Abstract
Evaluating trends in antibiotic resistance is a requisite. The study aimed to analyze the profile of multidrug-resistant organisms (MDROs) among hospitalized patients with bacteremia in intensive care units (ICUs) in a large geographical area. This is a 1-month cross-sectional survey for blood-borne pathogens in 57 ICUs from 24 countries with different income levels: lower-middle-income (LMI), upper-middle-income (UMI), and high-income (HI) countries. Multidrug-resistant (MDR), extensively drug-resistant (XDR), or pan-drug-resistant isolates were searched. Logistic regression analysis determined resistance predictors among MDROs. Community-acquired infections were comparable to hospital-acquired infections particularly in LMI (94/202; 46.5% vs 108/202; 53.5%). Although MDR (65.1%; 502/771) and XDR (4.9%; 38/771) were common, no pan-drug-resistant isolate was recovered. In total, 32.1% of MDR were Klebsiella pneumoniae, and 55.3% of XDR were Acinetobacter baumannii. The highest MDR and XDR rates were in UMI and LMI, respectively, with no XDR revealed from HI. Predictors of MDR acquisition were male gender (OR, 12.11; 95% CI, 3.025–15.585) and the hospital-acquired origin of bacteremia (OR, 2.643; 95%CI, 1.462–3.894), and XDR acquisition was due to bacteremia in UMI (OR, 3.344; 95%CI, 1.189–5.626) and admission to medical-surgical ICUs (OR, 1.481; 95% CI, 1.076–2.037). We confirm the urgent need to expand stewardship activities to community settings especially in LMI, with more paid attention to the drugs with a higher potential for resistance. Empowering microbiology laboratories and reports to direct prescribing decisions should be prioritized. Supporting stewardship in ICUs, the mixed medical-surgical ones in particular, is warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antimicrobial resistance (AMR) has become widespread over the past several decades. Infections caused by multidrug-resistant organisms (MDROs) lead to increased hospitalization and higher healthcare costs, require prolonged hospital stays, and result in higher mortality[1, 2]. MDROs are defined as microorganisms resistant to one or more antimicrobial agents, and are usually resistant to all but one or two commercially available antimicrobial agents [3]. The problem of AMR seems to represent a special challenge in low-income countries characterized by the scarcity of biological and epidemiological data [4, 5]. Antimicrobial stewardship programs (ASPs) have been shown to improve patient outcomes, reduce antimicrobial resistance, and decrease the spread of infections caused by these MDROs [6]. The prevalence of these pathogens varies temporally and geographically and by healthcare setting. Evaluating trends in antibiotic resistance and communicating the results to a broad audience are important for dealing with this global threat [7]. Detailed analysis of the factors affecting MDROs’ profiles can provide insight into potential solutions to the problem. Thus, the current report aims to analyze the profile of MDROs among hospitalized patients with bacteremia in intensive care units (ICUs) in a large geographical area.
Patients and methods
This is a cross-sectional survey, conducted by Infectious Diseases International Research Initiative (ID-IRI) between the 1st and 30th November 2019. The ethical approval of the study was obtained from Trabzon Kanuni Hospital by Serhat Uysal. No funding source was involved.
Study settings
A total of 57 ICUs representing 24 countries from various geographical regions); Europe (Turkey, Portugal, Italy, Slovak Republic, Serbia, France, Romania, Cyprus, Bosnia and Herzegovina, Kosovo, North Macedonia, Bulgaria, Belgium, Hungary), Middle East and North Africa (Egypt, Lebanon, Oman, Iran, Palestine), South Asia (Bangladesh, Pakistan, India), Latin America and the Caribbean (Puerto Rico), and East Asia (Thailand). Different economic levels were represented: lower-middle-income (LMI (n = 13), upper-middle-income (UMI) (n = 33), and high-income (HI) countries (n = 11), as per the World Bank [8]. The participating ICUs were medical (16), surgical (14), medical-surgical (20), and neonatal and pediatric units (7).
Case definition
All patients in the participating ICUs diagnosed with bacteremia were eligible for inclusion. Bacteremia was defined as the association of at least one positive blood culture and a prescription of a systemic antibiotic treatment to treat bacteremia. For common skin contaminants, such as coagulase-negative staphylococci (CoNS), at least two different sets of blood cultures were required. Only pathogenic bacteria isolated from blood cultures were included and contaminants were excluded from the analysis. Strict adherence to aseptic conditions during specimen collection was assured of by the research team.
Data collection
Clinical and microbiological data were collected regardless of the cause of bacteremia. An online questionnaire was prepared via Google forms for every enrolled patient. In each ICU, data were submitted by the researcher who is committed as per institutional agreement to collaborate in the study.
Antimicrobial susceptibility testing
Results of antimicrobial susceptibility testing were accepted if carried out in line with international standards, which were the European Committee on Antimicrobial Susceptibility Testing (EUCAST) or Clinical and Laboratory Standards Institute (CLSI) methodology and guidance. Automated systems and disc diffusion methods are accepted as per the mentioned guidelines. Colistin and vancomycin testing results were included in the analysis if microdilution method is used.
Definitions
Multidrug-resistant (MDR) pathogen is defined as non-susceptibility to > one agent in > three antimicrobial categories. Extensively drug-resistant (XDR) is defined as non-susceptibility to at least one agent in all but two or fewer antimicrobial categories (i.e., bacterial isolates remain susceptible to only one or two categories). Pan-drug-resistant (PDR) is defined as non-susceptibility to all agents in all antimicrobial categories (i.e., no agents tested as susceptible for that organism) [9]. Patients with bacteremia without detectable organ infection are included in bacteremia of unknown origin (BUO) category [10]. Specific resistance patterns were defined as reported earlier: methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococci (VRE), carbapenem-resistant Enterobacteriaceae (CRE), MDR Acinetobacter [11], and extended spectrum beta-lactamase-(ESBL) producing Gram-negative bacilli [12]. Positive specimens obtained on day 1 (admission date), day 2, and day 3 of admission were classified as community-onset, and positive specimens obtained on or after day 4 were classified as healthcare facility-onset [10].
Statistical analyses
Data were coded, validated, and analyzed using SPSS, version 22 (Armonk, NY: IBM Corp.). In minimizing the selection bias for the participating countries, we used cluster sampling method for the selection of ICUs. Each ICU itself is a mini-representation of the other ICUs in the countries since the selected ICUs’ population is heterogeneous. For this reason, selected ICUs represent the other ICUs in the population. In addition, we did not provide comparisons based on countries of the participating centers. Rather, we stratified the countries according to their economical statuses into three as LMI, UMI, and HI countries.
Continuous quantitative variables were expressed as the mean ± SD and median (range), and categorical qualitative variables were expressed as absolute frequencies (number) and relative frequencies (percentage). Continuous data were checked for normality by using the Shapiro–Wilk test. One-way ANOVA f-test was used to compare more than two groups of normally distributed data. Categorical data were compared using Chi-square test (χ2 test). All tests were two-sided. p < 0.05 was considered statistically significant, p < 0.001 was considered highly statistically significant (HS), and p-value ≥ 0.05 was considered insignificant (NS). We performed a univariate analysis for risk factors and a multivariate regression analysis for the factors predicting the acquisition of MDR or XDR microbes.
Results
A total of 771 patients were included in the study. Their ages ranged from 1 month to 93 years (57.8 ± 19.6). The patients were enrolled from different types of ICUs: medical (n = 311), surgical (n = 189), medical-surgical (n = 219), and neonatal and pediatric (n = 52). The investigated pathogens were isolated from various infections: BUO (n = 345, 44.7%); pneumonia (n = 179, 23.2%); UTI (n = 88, 11.4%), skin and soft tissue infections (n = 46, 6.0%); intra-abdominal infections (n = 50, 6.5%); cardiac infections (n = 18, 2.3%); septic shock (n = 18, 2.3%); arthritis (n = 4, 0.5%); other respiratory infections (n = 7, 0.9%); central nervous system (CNS) infections (n = 8, 1%); and others (n = 8, 1%).
Origin of infections
Two-thirds of the isolates (537/771, 69.6%) were recovered from hospital-acquired infections (LMI (n = 108, 20.1%), UMI (n = 325, 60.5%), HI (n = 104, 19.4%)) and the rest of them (234/771, 30.4%) were isolated from community-acquired infections (LMI (n = 94), UMI (n = 107), HI (n = 33)). A significant association was reported between the infection onset and income level (p = 0.001) (Fig. 1, Table 1). The most common hospital-acquired pathogens were Klebsiella pneumoniae (128/537, 23.8%), CoNS (96/537, 17.9%), Acinetobacter baumannii (59/537, 11%), and S. aureus (48/537, 8.9%) in descending order. On the other hand, Escherichia coli (50/234, 21.4%), K. pneumoniae (49/234, 20.9%), S. aureus (39/234, 16.7%), and CoNS (24/234, 10.2%) were the most common isolates in community-onset infections.
Antibiotic susceptibility patterns
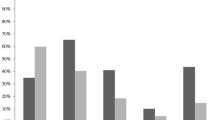
The susceptibility rates for Enterococcus faecalis (18/18, 100%), Enterococcus faecium (17/17, 100%), S. aureus (82/83, 98.8%), and CoNS (106/112, 94.6%) to linezolid were high. Vancomycin susceptibility rates were as follows: S. aureus (80/80, 100%), CoNS (109/110, 99.1%), E. faecalis (17/18, 94.4%), and E. faecium (10/19, 52.6%). Meropenem susceptibility rates were inconstant for E. coli (59/81, 72.8%), K. pneumoniae (72/171, 42.1%), and Pseudomonas aureginosa (21/57, 36.8%) as the prominent Gram-negative bacilli. Colistin showed varying susceptibility rates for E. coli (51/52, 98.1%), P. aureginosa (42/44, 95.5%), A. baumannii (42/44, 95.5%), and K. pneumoniae (108/123, 87.8%) (Tables 2, 3).
Multidrug resistance patterns
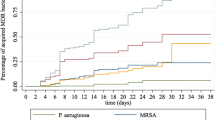
Five hundred and forty-one isolates (541/771, 70%) were MDROs including 502 isolates that showed MDR type of resistance (502/771, 65.1%) while 38 isolates showed the XDR profile (38.771, 4.9%). No PDR bacteria were isolated. Among the MDR pathogens, K. pneumoniae (161/502, 32.1%) was the commonest followed by CoNS (68/502, 13.6%), E. coli (64/502, 12.7%), and S. aureus (60/502, 12%). Out of XDR isolates, 55.3% (21/38) were A. baumannii (Fig. 2). Isolated MDROs showed a statistically significant distribution across different income levels, p < 0.001: MDR rates were as follows: LMI (n = 130/502, 25.9%), UMI (n = 295/502, 58.8%), and HI (n = 77/502, 15.3%). XDR rates were 44.8% in LMI (n = 17/38) and 55.3% in UMI (n = 21/38).
Certain resistance profiles
ESBL producers (255/771, 33.1%), MRSA (54/771, 7%), CRE (32/771, 4.2%), and VRE (12/771, 1.6%) were not rare in this study. The majority of ESBL producers, CREs, and VRE isolates were K. pneumoniae (n = 94/185, 50.8%), E. coli (n = 24/32, 75%), and E. faecium (n = 11/12, 91.7%) strains in descending order (Table 4).
MDRs and infectious syndromes
The MDROs showed a statistically significant association with different infectious syndromes (p < 0.001). Among BUO cases, 61.5% (212/345) were infected with MDR pathogens and 3.4% (12/345) of the cases were infected with bacteria of XDR type. Higher rates of MDR pathogens were reported from pneumonia (133/179, 74.3%) and 7.8% (14/179) were of XDR isolates. In UTIs, 68.2% (60/88) of the isolates were MDRs and 5.7% (5/88) were XDR types. Skin and soft tissue infection cases showed that 60.9% isolates (28/46) were MDRs and 6.5% of the isolates (3/46) were XDR types. Only one XDR isolate was detected from each of intra-abdominal infections, and cardiac and CNS infections with rates of 2% (n = 1/50), 5.6% (1/18), and 12.5% (1/8) respectively. The MDR rates of the aforementioned infections were 76% (38/50), 33.3% (6/18), and (4/8) 50% respectively. Although no XDR isolates were detected from septic shock and arthritis, MDR rates were 77.8% (14/18) and 50% (2/4), respectively.
Resistance and its relations
Significant association was seen between specific resistance pattern and each of income level and infection origin (p = 0.000). MDR acquisition is significantly associated with male gender (X2 = 3.99, p = 0.045), getting infected in upper-middle-income setting (X2 = 6.56, p = 0.038), admission to medical ICUs (3) (X2 = 21.47, p = 0.000), and the hospital-acquired origin of bacteremia (X2 = 7.04, p = 0.0008). For XDR isolates, a statistically significant association was recorded for acquiring bacteria in UMI setting (X2 = 12.36, p = 0.002), and admission to medical-surgical ICU (X2 = 15.79, p = 0.001) (Table 5).
Predictors of MDR/XDR acquisition
Male gender (odds ratio [OR], 12.11; 95% confidence interval [CI], 3.025–15.585) and bacteremia from hospital-acquired origin (OR, 2.643; 95%CI, 1.462–3.894) were the predictors of MDR acquisition. On the other hand, bacteremia in UMI setting (OR, 3.344; 95%CI, 1.189–5.626) or in medical-surgical ICUs (OR, 1.481; 95%CI, 1.076–2.037) was the predictor of XDR acquisition (Table 6).
Discussion
Our study is based on patients hospitalized in ICUs, and we analyzed the patterns of MDROs among bacteremic patients in a large geographic area. We presented the variations in rates and patterns across various clinical presentations, income levels, and origins of infections. Our study sheds light on the many challenges in appropriate antimicrobial usage. In this study, the most common pathogens with MDR profile were Klebsiella strains while Acinetobacter species had presented the highest XDR profiles. In addition, we have disclosed that community-acquired infections made up one-third of the infections inside the ICUs, and were comparable to hospital-acquired infections in LMI countries in particular. Although MDR bacteria were isolated from LMI and HI countries most commonly, XDR bacteria were isolated from LMI countries solely. Fortunately, PDR bacteria were not isolated. Although high susceptibility rates were recorded for last resort antibiotics, the efficacy of vancomycin was hampered in Enterococcal infections compared to linezolid, and carbapenems were troubled in common Gram-negative bacillary infections. Nearly half the investigated subjects were diagnosed as BUO lacking specified diagnoses, where source control attempts will be blurred. Pneumonia was the most common infection following BUO. We found that predictors of MDRO acquisition were male gender and hospital-acquired infections for MDR pathogens while UMI country settings and medical-surgical ICUs were for XDR pathogens.
The difference in microorganisms and resistance patterns for infections acquired in the community compared with hospital settings may deliver data for national, healthcare facility, and community AS programs [13]. Patients with community-acquired infections taken directly from the emergency departments to the ICUs are a nonnegligible group [14]. In this report, the rate of community-acquired infections was 30.4%, highlighting the need to support the prevention efforts in community settings. The rates of MDR and XDR pathogens in community and hospital settings were significantly higher in LMI and UMI settings than the settings in HI countries. Similar to our findings, a previous report of the International Nosocomial Infection Control Consortium showed that the prevalence of AMR organisms causing hospital-acquired infections in ICUs in LMI countries was much higher than those in the USA [15]. Affordable antimicrobials and the lack of ASPs either in the hospitals or in the community with poor control of over-the-counter sales could be the main drives for such emergence in LMI countries [16]. Since AMR is one of the greatest public health threats, bullying the practice of clinical medicine, reducing AMR requires organized and multidisciplinary ASPs supported by political commitment, resources, and practical managements.
Our analysis revealed high susceptibility rates for “last resort” antibiotics such as linezolid (94.6–100%) and colistin (87.8–98.1%). Relatively lower susceptibility rates to antibiotics that are more prone to be a target of antibiotic resistance as vancomycin (52.6–100%), meropenem (36.8–72.8%), and ciprofloxacin (3.4–69.7%) were reported. On the other hand, tigecycline showed higher susceptibility rates for both Gram-negative (81.8–97.9%) and Gram-positive (84.6–100%) isolates. Because of the increasing prevalence of Gram-negative MDROs worldwide, previously discarded antibiotics are being re-evaluated [17]. Accordingly, we have disclosed that doxycycline showed 97.6% susceptibility rate against staphylococcal isolates in this report. On the other hand, gentamycin displayed (62.8–92.8%) susceptibility rates for Gram-negative bacilli other than A. baumannii. These rates alarm the need to adapt empirical therapeutic treatments in accordance with the local antibiotic-resistance epidemiology and monitor the use of antibiotics. The WHO AWaRe categorization list could guide the proper antibiotic selection by illustrating which are the preferred antibiotic options for each syndrome, balancing benefits, harms, and the potentials for resistance [18].
The prevalence of infections sustained by MDR bacteria in ICU patients varies in the different regions of the world. An earlier worldwide study showed on average a 47.8% MDRO rate, including 20.5% and 0.5% of isolated microorganisms with XDR and PDR patterns, respectively, with a consistent variability among participating countries ranging from 8% to more than 75–80% [19]. We reported a higher rate of MDROs (70%), including 65.1% and 4.9% of isolates with MDR and XDR patterns. No PDR isolates were recorded in our analysis, yet the limited number of agents tested in routine susceptibility testing may provide an explanation, e.g., colistin, ciprofloxacin, tigecycline, and vancomycin were not tested for each isolate. As evident in our study, the variation between centers in antibiotics used for routine testing may be a limitation in reporting the actual rates of MDR and XDR isolates [9]. Another challenge that may have led to underestimation of the exact resistance rates was the necessity of micro-inhibitory concentration (MIC) testing to certain types of antibiotics as colistin, which is not usually available in routine susceptibility testing. Thus, in this study, we excluded the susceptibility testing results against vancomycin and colistin if the testing method was not a MIC technique. Although previously recommended [2, 20], we still need to support the use of microbiology reports to direct prescribing decisions, particularly in LMI countries where the standard international definitions may not be applied due to weaker laboratory capacity or insufficient resources for the sustainability of international definitions [21]
Predictors for the acquisition of MDROs as the agents of bloodstream infections would actually be valuable, assisting empirical treatment when infection occurs in ICUs. We reported male gender as the independent predictor of MDR acquisition (OR, 12.11; 95%CI, 3.025–15.585). Similar results were published by Wang et al. [22]. The hospital-acquired bacteremia was another predictor (OR, 2.643; 95%CI, 1.462–3.894) that was published in earlier studies where researchers concluded the carriage of MDROs, invasive devices, and excessive use of broad-spectrum antibiotics among hospitalized patients as principal sources of MDRO acquisition [23]. Routine screening of MDROs and limiting unnecessary use of broad-spectrum antimicrobial treatment should be encouraged [23, 24]. By regression analysis, we revealed that ICU type “medical-surgical ICU” is an independent predictor (OR, 1.481; 95%CI, 1.076–2.037) for XDR acquisition. This may be due to the fact that this type of ICUs usually specializes in the care and treatment of complex conditions, and treat patients who need advanced respiratory and circulation support by entailing excessive use of invasive devices in the critically ill patients [25].
In our report, a majority of cases (44.7%) were diagnosed as bacteremia without detectable organ infection for which 61.5% were of MDR and 3.4% were of XDR types. Although the decision basically includes identifying the infectious focus for proper antibiotic use, there are many comorbid conditions in patients in the ICUs ranging from infarcts to hematomas which may mask detecting the primary infectious site, and this may lead to inappropriate or excessive antibiotic administration [10]. Hence, rigorous diagnostic workup seems necessary for clarification. In addition, considering pneumonia as the most common established clinical diagnosis and with its specific features in the ICUs [26, 27], use of microbiological data as a marker to guide antimicrobial treatment would, then, be of great help.
Several limitations of the current study are worth mentioning. First, the distribution of the participating ICUs is not representative of the populations or healthcare systems in the 24 participating countries. In some countries, the number of included patients was small. Hence, we stratified our data in accordance with the economic status of the countries to overcome this limitation. Second, each participating center performed laboratory tests according to their own local protocols. Neither were all the isolates tested against the same antibiotics nor the panels of tested antibiotics uniform in all ICUs. Finally, clinical outcomes, severity status (e.g., SOFA score), and comorbidity data are not included and the study mostly focused on microbiological parameters. In conclusion, our report confirms that the current high rate of MDROs is alarming and there is an urgent need to expand ASPs to community settings, particularly in low-income settings. Appropriate antibiotic use is a must, with high priority to antibiotics with a higher potential for resistance. Equipping and empowering microbiology laboratories should be prioritized in AS plans. The use of microbiology reports to direct prescribing decisions should be a central focus of AS activities, particularly in LMI countries. Continuous efforts to implement ASPs in ICUs are warranted.
References
CDC (2013) Antibiotic resistant threats in the United States. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed [2nd Apr 2020]
Erdem H, Inan A, Altindis S, Carevic B, Askarian M, Cottle L et al (2014) Surveillance, control and management of infections in intensive care units in Southern Europe, Turkey and Iran - a prospective multicenter point prevalence study. J Infect 68:131–140. https://doi.org/10.1016/j.jinf.2013.11.001
Health Research & Educational Trust (2017) Multi-drug resistant organism infection change package: 2017 update. Chicago: Health Research & Educational Trust [Internet]. 2017. www.hret-hiin.org
Hope D, Ampaire L, Oyet C, Muwanguzi E, Twizerimana H, Apecu RO (2019) Antimicrobial resistance in pathogenic aerobic bacteria causing surgical site infections in Mbarara regional referral hospital. Southwestern Uganda Sci Rep 29:17299
Keizer J, Braakman-Jansen LMA, Kampmeier S, Köck R, Al Naiemi N, Te Riet-Warning R, et al (2019) Cross-border comparison of antimicrobial resistance (AMR) and AMR prevention measures: the healthcare workers’ perspective. Antimicrob Resist Infect Control 8. https://doi.org/10.1186/s13756-019-0577-4
Barlam TF, Cosgrove SE, Abbo LM, Macdougall C, Schuetz AN, Septimus EJ et al (2016) Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 62:e51-77. https://doi.org/10.1093/cid/ciw118
Klein EY, Tseng KK, Pant S, Laxminarayan R (2019) Tracking global trends in the effectiveness of antibiotic therapy using the Drug Resistance Index. BMJ Glob Heal 4:e001315. https://doi.org/10.1136/bmjgh-2018-001315
Updated country income classifications for the World Bank’s 2020 fiscal year (2020) https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups Accessed [2 Feb 2020] [Internet]. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups Accessed [2 Feb 2020]
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG et al (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect [Internet] 18:268–281. https://doi.org/10.1111/j.1469-0691.2011.03570.x
Courjon J, Demonchy E, Degand N, Risso K, Ruimy R, Roger PM (2017) Patients with community-acquired bacteremia of unknown origin: clinical characteristics and usefulness of microbiological results for therapeutic issues: a single-center cohort study. Ann Clin Microbiol Antimicrob 16. https://doi.org/10.1186/s12941-017-0214-0
CDC (2020) Multidrug-resistant organism & Clostridioides difficile infection (MDRO/CDI) Module. https://www.cdc.gov/nhsn/PDFs/pscManual/12pscMDRO_CDADcurrent.pdf [2nd Apr 2020]
Rawat D, Nair D (2010) Extended-spectrum ß-lactamases in gram negative bacteria. J Glob Infect Dis 2:263. https://doi.org/10.4103/0974-777x.68531
Weinshel K, Dramowski A, Hajdu Á, Jacob S, Khanal B, Zoltán M et al (2015) Gap analysis of infection control practices in low- and middle-income countries. Infect Control Hosp Epidemiol 36:1208–1214. https://doi.org/10.1017/ice.2015.160
Erdem H, Hargreaves S, Ankarali H, Caskurlu H, Alkan-Ceviker S, Bahar-Kacmaz A et al (2021) Managing adult patients with infectious diseases in emergency departments: international ID-IRI study. J Chemother. https://doi.org/10.1080/1120009X.2020.1863696
Rosenthal VD, Maki DG, Mehta Y, Leblebicioglu H, Memish ZA, Al-Mousa HH et al (2014) International Nosocomial Infection Control Consortium (INICC) report, data summary of 43 countries for 2007–2012. Device-associated module Am J Infect Control 42:942–956. https://doi.org/10.1016/j.ajic.2014.05.029
Lim C, Takahashi E, Hongsuwan M, Wuthiekanun V, Thamlikitkul V, Hinjoy S et al (2016) Epidemiology and burden of multidrug-resistant bacterial infection in a developing country. Elife 5:1–18. https://doi.org/10.7554/eLife.18082
Zilahi G, Artigas A, Martin-Loeches I (2016) What’s new in multidrug-resistant pathogens in the ICU? Ann Intensive Care 6. https://doi.org/10.1186/s13613-016-0199-4
WHO Antibiotic Categorization (2020) https://aware.essentialmeds.org/list. Last accessed 22nd Dec
Tabah A, Koulenti D, Laupland K, Misset B, Valles J, Bruzzi De Carvalho F et al (2012) Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: The EUROBACT International Cohort Study. Intensive Care Med 38:1930–1945. https://doi.org/10.1007/s00134-012-2695-9
El-Sokkary RH, Negm EM, Othman HA, Tawfeek MM, Metwally WS (2020) Stewardship actions for device associated infections: an intervention study in the emergency intensive care unit. J Infect Public Health [Internet] 13:1927–1931. https://doi.org/10.1016/j.jiph.2020.10.003
Forde CA, Martindale Y, Patel S (2019) The real scenario in infection prevention and control in low- and middle-income countries: the challenge of “starting from scratch.” Curr Treat Options Infect Dis 11:281–291. https://doi.org/10.1007/s40506-019-00196-3
Wang L, Huang X, Zhou J, Wang Y, Zhong W, Yu Q, et al (2020) Predicting the occurrence of multidrug-resistant organism colonization or infection in ICU patients: development and validation of a novel multivariate prediction model. Antimicrob Resist Infect Control 9. https://doi.org/10.1186/s13756-020-00726-5
Mascitti H, Duran C, Nemo EM, Bouchand F, Câlin R, Descatha A, et al (2018) Factors associated with bacteraemia due to multidrug-resistant organisms among bacteraemic patients with multidrug-resistant organism carriage: a case control study 11 Medical and Health Sciences 1103 Clinical Sciences. Antimicrob Resist Infect Control 7. https://doi.org/10.1186/s13756-018-0412-3
Goodman KE, Lessler J, Cosgrove SE, Harris AD, Lautenbach E, Han JH et al (2016) A Clinical decision tree to predict whether a bacteremic patient is infected with an extended-spectrum β-lactamase-producing organism. Clin Infect Dis 63:896–903. https://doi.org/10.1093/cid/ciw425
Tosi M, Roat E, De Biasi S, Munari E, Venturelli S, Coloretti I et al (2018) Multidrug resistant bacteria in critically ill patients: a step further antibiotic therapy. J Emerg Crit Care Med 2:103–103. https://doi.org/10.21037/jeccm.2018.11.08
Erdem H, Turkan H, Cilli A, Karakas A, Karakurt Z, Bilge U et al (2013) Mortality indicators in community-acquired pneumonia requiring intensive care in Turkey. Int J Infect Dis 17:e768–e772. https://doi.org/10.1016/j.ijid.2013.03.015
Erdem H, Cag Y, Gencer S, Uysal S, Karakurt Z, Harman R et al (2020) Treatment of ventilator-associated pneumonia (VAP) caused by Acinetobacter: results of prospective and multicenter ID-IRI study. Eur J Clin Microbiol Infect Dis 39:45–52. https://doi.org/10.1007/s10096-019-03691-z
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Yes, it is obtained from Trabzon Kanuni Hospital’s Review Board.
Informed consent
Obtained.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El-Sokkary, R., Uysal, S., Erdem, H. et al. Profiles of multidrug-resistant organisms among patients with bacteremia in intensive care units: an international ID-IRI survey. Eur J Clin Microbiol Infect Dis 40, 2323–2334 (2021). https://doi.org/10.1007/s10096-021-04288-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-021-04288-1