STEC O26:H11, seven were Enteropathogenic E. coli (EPEC) O21, eight were EPEC of unknown serotype, and three were Enteoaggregative E. coli (EAEC) O104:H4. On CHROMagar STEC, only EPEC O21 and EAEC O104:H4 were detected (Fig. 1).
Loss of stx across stages of infection and after in vitro culture
The general pattern for all patients was a decrease in both stx and O/H/lysP from time point 1 to time point 4, and hence a reduction of STEC over time. In two patients (patients 5 and 8), neither stx nor the O serogroup target gene was detected in the follow-up samples. In five patients (patients 1, 6, 8, 10, and 11), the relative quantities of stx were stable or higher in the follow-up samples (Fig. 4). Samples from patient 7 diverged from this pattern. Here, stx was not detected at time points 2, 3, and 4, while lysP (E. albertii specific target) was detected at every time point, indicating the presence of stx-negative E. albertii and in vivo stx loss (Fig. 4). Subsequent to agar culture, decreased stx quantities relative to the O serogroup target gene were observed for three of the samples (patients 1, 5, and 7, log2-fold change > 2.5). This suggests in vitro loss of stx (Fig. 5).
Discussion
The aim of the present study was to provide new insight into in vivo and in vitro stx loss in order to improve diagnostic procedures. A proposed theory for loss of Stx-encoding phages from STEC is that this offers a selective advantage for the cell and favors bacterial survival [11]. Both strain-related and patient-related factors, as well as environmental factors, may influence this process. Most available information at the present time supports the theory that stx loss is related to serotype or stx subtype. Mellmann and colleagues [11] documented progressive stx loss in seven different patients infected with STEC O26:H11 and O157:NM. Another study from the same group found that 5% of HUS patients had shed STEC-LST O26:H11, O103:H2, O145:H28, and O157:H7 by the time of testing [10]. stx loss was identified in STEC O145:H28 also in the present study and indicated, but not verified in STEC O26:H11. Two different STEC isolates were identified in one of the patient samples. These were STEC O145:H28 containing stx2a and STEC O103:H2 containing stx1. Only STEC O145:H28 existed as stx-positive and stx-negative variants, providing support for the hypothesis that stx loss is related to strain or stx type, and not induced by patient factors. In our work, stx loss was also detected in a patient infected with stx2a-positive E. albertii. This isolate was obtained from a patient suffering from bloody diarrhea [27]. To our knowledge, this study is the first to illustrate that stx2 may be lost from E. albertii. Several studies have shown that stx genes are more stably maintained in STEC O157:H7 strains than in non-O157 strains [11, 28]. This applies also to the STEC O157 isolates in the present study. Loss of stx1 is less well known, and our data support the idea that stx is lost only in certain serotypes and stx-subtypes.
The median length of STEC carriage was in a recent study measured to 24 days [29]. The present work detected stx after 3 or 4 weeks in samples from seven patients, but for all patients, a progressive reduction of STEC was observed. For the patient with E. albertii, stx was not found 7 days after the onset of disease, whereas lysP (E. albertii-specific target) was detected at 7 and 12 days. This finding suggests stx loss in vivo. Following laboratory culture of primary samples on agar plates, the sample with E. albertii displayed decreased quantities of stx relative to lysP, indicating stx loss also in vitro. Several studies have shown that STEC is prone to loss of stx after in vitro manipulation [12, 30]. Joris et al. [28] showed that stx genes may be lost already during the first subcultivation step; the present study illustrates that not only subcultivation, but primary cultivation may have the same effect. We cannot exclude that in vivo coexistence of stx-positive and stx-negative cells may have resulted in bias during the culture stage, as preexisting stx-negative variants may be more easily cultured than stx-positive variants. A progressive stx loss in this strain in a plausible explanation.
Whole genome sequencing demonstrated that different genomic rearrangements may lead to stx loss. Isolates of stx2-positive E. albertii and stx2-negative E. albertii were sequenced and bioinformatics analysis showed that these were the same strain, with only one allelic difference using wgMLST. The stx2-negative isolate had lost the entire stx2 encoding phage. In this strain, free bacteriophages may have co-existed with E. albertii in vivo and bidirectional conversion of the Stx phage between stx-positive and stx-negative variants may have occurred [9]. Results from the present study also suggest in vitro loss of stx in samples from the patient with stx-positive and stx-negative E. coli O145:H28. Whole genome sequencing revealed that these were the same strain, but that one of them was missing some of the stx prophage, including the stx genes. Remnants of the prophage, including the late gene regulator Q and the genes encoding the phage structural proteins, were still present in the stx-negative isolate. This would be in line with a different genomic rearrangement rather than loss of the complete prophage. Since the prophages are not intact when the phage DNA is excised, these are not likely to be transferred to new cells. The reason for only partial loss of the Stx prophage is not clear. Theoretically, it could be related to bacterial survival and adaptation and lower virulence expression. The patient from which this isolate was identified suffered from bloody diarrhea and was hospitalized for 4 days. He was co-infected with STEC O103:H2. Of diagnostic interest, the results illustrate that sequence analysis of more STEC-LST isolates may reveal new diagnostic targets. For example, a PCR assay for the late gene regulator Q could be used to detect remnants of Stx prophages and identify stx loss in some E. coli isolates.
Culture on selective agars
STEC diagnostics is often based on detection of stx in E. coli isolates or directly in stool samples using PCR. An indication of the presence of STEC in a patient sample may be the patient’s clinical presentation or a positive stx PCR-result from stool samples. A routine procedure for STEC detection used at many hospital laboratories is to test 2–8 colonies isolated from stool samples for the presence of stx [13, 31].
It can be technically challenging to culture low quantities of STEC in samples with co-occurring STEC-LST and other E. coli. In this study, analysis of the two patient samples with pairs of stx-positive and stx-negative E. coli showed that only a minority of the colonies growing on lactose agar and CHROMagar STEC were STEC. The majority of colonies growing from these samples were STEC-LST, competing microflora or commensal E. coli. Therefore, detection of 2–8 stx-negative colonies in a stx-positive stool sample should not lead to termination of the STEC “search”. STEC isolates are likely to be present in samples where stx loss has occurred, although they will probably exist in small numbers. Furthermore, it would be unwise to assume that stx-negative, eae-positive colonies are possible STEC-LST without further analysis. In this study, one patient was co-infected with STEC O26:H11, EPEC O21, an EPEC of unknown serotype, and EAEC O104:H4. Only EPEC O21 and EAEC O104:H4 grew on CHROMagar STEC. If CHROMagar STEC were to be used as the only culture medium, the eae-positive EPEC O21 could be mistaken for STEC-LST. Although several studies have shown that CHROMagar STEC is a suitable medium for STEC O26 [32,33,34], it is clear that the commonly used selective culture media for STEC do not support growth of all STEC variants. Conversely, the less selective lactose agar also supports growth of commensal E. coli. In the present study, commensal E. coli suppressed STEC O157:H7 from one sample at the primary cultivation stage. Dual plating on lesser and more selective agars should be performed if STEC isolates are not recovered after a positive stx PCR result from stool or mixed culture. Our data illustrate that a high number of colonies need to be screened for stx if the patient’s clinical presentation suggests the presence of STEC.
The present work also showed that STEC may not be recovered if lytic induction occurs during cultivation. In two of the samples (patients 3 and 9), a positive stx-PCR was obtained directly from stool and from a culture swipe from lactose agar. Representative samples of the colonies on the plate were tested for stx; however, no STEC isolates were identified. It has previously been shown that free Stx phages may exist in patient stool samples and lead to positive stx-PCR results [35]. Since stx was detected in mixed culture on agar and not only from stool samples in patients 3 and 9, the results are in line with lytic induction upon subculture, rather than the presence of free Stx phages in the sample. In such cases, DNA from culture swipes could be used to search for common STEC serotypes or other genetic STEC markers. Although single isolates cannot be characterized using this approach, a possible STEC infection may be identified and the STEC serotype recorded for infection control purposes. If stx is not detected in culture swipes, free Stx phages are more likely to have caused the stx-positive PCR result.
The present study has some limitations which should be considered. The fecal samples had been frozen prior to analysis, which may have influenced stx loss. The small sample size of the study is also a limiting factor.
References
Tarr PI, Gordon CA, Chandler WL (2005) Shiga-toxin-producing Escherichia coli and haemolytic uremic syndrome. Lancet 365:1073–1086
Bai X, Fu S, Zhang J, Fan R, Xu Y, Sun H, He X, Xu J, Xiong Y (2018) Identification and pathogenomic analysis of an Escherichia coli strain producing a novel Shiga toxin 2 subtype. Sci Rep 8:6756. https://doi.org/10.1038/s41598-018-25233-x
Naseer U, Løbersli I, Hindrum M, Bruvik T, Brandal LT (2017) Virulence factors of Shiga toxin-producing Escherichia coli and the risk of developing haemolytic uremic syndrome in Norway, 1992-2013. Eur J Clin Microbiol Infect Dis 36:1613–1620
Fasel D, Mellmann A, Cernela N, Hächler H, Fruth A, Khanna N, Egli A, Beckmann C, Hirsch HH, Goldenberger D, Stephan R (2014) Hemolytic uremic syndrome in a 65-year-old male linked to a very unusual type of stx 2e - and eae-harboring O51:H49 Shiga toxin-producing Escherichia coli. JCM 52(4):1301–1303
Saupe A, Edel B, Pfister W, Löffler B, Ehricht R, Rödel J (2017). Acute diarrhea due to a Shiga toxin 2e-producing Escherichia coli O8:H19. JMM Case Rep 4. doi:https://doi.org/10.1099/jmmcr.0.005099
Herold S, Karch H, Schmidt H (2004) Shiga toxin-encoding bacteriophages — genomes in motion. Int J Med Microbiol 294:115–121
de Sablet T, Bertin Y, Vareille M, Girardeau JP, Garrivier A, Gobert AP, Martin C (2008) Differential expression of stx2 variants in Shiga toxin-producing Escherichia coli belonging to seropathotypes a and C. Microbiology 154(Pt 1):176–186
Waldor MK, Friedman DI (2005) Phage regulatory circuits and virulence gene expression. Curr Opin Microbiol 8(4):459–465
Bielaszewska M, Prager R, Köck R, Mellmann A, Zhang W, Tschäpe H, Tarr PI, Karch H (2007) Shiga toxin gene loss and transfer in vitro and in vivo during enterohemorrhagic Escherichia coli O26 infection in humans. Appl Environ Microbiol 73(10):3144–3150
Bielaszewska M, Köck R, Friedrich AW, von Eiff C, Zimmerhackl LB, Karch H, Mellmann A (2007) Shiga toxin-mediated hemolytic uremic syndrome: time to change the diagnostic paradigm? PLoS One 2(10):e1024
Mellmann A, Lu S, Karch H, Xu JG, Harmsen D, Schmidt MA, Bielaszewska M (2008) Recycling of Shiga toxin 2 genes in sorbitol-fermenting enterohemorrhagic Escherichia coli O157:NM. Appl Environ Microbiol 74(1):67–72
Karch H, Meyer T, Rüssmann J (1992) Frequent loss of Shiga-like toxin genes in clinical isolates of Escherichia coli upon subcultivation. Infect Immun 60:3464–3467
Tunsjø HS, Kvissel AK, Follin-Arbelet B, Brotnov B-M, Ranheim TE, Leegaard TM (2015) Suitability of stx-PCR directly from fecal samples in clinical diagnostics of STEC. APMIS 123(10):872–878
Scheutz F, Teel LD, Beutin L, Piérard D, Buvens G, Karch H, Mellan A, Caprioli A, Tozzoli R, Morabito S et al (2012) Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. JCM 50(9):2951–2963
Nielsen EM, Andersen MT (2003) Detection and characterization of verocytotoxin-producing Escherichia coli by automated 50 nuclease PCR assay. J Clin Microbiol 41:2884–2893
Lin A, Sutton O, Lau HK, Wong E, Hartman G, Lauzon CR (2011) O-serogroup specific real-time PCR assays for the detection and identification of nine clinically relevant non O-157 STECs. Food Microbiol 28:478–483
Guy RA, Tremblay D, Beausoleil L, Harel J, Champagne MJ (2014) Quantification of E. coli O157 and STEC in feces of farm animals using direct multiplex real time PCR (qPCR) and a modified most probable number assay comprised of immunomagnetic bead separation and qPCR detection. J Microbiol Methods 99:44–53
Beutin L, Delannoy S, Fach P (2015) Sequence variations in the flagellar antigen genes fliCH25 and fliCH28 of Escherichia coli and their use in identification and characterization of Enterohemorrhagic E. coli (EHEC) O145:H25 and O145:H28. PLoS One 10(5):e0126749
Hyma KE, Lacher DW, Nelson AM, Bumbaugh AC, Janda JM, Strockbine NA, Young VB, Whittam TS (2005) (2005). Evolutionary genetics of a new pathogenic Escherichia species: Escherichia albertii and related Shigella boydii strains. J Bacteriol 187(2):619–628
Larsen MV, Cosentino S, Lukjancenko O, Saputra D, Rasmussen S, Hasman H, Sicheritz-Pontén T, Aarestrup FM, Ussery DW, Lund O (2014) Benchmarking of methods for genomic taxonomy. J Clin Microbiol 52(5):1529–1539
Cosentino S, Voldby Larsen M, Møller Aarestrup F, Lund O (2013) PathogenFinder – distinguishing friend from foe using bacterial whole genome sequencing data. PlosOne 8(10):e77302
Joensen KGAM, Tetzschner A, Iguchi FM, Aarestrup M, Scheutz F (2015) Rapid and easy in silico serotyping of Escherichia coli using whole genome sequencing (WGS) data. J Clin Microbiol 53(8):2410–2426
Larsen MV, Cosentino S, Rasmussen S, Friis C, Hasman H, Marvig RL, Jelsbak L, Sicheritz-Pontén T, Ussery DW, Aarestrup M, Lund O (2012) Multilocus sequence typing of Total genome sequenced bacteria. J Clin Micobiol 50(4):1355–1361
Carattoli A, Zankari E, Garcia-Fernandez A, Voldby Larsen M, Lund O, Villa L, Aarestrup FM, Hasman H (2014) In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 58(7):3895–3903
Joensen KG, Scheutz F, Lund O, Hasman H, Kaas RS, Nielsen EM, Aarestrup FM (2014) Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J Clin Microbiol 52(5):1501–1510
Darling AC, Mau B, Blattner FR, Perna NT (2004) Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res 14:1394–1403
Brandal LT, Tunsjø HS, Ranheim TE, Løbersli I, Lange H, Wester AL (2015) Shiga toxin 2a in Escherichia albertii. JCM 53(4):1454–1455
Joris M-A, Verstraete K, De Reu K, De Zutter L (2011) Loss of vtx genes after the first subcultivation step of Verocytotoxigenic Escherichia coli O157 and non-O157 during isolation from naturally contaminated fecal samples. Toxins (Basel) 3(6):672–677
Matussek A, Jernberg C, Einemo I-M, Monecke S, Ehricht R, Engelmann I, Löfgren S, Mernelius S (2017) Genetic make-up of Shiga toxin-producing Escherichia coli in relation to clinical symptoms and duration of shedding: a microarray analysis of isolates from Swedish children. Eur J Clin Microbiol Infect Dis 36:1433–1441
Shaikh N, Tarr PI (2003) Escherichia coli O157:H7 Shiga toxin-encoding bacteriophages: integrations, excisions, truncations, and evolution-ary implications. J Bacteriol 185:3596–3605
Strategy report of enteropathogens, Norwegian public health, 2011. Available from https://www.fhi.no/globalassets/dokumenterfiler/rapporter/strategirapporter/strategirapport-nr-10-1996-bakteriologiske-faecesundersokelser-med-revisjoner-fra-2007-og-2011-.pdf
Hirvonen JJ, Siitonen A, Kaukoranta SS (2012) Usability and performance of CHROMagar STEC medium in detection of Shiga toxin-producing Escherichia coli strains. J Clin Microbiol 50(11):3586–3590
Gill A, Huszczynski G, Gauthier M, Blais B (2014) Evaluation of eight agar media for the isolation of Shiga toxin-producing Escherichia coli. J Microbiol Methods 96:6–11
Gouali M, Ruckly C, Carle I, Lejay-Collin M, Weill F-X (2013) Evaluation of CHROMagar STEC and STEC O104 chromogenic agar Media for Detection of Shiga toxin-producing Escherichia coli in stool specimens. J Clin Microbiol 51(3):894–900
Martinez-Castillo A, Quirós P, Navarro F, Miró E, Muniesa M (2013) Shiga toxin 2-encoding bacteriophages in human fecal samples from healthy individuals. Appl Environ Microbiol 79:4862–4868
Acknowledgements
We thank colleagues at the Department of Microbiology and Infection Control at Ahus for laboratory assistance. Parts of the contents have been presented in a report to the National Institute of Public Health. The sequencing service was provided by the Norwegian Sequencing Centre (www.sequencing.uio.no), a national technology platform hosted by the University of Oslo and supported by the “Functional Genomics” and “Infrastructure” programs of the Research Council of Norway and the Southeastern Regional Health Authorities.
Funding
This study received funding from Akershus University Hospital, National Institute of Public Health and Oslo Metropolitan University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Data protection manager at Ahus (Project number12-042) and by the Regional Committees for Medical and Health Research Ethics (REK), South East, Norway (Project number 2012-102).
Informed consent was obtained from all participants.
ᅟ
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Senthakumaran, T., Brandal, L.T., Lindstedt, BA. et al. Implications of stx loss for clinical diagnostics of Shiga toxin-producing Escherichia coli. Eur J Clin Microbiol Infect Dis 37, 2361–2370 (2018). https://doi.org/10.1007/s10096-018-3384-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-018-3384-6