Abstract
The objective of this study was to explore whether the percentage of inappropriate empirical antibiotic treatment in patients with bacteremia changed over time and to understand the factors that brought on the change. Three prospective cohorts of patients with bacteremia in three different periods (January 1st, 1988 to December 31st, 1989; May 1st, 2004 to November 30, 2004; May 1st, 2010 to April 30, 2011) were compared. Analysis was performed on a total of 811 patients. In 2010–2011, 55.9% (76/136) of patients with bacteremia received inappropriate empirical treatment, compared with 34.5% (170/493) and 33.5% (55/164) in the first and second periods, respectively, in a significant upward trend (p = 0.001). Resistance to antibiotics increased significantly during the study period. The following variables were included in the multivariate analysis assessing risk factors for inappropriate empirical treatment: study period (third period) [odds ratio, OR = 2.766 (95% confidence interval, CI, 1.655–4.625)], gender (male) [OR = 1.511 (1.014–2.253)], pathogen carrying extended-spectrum beta-lactamases [OR = 10.426 (4.688–23.187)], multidrug-resistant Acinetobacter baumannii [OR = 5.428 (2.181–13.513)], and skin/soft infections [OR = 3.23 (1.148–9.084)]. A model excluding microbiological data included: gender (male) [OR = 1.648 (1.216–2.234)], study period (third period) [OR = 2.446 (1.653–3.620)], hospital-acquired infection [OR = 1.551 (1.060–2.270)], previous use of antibiotics [OR = 1.815 (1.247–2.642)], bedridden patient [OR = 2.019 (1.114–3.658)], and diabetes mellitus [OR = 1.620 (1.154–2.274)]. We have observed a worrisome increase in the rate of inappropriate empirical treatment of bacteremia. We need tools that will allow us better prediction of the pathogen and its susceptibilities during the first hours of managing a patient suspected of a severe bacterial infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacteremia is a major cause of morbidity and mortality in both hospitalized and community-dwelling patients. In Europe, the annual number of bacteremia episodes is estimated at over 1.2 million, with more than 157,000 deaths per year [1]. Delays in appropriate antimicrobial treatment for severe bacterial infections are associated with higher mortality rates [2]. Consequently, when identifying a patient with a suspected severe bacterial infection, the attending physician needs to decide on an empirical treatment as soon as possible while considering the benefits of the treatment against the potential resistance selection.
A systematic review of prospective studies reporting the association between appropriate empirical antibiotic treatment and all-cause mortality among adult inpatients with sepsis demonstrated a concerning rate of 46.5% of inappropriate empirical antibiotic treatment [3]. Inappropriate empirical antibiotic treatment was associated with a higher 30-day mortality [odds ratio, OR = 1.60 (95% confidence interval, CI, 1.37–1.86)] [3].
Among the factors that contribute to high rates of inappropriate empirical treatment are the lack of knowledge of pathogen resistance patterns, pathogen distribution, and patients’ risk factors. These factors have changed over the years and we cannot be sure that physicians are aware of these factors and take the changes into account when prescribing empirical antibiotic treatment.
In the present study, we aimed to explore whether there was a specific trend in the percentage of inappropriate empirical antibiotic treatment in patients with bacteremia over time and to understand the factors that brought on the change.
Materials and methods
Design and setting
We examined three prospective cohorts in three different periods from 1988 to 2011.
The first cohort was collected between January 1st, 1988 and December 31st, 1989; the second between May 1st, 2004 and November 30, 2004; and the third between May 1st, 2010 and April 30, 2011. The first two cohorts were a part of previous studies [4,5,6,7,8]. The third cohort was assembled for this study. The study population consisted of inpatients with a suspected bloodstream infection from six departments of medicine in Beilinson Hospital, Petah Tikva, Israel. Data were collected at four time points: the beginning of the episode (day 0), day 2, day 4, and day 30. The study protocol was approved by the research ethics committee of the hospital.
Inclusion/exclusion criteria
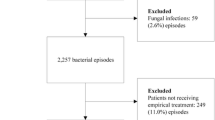
We included patients older than 17 years of age with clinically significant positive blood cultures that fulfilled the systemic inflammatory response syndrome diagnostic criteria. Isolates such as coagulase-negative staphylococci and other skin microorganisms were defined as contaminants if they were isolated from a single set of blood cultures. Exclusion criteria were suspected travel infection and pregnancy.
Data collection
Patients fulfilling the inclusion/exclusion criteria were prospectively identified by daily review of patient charts. The following details were collected: background conditions, devices, signs and symptoms, and all available laboratory data. At follow-up, data on the final diagnosis, treatment, and microbiological cultures were collected.
The primary outcome was inappropriate empirical antibiotic treatment, which was defined as inappropriate if the antibiotic treatment given within the first 24 h after blood cultures were taken did not match the in vitro susceptibility of the pathogen.
In order to assess the extent of bacterial resistance, two variables were created: Gram-positive bacteria resistance index and Gram-negative bacteria resistance index. The variables were computed with an arithmetic summation of resistance: susceptible, 0; intermediate, 1; resistant, 2, and divided by the number of antibiotics tested for each isolate.
Statistical analysis
A sample of at least 131 patients in each period was sufficient to detect a statistically significant difference in the primary outcome (α = 0.05, 1 − β = 0.8).
Analyses were performed using the Statistical Package for the Social Sciences 19 (SPSS Inc.). Proportions were tested by univariate analysis: χ2 or Fisher’s exact test for comparison of categorical variables, Student’s t-test for comparison of two independent continuous variables, Mann–Whitney test for comparison of two independent variables with abnormal distributions, and analysis of variance (ANOVA) test for comparison of three continuous variables. The significance of differences in the ORs between different variables and inappropriate empirical antibiotic treatment in each period was examined with the Breslow–Day test.
Logistic regression in the stepwise method was used for multivariate analysis to assess the impact of risk factors on inappropriate antibiotic treatment with and without microbiological variables. Due to the similar rates of inappropriate antibiotic treatment in the first and second periods, we merged those two periods and recoded the study period as a dichotomous variable (0, first and second periods; 1, third period). We entered all variables significantly associated with the outcome on univariate analysis (p < 0.1) and not correlated (Spearman correlation > 0.5). We examined interactions that seemed reasonable to us, but none reached significance. The Hosmer–Lemeshow statistic was used for goodness of fit.
Results
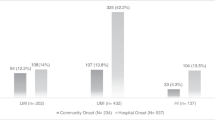
Analysis was performed on 811 patients, comprising 493 patients in the first period, 171 patients in the second period, and 147 patients in the third period. In 2010–2011, 55.9% (76/136) of patients with bacteremia received inappropriate empirical treatment, compared with 34.5% (170/493) and 33.5% (55/164) in the first and second periods, respectively, in a significant upward trend (p = 0.001).
Descriptive epidemiology
The baseline characteristics of bacteremic patients in each period and the results of the χ2 test for trends are presented in Table 1. A significant downward trend has been seen in hospital-acquired infections [from 21.9% (108/493) to 14.3% (21/147), p = 0.023] and urinary tract infections (UTI) [from 45.2% (223/493) to 32.7% (48/147), p = 0.004]. A significant upward trend has been seen in infections of a primary/unknown source [from 30.2% (149/493) to 43.5% (64/147), p = 0.002], presence of central line [from 2.9% (14/488) to 8.2% (12/146), p = 0.003], and rate of mechanical ventilation [from 0.2% (1/493) to 12.9% (19/147), p = 0.000]. The most common Gram-negative bacteria in all three periods were Escherichia coli. Three Gram-negative pathogens have shown a significant upward trend: Acinetobacter sp. [from 2.6% (13/493) to 10.2% (15/147), p = 0.000], Klebsiella sp. [from 11.4% (56/493) to 20.4% (30/147), p = 0.003], and Pseudomonas sp. [from 9.1% (45/493) to 22.4% (33/147), p = 0.000]. The most common Gram-positive bacterium in all three periods was Staphylococcus aureus. Enterococcus sp. showed a significant upward trend [from 4.7% (23/493) to 13.6% (20/147), p = 0.000]. Resistance to antibiotics increased significantly in all three periods.
Subgroup differences according to appropriateness of empirical treatment throughout the study periods
We looked for factors associated with inappropriate empirical antibiotic treatment in each of the three periods (Table 2). It is interesting to note that septic shock, old age, nursing home residence, and diabetes mellitus were more closely related to inappropriate treatment in the third period. The mortality rate was significantly higher in the inappropriate treatment subgroup in all three periods (an absolute difference of 14.5–19.9%).
Risk factors for inappropriate empirical treatment
The univariate analysis for appropriateness of empirical treatment is displayed in Table 3. UTI was excluded from the multivariate analysis due to significant correlation with primary/unknown source of infection (r = − 0.599, p = 0.000).
The following risk factors for inappropriate empirical treatment were included in the final logistic model: study period (third period) [OR = 2.766 (1.655–4.625)], gender (male) [OR = 1.511 (1.014–2.253)], pathogen carrying extended-spectrum beta-lactamases (ESBLs) [OR = 10.426 (4.688–23.187)], multidrug-resistant (MDR) Acinetobacter baumannii [OR = 5.428 (2.181–13.513)], and skin/soft infections [OR = 3.23 (1.148–9.084)] (Table 4).
In order to assess risk factors that were associated with clinical decision-making, we analyzed the same variables excluding microbiological data. The final model of the multivariate analysis included: gender (male) [OR = 1.648 (1.216–2.234)], study period (third period) [OR = 2.446 (1.653–3.620)], hospital-acquired infection [OR = 1.551 (1.060–2.270)], previous use of antibiotics [OR = 1.815 (1.247–2.642)], bedridden patient [OR = 2.019 (1.114–3.658)], and diabetes mellitus [OR = 1.620 (1.154–2.274)] (Table 5).
Discussion
The prescription of inappropriate empirical antibiotic treatment has risen by more than 20% (up to 55.9% in 2010–2011) in the last 20 years, along with a significant increase in resistant bacteria.
We observed trends over time in patients with bacteremia. The ratio of hospital-acquired to community-onset episodes has decreased over the years. The marked rise in mechanical ventilation and the increased use of central catheters as underlying conditions are consistent with previous reports [9, 10]. A major concern is the rise in infections that are defined as primary or unknown due to the difficulty of defining the source of infection, found to be a risk factor of mortality [11,12,13]. Almost all antibiotics showed an upward trend in resistance. The most prominent trend was of carbapenem-resistant Enterobacteriaceae (CRE), which was barely present in the late 1980s and reached 9.5% of patients in 2010–2011. The increased prevalence of MDR A. baumannii came in tandem with the increase in prevalence of Acinetobacter sp.
In a stratified analysis, we assessed whether the association of risk factors with inappropriate treatment changed over time. Nursing home residents were increasingly given inappropriate treatment: the odds for inappropriate treatment increased from 0.93 in the first period to 4.3 in the third, probably reflecting the prevalence of resistant bacteria in nursing home residents [14], the increasing use of antibiotics in these institutions [15], and the inability of physicians to take that into account. We observed the same trend over time in diabetic patients: while the percentage of diabetes mellitus among patients given appropriate antibiotic treatment remained stable over time, the percentage of patients with diabetes among patients given inappropriate treatment increased from 23% in the first period to 32% in the second period and 43% in the third period.
The strong risk factors for inappropriate treatment were stable over the years: patients infected with ESBL-carrying Enterobacteriaceae, MDR A. baumannii, and methicillin-resistant Staphylococcus aureus were at high risk for inappropriate early antibiotic treatment. Carbapenem resistance joined these risk factors in the third period.
In the multivariate regression for the risk of inappropriate empirical treatment including all patients, ESBL-producing bacteria had the highest impact (OR = 10.426). In our study, a marked increase in the incidence of infections due to ESBLs was observed over the years (from 4.7% up to 10.2%) [16,17,18,19,20]. A possible association with the surge in ESBLs is the increase over time in the use of third-generation cephalosporins. The study period was entered in our final model and was not explained by the other risk factors. This might be explained by a general rise in antibiotic resistance that was not fully accounted for by the variables we have used. A second explanation might be stricter restrictions on the use of broad-spectrum antibiotics over the years.
In conclusion, we have observed a worrisome increase in the rate of inappropriate empirical treatment of bacteremia, a trend similar to that observed in the published literature [21] (and unpublished data). Of special interest was the role of long-term care facilities as risk factors for inappropriate treatment; interventions to prevent the spread of resistant bacteria and avoid abuse of antibiotics should target these institutions [22]. We need tools that will allow us better prediction of the pathogen and its susceptibilities during the first hours of managing a patient suspected of a severe bacterial infection. This can be done by better use of the patient’s data [23, 24] or by rapid, point-of-care, culture-free tests. These tools should focus on the main culprits: ESBL-carrying bacteria, A. baumannii, methicillin-resistant Staphylococcus aureus, and carbapenem-resistant Enterobacteriaceae.
References
Schwaber MJ, Navon-Venezia S, Kaye KS, Ben-Ami R, Schwartz D, Carmeli Y (2006) Clinical and economic impact of bacteremia with extended-spectrum-beta-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 50:1257–1262
Leibovici L, Shraga I, Andreassen S (1999) How do you choose antibiotic treatment? BMJ 318:1614–1618
Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L (2010) Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother 54:4851–4863
Leibovici L, Samra Z, Konisberger H, Kalter-Leibovici O, Pitlik SD, Drucker M (1991) Bacteremia in adult diabetic patients. Diabetes Care 14(2):89–94
Leibovici L, Konisberger H, Pitlik SD, Samra Z, Drucker M (1992) Bacteremia and fungemia of unknown origin in adults. Clin Infect Dis 14(2):436–443
Leibovici L, Konisberger H, Pitlik SD, Samra Z, Drucker M (1992) Patients at risk for inappropriate antibiotic treatment of bacteraemia. J Intern Med 231(4):371–374
Paul M, Gafter-Gvili A, Leibovici L, Bishara J, Levy I, Yaniv I, Shalit I, Samra Z, Pitlik S, Konigsberger H, Weinberger M (2007) The epidemiology of bacteremia with febrile neutropenia: experience from a single center, 1988–2004. Isr Med Assoc J 9(6):424–429
Fraser A, Paul M, Almanasreh N, Tacconelli E, Frank U, Cauda R, Borok S, Cohen M, Andreassen S, Nielsen AD, Leibovici L; TREAT Study Group (2006) Benefit of appropriate empirical antibiotic treatment: thirty-day mortality and duration of hospital stay. Am J Med 119(11):970–976
Endimiani A, Tamborini A, Luzzaro F, Lombardi G, Toniolo A (2003) A two-year analysis of risk factors and outcome in patients with bloodstream infection. Jpn J Infect Dis 56(1):1–7
Gasch O, Ayats J, Angeles Dominguez M, Tubau F, Liñares J, Peña C, Grau I, Pallarés R, Gudiol F, Ariza J, Pujol M (2011) Epidemiology of methicillin-resistant Staphylococcus aureus (MRSA) bloodstream infection: secular trends over 19 years at a university hospital. Medicine (Baltimore) 90(5):319–327
Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Kim EC, Oh MD, Choe KW (2005) Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother 49:760–766
Tumbarello M, Sanguinetti M, Montuori E, Trecarichi EM, Posteraro B, Fiori B, Citton R, D’Inzeo T, Fadda G, Cauda R, Spanu T (2007) Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother 51:1987–1994
Suppli M, Aabenhus R, Harboe ZB, Andersen LP, Tvede M, Jensen JU (2011) Mortality in enterococcal bloodstream infections increases with inappropriate antimicrobial therapy. Clin Microbiol Infect 17(7):1078–1083
Kahvecioglu D, Ramiah K, McMaughan D, Garfinkel S, McSorley VE, Nguyen QN, Yang M, Pugliese C, Mehr D, Phillips CD (2014) Multidrug-resistant organism infections in US nursing homes: a national study of prevalence, onset, and transmission across care settings, October 1, 2010–December 31, 2011. Infect Control Hosp Epidemiol 35:S48–S55
Sundvall PD, Stuart B, Davis M, Roderick P, Moore M (2015) Antibiotic use in the care home setting: a retrospective cohort study analysing routine data. BMC Geriatr 15:71
Van Aken S, Lund N, Ahl J, Odenholt I, Tham J (2014) Risk factors, outcome and impact of empirical antimicrobial treatment in extended-spectrum β-lactamase-producing Escherichia coli bacteraemia. Scand J Infect Dis 46(11):753–762
Denis B, Lafaurie M, Donay JL, Fontaine JP, Oksenhendler E, Raffoux E, Hennequin C, Allez M, Socie G, Maziers N, Porcher R, Molina JM (2015) Prevalence, risk factors, and impact on clinical outcome of extended-spectrum beta-lactamase-producing Escherichia coli bacteraemia: a five-year study. Int J Infect Dis 39:1–6
Nguyen ML, Toye B, Kanji S, Zvonar R (2014) Risk factors for and outcomes of bacteremia caused by extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella species at a Canadian tertiary care hospital. Can J Hosp Pharm 68(2):136–143
Freeman JT, McBride SJ, Nisbet MS, Gamble GD, Williamson DA, Taylor SL, Holland DJ (2012) Bloodstream infection with extended-spectrum beta-lactamase-producing Enterobacteriaceae at a tertiary care hospital in New Zealand: risk factors and outcomes. Int J Infect Dis 16(5):e371–e374
Nasa P, Juneja D, Singh O, Dang R, Singh A (2012) An observational study on bloodstream extended-spectrum beta-lactamase infection in critical care unit: incidence, risk factors and its impact on outcome. Eur J Intern Med 23(2):192–195
Kariv G, Paul M, Shani V, Muchtar E, Leibovici L (2013) Benchmarking inappropriate empirical antibiotic treatment. Clin Microbiol Infect 19(7):629–633
Dyar OJ, Pagani L, Pulcini C (2015) Strategies and challenges of antimicrobial stewardship in long-term care facilities. Clin Microbiol Infect 21(1):10–19
Paul M, Nielsen AD, Goldberg E, Andreassen S, Tacconelli E, Almanasreh N, Frank U, Cauda R, Leibovici L; TREAT Study Group (2007) Prediction of specific pathogens in patients with sepsis: evaluation of TREAT, a computerized decision support system. J Antimicrob Chemother 59(6):1204–1207
Paul M, Andreassen S, Tacconelli E, Nielsen AD, Almanasreh N, Frank U, Cauda R, Leibovici L; TREAT Study Group (2006) Improving empirical antibiotic treatment using TREAT, a computerized decision support system: cluster randomized trial. J Antimicrob Chemother 58(6):1238–1245
Acknowledgements
LL and MP are active members of the European Society of Clinical Microbiology and Infectious Diseases—Study Group for Infections in the Elderly (ESGIE) and acknowledge the ESGIE’s contribution to the conception of this study.
Funding
This work was supported by ‘The Israel National Institute for Health Policy Research’.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Daitch, V., Akayzen, Y., Abu-Ghanem, Y. et al. Secular trends in the appropriateness of empirical antibiotic treatment in patients with bacteremia: a comparison between three prospective cohorts. Eur J Clin Microbiol Infect Dis 37, 455–462 (2018). https://doi.org/10.1007/s10096-018-3190-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-018-3190-1