Abstract
The role of metformin (MET) on treatment effect of diabetic tuberculosis (TB) patients has not been studied in China. Thus, we conducted a retrospective study to investigate whether MET exhibited more efficacy in combination with anti-TB regimens for diabetic TB patients. All patients recruited came from five tuberculosis control and prevention institutes from July 2009 to July 2016 and completed 3 years of follow-up. We used chi-square test or Fisher’s exact test to evaluate the demographic characteristics and the frequency of clinical outcome between MET and non-MET group. A total of 58 TB patients with diabetes mellitus (DM), of these 27.6% (16/58) patients in the MET group and 72.4% (42/58) patients in the non-MET group, there was no significant difference in blood glucose level between MET and non-MET group (P = 0.494), in addition, there was a higher proportion of treatment success (93.8 vs. 71.4%) and culture conversions by the end of 2 months (87.5 vs. 71.4%) among MET group; the relapse rates of patients in MET and non-MET group were 6.3% (1/16) and 35.7% (15/42) through a 3-year follow-up (P = 0.045). Our data revealed that the use of MET as a combination drug with existing regimen improved the success rate of anti-TB treatment and reduced the relapse rate in TB patients with DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberculosis remains a leading global health concern, especially in developing countries, with an estimated incidence of 10.4 million new cases in 2016 by World Health Organization [1].Despite having an average cure rate of 83% for TB patient worldwide, treatment of TB can be complicated by the presence of concurrent diseases, such as HIV infection and diabetes mellitus (DM) [2,3,4]. Previous studies showed that 5.4–44% PTB patients also have DM [5]; China has the highest DM burden with about 110 million people, accounts for a quarter of DM burden in the world [6]. Therefore, the high prevalence of DMs in China becomes a major threaten to achieve the goals of the WHO post-2015 END TB strategy goal in the future.
Owing to diminished innate and adaptive immune responses, latent TB patients with DM have higher risk for developing active cases compared with non-diabetics [7, 8]. In addition, several clinical trials have confirmed that diabetic TB patients are associated with unfavorable treatment outcomes such as delayed sputum culture conversion, higher mortality, and increased risk of TB relapse after successful anti-TB treatment [9]. Hence, patients suffering from both TB and DM make up a special population with higher vulnerability.
Metformin (MET) is the recommended first-line antihyperglycemic drug for the treatment of type 2 diabetes [10]. It reduces the blood glucose levels by impeding the production of hepatic glucose, inhibiting intestinal glucose absorption, and enhancing glucose utilization [11]. Notably, the potential role of metformin on improving the effective treatment of TB is noted by recent data, indicating that MET can be used as a promising candidate host-adjunctive therapy for TB cases [10]. In view of these published evidence, the favorable outcome can be expected among diabetic TB patients treated with MET, whereas the current data on this issue is limited. In this study, we conducted a retrospective study to investigate whether MET exhibited more efficacy in combination with anti-TB regimens for diabetic TB patients compared with those without MET. In addition, the 3-year follow-up was completed to assess the long-term effect of anti-TB treatment.
Methods
Patient enrollment
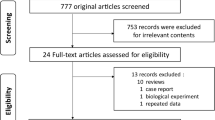
We retrospectively reviewed the medical records of all culture-positive retreatment pulmonary TB patients with type 2 DM at Beijing Chest Hospital, Henan Center for Disease Control and Prevention, Shenyang Chest Hospital, the 309th Hospital of Chinese People’s Liberation Army, Beijing Research Institute for tuberculosis control between July 2009 and July 2013. The patients were excluded if they had any clinical indication as follows: (1) multidrug-resistant TB or extensively drug-resistant TB, (2) extra-pulmonary TB, and (3) patients with other serious comorbidity such as pneumoconiosis and insufficiency in the liver or kidney. The study was approved by the Ethics Committee of the Beijing Chest Hospital, affiliated to Capital Medical University; all participants enrolled signed the informed consent in the study.
Microbiological examination
Sputum smear and solid mycobacterial culture were performed according to the standard methods as described previously in the guidelines of China’s National TB Control Program (NTP) [12]. Briefly, the smears were examined by fluorescence method. Two sputum samples were decontaminated using N-acetylcysteine/NaOH. After neutralization with PBS, the concentrated specimens were inoculated on the Löwenstein-Jensen (L-J) medium. Growth on any L-J medium tube was considered as culture-positive. In addition, the absolute concentration method was performed to determine in vitro drug susceptibility to anti-tuberculosis antimicrobial agents. The critical concentrations were 40 mg/L for rifampicin (RIF, R), 0.2 mg/L for isoniazid (INH,H), 10 mg/L for streptomycin (SM,S), 2 mg/L for ethambutol (EMB,E), 30 mg/L for kanamycin (KAN), 40 mg/L for capreomycin (CPM), 30 mg/L for amikacin (AMK), 2 mg/L for ofloxacin (OFLX), and 2 mg/L for levofloxacin (LFX), respectively. Media supplied separately with 500 mg/L of paranitrobenzoic acid and 5 mg/L of thiophen-2-carboxylic acid hydrazide were used to identify the mycobacterial species.
Clinical treatment
Treatment regimens of TB patients followed the guidelines of China’s NTP [12], that was 2HREZS/6HRE with 8-month chemotherapy and consists of H, R, E, Z (pyrazinamide (Z)), and S in 2-month intensive phases; meanwhile, H, R E in 6-month continuation phases, patients need to take the medicine daily for 8 months continuously in the present study. The primary treatment outcomes of patients were monitored monthly for sputum culture during anti-TB treatment. In the present study, the treatment outcome was divided into five categories: cured, completed, failure, died, and defaulted treatment. Definitions were referred according to the guidelines of NTP in China [12]; (1) cured: a patient who has completed prescriptive treatment, is sputum smear negative with continuing two-time and at least one after completed; (2) completed treatment: a smear-positive patient who has completed treatment, is sputum smear negative once at the end of intensive phase, none or once in continuing phase of treatment, and does not do sputum smear in the last month during treatment; (3) failure: a smear-positive patient is still sputum smear positive at 5 months; a smear-negative patient becomes sputum smear positive during his/her treatment. (4) died: a patient who dies for TB or other reasons during treatment; (5) defaulted (interrupted treatment): a patient whose treatment was interrupted for 2 months or more, doctors do their best but the patient does not continue to receive treatment. The second treatment outcomes of patients were evaluated on the basis of 3-year follow-up. The poor outcome was defined as the cured patients with the occurrence of relapse during the follow-up period. In addition to anti-TB treatment, these diabetes patients also received the treatment to improve the control of blood glucose. According to the treatment regimens for diabetes, the patients were divided into MET and non-MET group, respectively.
Statistical analysis
We carried out a chi-square test or Fisher’s exact test to evaluate the demographic characteristics and the frequency of clinical outcome between MET and non-MET group. Statistical analysis was performed with SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). The differences were declared as significant if P values were lower than 0.05.
Results
Demographic characteristics and drug resistance patterns
A total of 58 culture-positive TB patients with DM were enrolled in this study, including 16 patients in the MET group and 42 patients in the non-MET group, respectively. Of the 58 TB patients, 82.8% were male. Statistical analysis revealed that there was no significant difference in the proportion of male between MET and non-MET group (P = 0.999). In addition, the median age of MET and non-MET group was 56.5 and 49.9 years. Patients from MET group had a median blood glucose level of 8.89 ± 4.98 mg/L, which had no significant difference compared with that from non-MET group (8.48 ± 3.56 mg/L, P = 0.494). We further analyzed the drug susceptibility patterns of TB patients enrolled in this study. As shown in Table 1, two thirds of the patients (65.5%, 38/58) were susceptible to anti-TB drugs tested, while there were also 20 patients (34.5%) exhibiting resistance to anti-TB drugs, respectively. No significant difference was identified in the distribution of susceptible and drug-resistant TB between two groups (P = 0.749) (Table 1).
Treatment outcomes
Of the 58 TB patients, 38 (65.5%) were cured, 7 (12.1%) completed treatment, no patient died, 9 (15.5%) had treatment failure, and 4 (6.9%) were lost to follow-up (Table 2). Of 16 patients receiving treatment with MET, as expected, there was a higher proportion of treatment success (93.8 vs. 71.4%) among MET group compared with non-MET group at the end of anti-TB treatment. We further compared sputum culture conversion rate at the end of the second month of treatment between MET and non-MET group, though had no significant statistical difference (P = 0.308), similarly, the proportion of patients exhibited culture conversation of MET group (87.5 vs. 71.4%) was higher (Table 2). We also analyzed the relapse rate of patients enroll in this study during the subsequent 3-year follow-up period. Overall, a total of 48 patients completed the follow-up, and the relapse rates of patients in MET and non-MET groups were 6.3% (1/16) and 35.7% (15/42), respectively. Statistically significant difference was found in the occurrence rate among patients in MET group compared with those in non-MET group (P = 0.045) (Table 3).
Discussion
In this retrospective cohort analysis of TB patients that comorbided with type 2 diabetes in China, we found that the use of MET as a combination drug with existing regimen improved sputum culture conversion rate at the end of 2nd month of TB treatment, in consistent to a study from Korea [13], in addition, MET also increased the success rate of anti-TB treatment compared with other antihyperglycemic drugs. It is interesting to investigate how MET improves the treatment efficacy of the universal regimens. On one hand, the experimental evidence from Singhal and colleagues revealed that MET reduces inflammation by activating AMP kinase, thereby reducing mycobacterial growth and tissue pathology [10]. Hence, we speculate that MET serves as an important immunomodulator that enhances TB immunity, which contributes the improved clinical outcome of TB patients. On the other hand, a recent comprehensive system level mapping of metabolic complexity in MTB indicates that MET may target proteins encoding for respiratory chain complex NDH-I biosynthesis pathway, further preventing the formation of a persistence phenotype [10]. Given that the persistence phenotype of MTB produces tolerance to anti-TB drugs, the higher proportion of patients suffering from poor clinical outcome in non-MET group can be expected.
Notably, we also observed that MET brought benefits to reduce relapse following TB cure, suggesting the potential role of MET in killing the latent bacteria living in macrophages. Although the exact reason remains unclear, a previous report has demonstrated that MET could enhance phagocytosis, phagolysosome fusion, and autophagy in macrophages [14], which are essential for killing intracellular MTB. In addition, as reported by Singhal et al., macrophages in vitro exposed to MET demonstrate increased capability to produce mitochondrial reactive oxidative species, thereby resulting in higher mycobactericidal capacity against both intracellular and extracellular bacteria [10]. Taken together, our results revealed that the utilization of MET as a combination drug with existing antibiotics for diabetic TB patients may optimize both short-term and long-term treatment outcomes.
The findings of the present analysis are subject to several obvious limitations. First, the small sample size of diabetic TB patients is a major limitation of this study, which may undermine reliability of the statistical analysis. Further study is urgently needed to verify our findings regarding the attractive effect of MET in the treatment of TB. Second, due to the lack of genotyping data, we could not distinguish whether recurrent disease was contributing to relapse or reinfection, especially in the setting with high TB prevalence. Hence, the relapse rate may be overestimated during the follow-up period. Despite these limitations, our study has several strengths. As far as we know, this is the first study to investigate treatment effect of MET in pulmonary tuberculosis patients with DM, and relapse through a 3-year follow-up period in China, our study provides a clinical indication of the important role of MET in the treatment of diabetic TB patients, which echoes the previous experimental evidence.
In conclusion, our data demonstrate that the use of MET as a combination drug with existing regimen increases the success rate of anti-TB treatment and reduces the relapse rate. Further clinical trials will be carried out to verify our findings regarding the attractive effect of MET in the treatment of TB.
References
World Health Organization (2017) Global tuberculosis report 2017. Geneva: WHO 2017; WHO/HTM/TB/2017.23. Retrieved November 2017 from http://apps.who.int/iris/bitstream/10665/259366/1/9789241565516-eng.pdf?ua=1
Restrepo BI (2007) Convergence of the tuberculosis and diabetes epidemics: renewal of old acquaintances. Clin Infect Dis 45(4):436–438. https://doi.org/10.1086/519939
Park SW, Shin JW, Kim JY, Park IW, Choi BW, Choi JC, Kim YS (2012) The effect of diabetic control status on the clinical features of pulmonary tuberculosis. Eur J Clin Microbiol Infect Dis 31(7):1305–1310. https://doi.org/10.1007/s10096-011-1443-3
Reid A, Scano F, Getahun H, Williams B, Dye C, Nunn P, De Cock KM, Hankins C, Miller B, Castro KG, Raviglione MC (2006) Towards universal access to HIV prevention, treatment, care, and support: the role of tuberculosis/HIV collaboration. Lancet Infect Dis 6(8):483–495. https://doi.org/10.1016/S1473-3099(06)70549-7
Ma Y, Huang ML, Li T, Du J, Shu W, Xie SH, Wang HH, Zhu GF, Tan SY, Fu YY, Ma LP, Zhang LY, Liu FY, Hu DY, Zhang YL, Li XQ, Liu YH, Li L (2017) Role of diabetes mellitus on treatment effects in drug-susceptible initial pulmonary tuberculosis patients in China. Biomed Environ Sci 30(9):671–675. https://doi.org/10.3967/bes2017.089
World Health Organization (2016) Global report on diabetes. WHO, Geneva
Jeon CY, Murray MB (2008) Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 5(7):e152. https://doi.org/10.1371/journal.pmed.0050152
Dooley KE, Chaisson RE (2009) Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis 9(12):737–746. https://doi.org/10.1016/S1473-3099(09)70282-8
Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, Lonnroth K, Ottmani SE, Goonesekera SD, Murray MB (2011) The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med 9:81. https://doi.org/10.1186/1741-7015-9-81
Vashisht R, Brahmachari SK (2015) Metformin as a potential combination therapy with existing front-line antibiotics for tuberculosis. J Transl Med 13:83. https://doi.org/10.1186/s12967-015-0443-y
Wu T, Xie C, Wu H, Jones KL, Horowitz M, Rayner CK (2017) Metformin reduces the rate of small intestinal glucose absorption in type 2 diabetes. Diabetes Obes Metab 19(2):290–293. https://doi.org/10.1111/dom.12812
Department of Disease Control, Ministry of Health, Department of Medical Administration, Ministry of Health, Chinese Center for Disease Control and Prevention (2008) Guideline of implementing tuberculosis control programme China, vol 2008. Pecking Union Medical College Press, Beijing, p 89
Lee YJ, Han SK, Park JH, Lee JK, Kim DK, Chung HS, Heo EY (2018) The effect of metformin on culture conversion in tuberculosis patients with diabetes mellitus. Korean J Intern Med. https://doi.org/10.3904/kjim.2017.249
Labuzek K, Liber S, Gabryel B, Adamczyk J, Okopien B (2010) Metformin increases phagocytosis and acidifies lysosomal/endosomal compartments in AMPK-dependent manner in rat primary microglia. Naunyn Schmiedebergs Arch Pharmacol 381(2):171-186. https://doi.org/10.1007/s00210-009-0477-x
Acknowledgements
We would like to thank all the members from Beijing Chest Hospital, Henan Center for Disease Control and Prevention, Shenyang Chest Hospital, the 309th Hospital of Chinese People’s Liberation Army, Beijing Research Institute for tuberculosis control in this study for their hard work.
Funding
This study was supported by the National Science and Technology Major Project of China (2008ZX10003-009) and the National Science and Technology Major Project of China (2008ZX10003-008-02).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This study was approved by the Ethic Committee of Beijing Chest Hospital affiliated to Capital Medical University and the four provincial TB Control and Prevention Centers (TB hospitals).
Rights and permissions
About this article
Cite this article
Ma, Y., Pang, Y., Shu, W. et al. Metformin reduces the relapse rate of tuberculosis patients with diabetes mellitus: experiences from 3-year follow-up. Eur J Clin Microbiol Infect Dis 37, 1259–1263 (2018). https://doi.org/10.1007/s10096-018-3242-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-018-3242-6