Abstract
In human congenital toxoplasmosis the effects of parasite burden and pregnancy time at infection on clinical outcome are well known, but there is controversy regarding the role of Toxoplasma gondii type. Through a systematic review of the literature, we aimed to discern if T. gondii type has a role on clinical outcome in human congenital toxoplasmosis. We built up a database of congenital toxoplasmosis from reports of cases, case series and screening-based cohorts, which had information about parasite type, gestation time at maternal infection and/or clinical outcome in the product. Then, we obtained frequencies for loci used to genotype geographical origin of cases and types found. Also, odds ratios were calculated for association between time of maternal infection or parasite type on outcome. Type II parasites were the most common in Europe, Asia and Africa, while in America there were mainly atypical strains. More newborns with clinical problems were born from mothers infected during the first half of gestation than from those acquiring the parasite after week 24, regardless of parasite genotype (92.9 vs. 16.1 %, OR = 67.9, CI95 25.4–181.6). Type I and atypical parasites were associated with clinical problems as opposed to types II and III, regardless of pregnancy period at infection (86.9 vs. 72.9 %, OR = 2.47, CI95 1.1–5.4). A significant and remarkable tendency of type I parasites to be present during early pregnancy was also observed (94.4 vs. 5.6 %, P < 0.009). In addition to parasite burden and period of gestation, T. gondii genotype seems involved in CT clinical outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Toxoplasma gondii is one of the most common parasites worldwide, since it is an obligate microorganism that infects all homoeothermic animals [1]. This pathogen was originally found as a clonal population derived in three lineages (I, II and III) in Europe and North America [2], whereby those of type I are commonly virulent in mice, with LD100 ˂10 parasites; those of type II of low virulence (LD50 ̴103 parasites) in the majority of inbred mouse strains; and type III parasites are usually non-virulent, with LD50 ≥ 105 [3, 4]. Genotypes not fitting within the three dominant lineages were classified as “atypical”, some of them being virulent in mice at isolation [5, 6].
More recent studies have shown a greater variability [7–10]. One of the main studies on genetic T. gondii diversity was based on a collection of 956 isolates obtained from various animal species, and used a combination of genetic markers; this analysis led to 138 different genotypes grouped in 15 haplogroups, which collectively define six major clades, spread out around the world from a small number of ancestors [11–14]. Methods commonly used for molecular characterization of T. gondii in epidemiological studies are PCR-RFLP, microsatellite analysis and DNA sequencing [2, 11–13, 15, 16]. B1, SAG1 and SAG2 loci were the first utilized for this purpose [5, 17, 18]. Later SAG3, GRA6 and BTUB genes along with SAG2 were included and used to analyse T. gondii genotype in immunocompromised patients [19]. Then, c22-8, c29-2, L358, PK1 and Apico markers were incorporated into a multiplex nested PCR-RFLP [20]. This technique is useful when small amounts of DNA are available from valuable samples and facilitates understanding of the epidemiology and genetic diversity of the parasite [21]. During recent years, different sets of microsatellites have been utilized for this objective, with the more recent being a multiplex PCR which includes TUB2, W35, TgM-A, B18, B17, M33, IV.1, XI.1, M48, M102, N60, N82, AA, N61 and N83 [15, 22, 23].
Transmission of this protozoan occurs horizontally by ingestion of contaminated meals or water, or vertically from mother to embryo/foetus [1, 24]. Congenital toxoplasmosis (CT) frequency in humans depends on the period at which maternal infection occurs, i.e. if early, vertical transmission is of low probability, but it causes major damage, including abortions and still births. In the last weeks, the rate of transmission increases to nearly 72 %, but the foetal disease is clinically absent or less severe; thus, most neonates with CT are born asymptomatic. However, such infections may cause eye or central nervous system sequels later in life [25]. Besides this gestation period effect and that of the parasite burden, it would be important to determine if the parasite type has an effect on disease severity, because it may impact on clinical management; this topic, nevertheless, is still controversial [2, 17, 26].
In order to determine if there is evidence to support a role of T. gondii type on clinical aspects of congenital infection, we performed this systematic review of the literature with meta-analysis and are reporting as well as discussing the results herein.
Material and methods
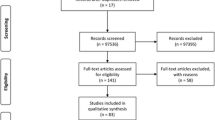
We performed a systematic review of the literature about human congenital T. gondii infection genotyping. Articles were sourced from the following publication databases: Google Scholar, ISIWeb, Lilacs, PuBMed, Science Direct, and Scopus using the Mesh: Toxoplasma gondii typing, genotyping, children, pregnant woman, toxoplasmosis, genotype, abortions, humans, and CT. Results of articles were included if they presented data on T. gondii genotype of confirmed congenital cases; they may also report gestation time at maternal infection or clinical outcome in the product/newborn.
Information was classified according to (a) the source of cases (prenatal/neonatal screening or case report); (b) the clinical outcome: asymptomatic till year of age, CNS/eye disease (cerebral calcification, hydrocephalus, ventriculomegaly, retinochoroidits, microphthalmy and uveitis), systemic damage (jaundice, hepatomegaly, splenomegaly, pneumonitis, purpure, hepatitis, pleural effusion, septic shock, ascites, hypotrophy) or fatal, i.e. abortion, stillbirth or newborn death; and (c) the time at which maternal infection occurred: only two periods could be clearly defined in order to maximize possibility of data analysis, i.e. first (≤24 weeks) and second (>24 weeks).
Genotypes were grouped in classical (type I, II or III), “atypical” (ToxoDB #8, #11, # 36, #41, #65, #67, #108, #162, #166, #206, #229, Africa 1 and TgCTBrca) and recombinant (I/II or I/III). Those types reported as “non-virulent” presented a (TG)7 microsatellite in the beta-tubulin gene, which corresponds to non-virulent strains for mice; also, due to the geographical location (France) they assumed these cases were due to type II parasites (n = 37) [27]. Mixed infections, i.e. combination of two different genotypes in the same sample, were also included in this study and labelled as “I,II” or “I,III”. Parasite genotypes from Argentina, Crete, Cyprus, France, Mexico and Serbia, from mothers with serological evidence of acute infection, were not included in this study, because congenital infection was not confirmed [22, 28–31]. We considered the studies performed with one or more molecular markers for genotyping by PCR-RFLP of protein coding sequences or microsatellite PCR analysis.
Statistical analysis
A database was built with individual values taken from the papers included, using time at maternal infection and parasite genotype as independent variables and clinical outcome as dependent. To measure risk, odds ratio was used, with a P ≤ 0.05 for statistical significance. The SPSS 22.0 software was used for these purposes.
Results
Thirty-two articles met the criteria of having hard data on genotype, clinical outcome, and/or gestation time at maternal infection (Table 1). From these papers we could gather 372 individual genotyping data, 61.0 % of which also had information about trimester of gestation at maternal infection, and 67.7 % on clinical outcome; 182 cases had both types of information (48.9 %).
Most T. gondii genotyping was performed in maternal samples (n = 217, 58 %), while the rest was done in foetal/newborn fluids or tissues (n = 70, 19 %), abortion products or placentas (n = 65, 17 %) or unidentified samples (n = 20, 5 %). The majority of cases were from screening programs (n = 224, 60 %), especially in Europe (n = 172) and America (n = 35), while clinical reports were collected mainly from Asia (n = 66) and Africa (n = 39).
As it can be seen in Table 1, most cases (297 out of 372) were infected by type I, II or III T. gondii classical types, with type II being the most common in CT (n = 246, 66.1 %), including the “non-virulent” strains reported by Costa et al. in 1997 [27], followed by atypical (n = 60, 16.1 %) and type I (n = 44, 11.8 %). Only 1.8 % genotypes were type III and mixed infections were reported in 2.7 % of the cases.
As expected, there were differences among continents (Fig. 1a). Genotypes I and II were reported in America, Europe, Africa and Asia. Atypical variants were present mainly in America and Africa, while genotype III was found in America and Europe only. Finally, mixed infections have been found in Africa, Europe, and America.
Toxoplasma gondii genotyping in human congenital infections worldwide. a Distribution of T. gondiiparasites by continent. b Number of genetic markers used. *PCR-RFLP; **Microsatellite analysis. Atypical genotypes included: ToxoDB #8, #11, # 36, #41, #65, #67, #108, #162, #166, #206, #229, Africa 1,TgCTBrca and recombinant I/II or I/III; Mixed infections were composed of a combination of I,II and I,III types
Genotypes were obtained by PCR-RFLP or microsatellite analysis using different genetic markers and variable number of loci (Fig. 1b). In America, 85.7 % of parasites were typed with three to nine protein-coding genetic markers by PCR-RFLP, while the majority of T. gondii genotyping in Europe (67.4 %) and Oceania (100 %, n = 2) was performed by microsatellite analysis. Furthermore, all variants from Asia and most from Africa (70.4 %) were identified with one coding-gene marker.
In general, type I (77.0 %) and II (44.0 %) variants were detected with one genetic marker (SAG2 gene), while atypical variants have been mainly classified using up to nine protein-coding genes by PCR-RFLP (58.3 %). Also, type II and atypical variants have been identified by multilocus microsatellite analysis in 45.5 % and 21.6 % of the cases. The most common genetic markers were those coding for surface or invasion/replication-related proteins such as: SAG2 (99.2 %), GRA6 (26.4 %), SAG1 (26.0 %), SAG3 (25.1 %) and BTUB (22.7 %).
Most genotyping has been performed in Europe (50.3 %) with type II parasites being prevalent, especially in France (90.5 %). In Spain, the majority of cases were caused by type I parasites. To a lesser extent, atypical and classical III genotypes have also been identified in this continent (Table 1). Genotype II was more frequent in Iran and Egypt as well. Atypical types were reported in Tunisia and French Polynesia. In America, type II parasites have been found in a large proportion in the United States, while in Colombia and Mexico type I parasites prevail. Those called “atypical”—due to the presence of “extra bands” corresponding to “new alleles” in the restriction patterns of SAG1, c22-8, BTUB or SAG2 loci—have been reported mainly in Brazil and Tunisia, but they have also been found in Mexico (Table 1) [8, 10, 31, 52].
During the first period of gestation, 56.4 % of cases were clinical findings (mainly deaths) and even among those diagnosed by screening the majority were ill or dead (Fig. 2a). The nine asymptomatic cases were due to type II or atypical T. gondii variants (Fig. 2c). Conversely, infections of the second half of gestation were mainly detected by prenatal/neonatal screening, most being newborns asymptomatic for up to one year (83.9 %), followed by cases with CNS/eye alterations and two newborns with systemic disease (Fig. 2b and d). Only five cases infected during the second half came from clinical reports, four with fatal outcome and one with CNS/eye disease. As expected and independently on parasite type, most cases infected before the 24th week of pregnancy presented clinical problems (90.8 %), while only 16.1 % of those infected during the second period had bad outcome (Table 2). Remarkable is that all cases infected by type I parasites were symptomatic regardless of time at infection (Fig. 2c and d).
Relation of clinical outcome in the newborns with period at maternal infection and origin of cases (a and b) or parasite genotype (c and d). Asymptomatic = newborns without clinical problems for up to 1 year after birth. CNS/eye disease = cerebral calcification, hydrocephalus, ventriculomegaly, retinochoroidits, microphthalmy, uveitis. Systemic disease = jaundice, hepatomegaly, splenomegaly, pneumonitis, purpure, hepatitis, pleural effusion, septic shock, ascites and hypotrophy. Death = abortion or fetal/newborn death
Besides pregnancy time at infection, parasite type (I or atypical) represented a risk for clinical problems in congenitally infected newborns (Table 2). Furthermore, type I parasites had a significant tendency to infect during the first period of gestation in comparison to the second one (Table 3). When type I variants were compared with variants II and III, this tendency increased. Finally, when infections by type I variants were grouped with atypical and compared to types II and III, the risk decreased to 1.7 (Table 3).
Since almost 37 % of the cases included in the present study came from France alone, we performed a more detailed analysis of the data published from this country. Only 88 individual data had parasite genotype, period of gestation at maternal infection and clinical outcome. Similar results to those of the global analysis arose: 77 cases were infected by type II parasites; all those of the first half presented clinical problems (n = 32), while only 4.4 % of the second period (2/45) had bad outcome. Moreover, the two type I and the seven atypical cases, presented bad outcome. The only two asymptomatic newborns infected with type III variants (Fig. 2d) were reported in this study.
Discussion
There has been a controversy regarding the importance of T. gondii type on clinical problems developed in the human host; some researchers have claimed there is no clear association between genotype and phenotype [26]. As a matter of fact, most adults with acquired toxoplasmosis are apparently healthy all their lives. But caution must still be taken, since chronic subtle (and not so subtle) alterations have been associated with “subclinical” T. gondii infection [55]. The host immune response profile seems important in these cases, with both innate and TH1(/TH17?)-regulated mechanisms involved in protection and clear disadvantage of those individuals who suffer an immune depression, either due to treatment or to infection, for example, with the human immunodeficiency virus [56–61]. Exceptions to this rule seem to be cases of ocular toxoplasmosis in South America, which develop problems in spite of immune competence; it is important to mention that they are caused by atypical T. gondii variants [7, 62].
The severity of the congenital disease is influenced by the time of gestation at which the mother became infected, as reported by Dunn et al. in 1999 [25]. So there was no surprise that the meta-analysis reinforced this notion, i.e. there were more cases with clinical problems in newborns of mothers infected during the first half of gestation than in those acquiring the parasitosis in the second part, with a strong and significant risk value. These could be explained by the fact that the foetal immune response is quite immature as compared to that in the newborn [63–65].
Besides pregnancy time at infection, clinical outcome in congenitally infected products depends on parasite burden. Romand et al. (2004) observed that a concentration larger than 100 parasites/mL in amniotic fluid was strongly associated with a severe outcome (OR = 25.1, CI95% 4.4–143.1), and that these parasite loads were mostly reached if infection occurred in the first half of gestation. Remarkably, the few cases with <100 tachyzoites/mL in early pregnancy were born asymptomatic or with few clinical problems. Moreover, the amniotic fluid of infections acquired after 20 weeks harboured relatively low parasite concentrations and very few cases were born with severe CT. Therefore, gestational age at maternal infection and parasite load in amniotic fluid can be used as early markers for CT severity. Unfortunately they did not type the parasites which infected those cases [66].
A less clear picture exists regarding the role of parasite type and clinical outcome. There is a great variety of T. gondii genotypes reported in CT cases around of world. Individual studies have failed to demonstrate either an association with clinical outcome or even a lack of relation. This is why we performed this review, and found evidence that supports this hypothesis. One possible bias that could be argued is that this review included clinical reports, but of the 372 individuals 60 % were detected through screening, in countries both in Europe (mainly France) and America (mainly Brazil) [8, 10, 22, 49, 67, 68]. It is important to mention that we are including literature published in a 20-year period (1995 to 2015), so we are comparing the first genotypes reported on the basis of only one marker—which discerned among three clonal lineages and “atypical” variants—with recent studies, which are based on a larger number of markers and thus allow better differentiation among “atypical” strains, more prevalent in Brazil or French Polynesia, as well as identification of recombinants [49, 54]. Despite this, the geographical distribution emerged in this review is similar to that reported by Su et al. (2012), who reported clonal lineages mainly present in the northern hemisphere and high polymorphism in South America; these data were gathered using samples collected from animals mainly, which are considered sentinels of T. gondii infection [14]. This gives support to the findings of the present work, which showed classical strains predominance in Europe-Asia-Africa and atypical genotypes in America-Oceania; especially considering that most cases of Europe (55 %) and America (88.9 %) were characterized with more than three genetic markers.
Although already 20 years have passed from the first T. gondii genotype was reported, the role of the parasite type on congenital toxoplasmosis is still controversial [26, 69]. Initially, virulence was defined in laboratory mice with acquired toxoplasmosis on the basis of LD50. Direct assays for the biological basis appeared later; for instance, type I parasites have high rates of multiplication, pathological events induction and crossing of biological barriers in mice [70, 71]. Later, a relation between T. gondii type and immune response profile was suggested, virulent strains associated with poorly controlled Th1 response (IFN-γ, TNF-α) [72], which may kill the host by inflammatory processes [73, 74]. Moreover molecules like IFN-γ, MIF or ICAM-1 indirectly may help the parasite to cross through the syncytiotrophoblast, arriving in the foetal blood stream during early stages of gestation [75–77]. Additionally, some type I parasite molecules may allow pathogen growth and host death by excessive parasite burden, such as ROP16 (which induces activation of STAT3/6) and ROP18 (which phosphorylates and inactivates Interferon Regulated GTPases) [78, 79]. Thus, larger numbers of tachyzoites could reach the embryo/foetus and cause damage.
In the present review we observed type I variants were more frequent among infections of the first half of gestation (see Fig. 2c and d) and all cases were clinically severe. In contrast, type II parasites are very effective in activating an early immune response which destroys tachyzoites and induces cyst formation [78, 80]. Those neonatal cases infected by type II strains during the first half of pregnancy presented severe clinical problems (see Fig. 2c), suggesting a crucial role of the immature status of the immune response of the foetus in disease susceptibility. It is important to mention that most of the cases analyzed in this study were monitored until one year of age, so it is not known if asymptomatic babies that were infected during the second period of gestation presented clinical problems later on.
Type III parasites inhibit the early production of pro-inflammatory cytokines and induce cyst formation, leading to chronic infection in humans and animals [80, 81]. Both cases infected by type III T. gondii reported herein were asymptomatic and were of the second half of gestation, but they are very few.
According to the present study, atypical parasites gave rise to both asymptomatic and severe CT, regardless of gestation period at infection; these parasites belong to three clades and compose the majority of genotypes worldwide [14]. So, with further classification into subgroups, a large number of cases from other parts of the world are needed to finely understand the relation between these genotypes and clinical outcome in congenital toxoplasmosis.
McLeod et al. performed serotyping of T. gondii using the GRA6 and GRA7 polymorphic peptides in a large cohort of cases with CT and found an association between type II parasites and better clinical outcome as compared to those with non-type II infections [82]. Although this study was not included in the datasheet because the article lacked information on pregnancy period at maternal infection, it would support the fact that the parasite type is important for generation of clinical problems in CT. In fact, the present review suggests that type II strains are not aggressive during first year of life unless infection occurs in the first half of gestation. It must be emphasized that there is no prenatal screening program in the United States, thus the results are reflecting what naturally occurs without maternal prophylactic treatment.
In the editorial of the same volume where McLeod et al. published their work, Ajzenberg argues that differences between the United States and Europe (especially France) may arise from the prenatal program established in the latter and not from the parasite type (>90 % of cases are type II in France) [26]. But as stated in the last part of the results section, the observations made for the whole world from the available data can also be gathered by analysis of France alone, i.e. that type II strains are inducing damage if they infect before the 24th week of gestation and not if they get infected later.
This review indicates that besides time of maternal infection, concentration of parasites and maternal/foetal immune response, T. gondii type is involved in the severity of congenital toxoplasmosis. In fact we think there is evidence to support these four aspects are related to each other.
If the parasite type is important for disease outcome, it can influence clinical management; especially because there are few therapeutic possibilities and the more efficient regimen nowadays has serious side-effects [83]. The peptide-based serological typing developed some years ago would simplify prognosis, and thus it would improve decision-making about dose therapy and duration [84].
Conclusions
The following conclusions could be gathered from analysis of articles included in the present review:
-
Gestation period at maternal infection is critical for the development of foetal damage in the case of infections with T. gondii type II.
-
Congenital infection due to genotype I parasites is apparently more frequent during the first period of gestation.
-
Atypical variants were not associated with clinical outcome, but they compose by far the largest group of T. gondii, thus, they deserve further analysis.
References
Weiss LM, Kim K (2013) Toxoplasma gondii. The model Apicomplexan; perspectives and methods. Elsevier, Amsterdam
Howe DK, Sibley LD (1995) Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis 172:1561–1566
Sibley LD, Howe DK (1996) Genetic basis of pathogenicity in toxoplasmosis. Curr Top Microbiol Immunol 219:3–15
Sibley LD, Boothroyd JC (1992) Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature 359:82–85
Grigg ME, Ganatra J, Boothroyd JC, Margolis TP (2001) Unusual abundance of atypical strains associated with human ocular toxoplasmosis. J Infect Dis 184:633–639
Darde ML (2008) Toxoplasma gondii, “new” genotypes and virulence. Parasite 15:366–371
Ferreira IM, Vidal JE, de Mattos CC, de Mattos LC, Qu D, Su C et al (2011) Toxoplasma gondii isolates: multilocus RFLP-PCR genotyping from human patients in Sao Paulo State, Brazil identified distinct genotypes. Exp Parasitol 129:190–195
Carneiro AC, Andrade GM, Costa JG, Pinheiro BV, Vasconcelos-Santos DV, Ferreira AM et al (2013) Genetic characterization of Toxoplasma gondii revealed highly diverse genotypes for isolates from newborns with congenital toxoplasmosis in southeastern Brazil. J Clin Microbiol 51:901–917
Döşkaya M, Caner A, Ajzenberg D, Değirmenci A, Dardé ML, Can H et al (2013) Isolation of Toxoplasma gondii strains similar to Africa 1 genotype in Turkey. Parasitol Int 62:471–474
Higa LT, Garcia JL, Su C, Rossini RC, Falavigna-Guilherme AL (2014) Toxoplasma gondii genotypes isolated from pregnant women with follow-up of infected children in southern Brazil. Trans R Soc Trop Med Hyg 108:244–246
Khan A, Fux B, Su C, Dubey JP, Darde ML, Ajioka JW et al (2007) Recent transcontinental sweep of Toxoplasma gondii driven by a single monomorphic chromosome. Proc Natl Acad Sci USA 104:14872–14877
Khan A, Dubey JP, Su C, Ajioka JW, Rosenthal BM, Sibley LD (2011) Genetic analyses of atypical Toxoplasma gondii strains reveal a fourth clonal lineage in North America. Int J Parasitol 41:645–655
Khan A, Miller N, Roos DS, Dubey JP, Ajzenberg D, Dardé ML et al (2011) A monomorphic haplotype of chromosome Ia is associated with widespread success in clonal and nonclonal populations of Toxoplasma gondii. MBio 2:e00228–00211
Su C, Khan A, Zhou P, Majumdar D, Ajzenberg D, Dardé ML et al (2012) Globally diverse Toxoplasma gondii isolates comprise six major clades originating from a small number of distinct ancestral lineages. Proc Natl Acad Sci USA 109:5844–5849
Blackston CR, Dubey JP, Dotson E, Su C, Thulliez P, Sibley D et al (2001) High-resolution typing of Toxoplasma gondii using microsatellite loci. J Parasitol 87:1472–1475
Ajzenberg D, Banuls AL, Tibayrenc M, Darde ML (2002) Microsatellite analysis of Toxoplasma gondii shows considerable polymorphism structured into two main clonal groups. Int J Parasitol 32:27–38
Howe DK, Honoré S, Derouin F, Sibley L (1997) Determination of genotypes of Toxoplasma gondii strains isolated from patients with toxoplasmosis. J Clin Microbiol 35:1411–1414
Grigg ME, Boothroyd JC (2001) Rapid identification of virulent type I strains of the protozoan pathogen Toxoplasma gondii by PCR-restriction fragment length polymorphism analysis at the B1 gene. J Clin Microbiol 39:398–400
Khan A, Su C, German M, Storch GA, Clifford DB, Sibley LD (2005) Genotyping of Toxoplasma gondii strains from immunocompromised patients reveals high prevalence of type I strains. J Clin Microbiol 43:5881–5887
Su C, Zhang X, Dubey JP (2006) Genotyping of Toxoplasma gondii by multilocus PCR-RFLP markers: A high resolution and simple method for identification of parasites. Int J Parasitol 36:841–848
Su C, Shwab EK, Zhou P, Zhu XQ, Dubey JP (2010) Moving towards an integrated approach to molecular detection and identification of Toxoplasma gondii. Parasitol 137:1–11
Ajzenberg D, Cogné N, Paris L, Bessières MH, Thulliez P, Filisetti D et al (2002) Genotype of 86 Toxoplasma gondii isolates associated with human congenital toxoplasmosis, and correlation with clinical findings. J Infect Dis 186:684–689
Ajzenberg D, Collinet F, Mercier A, Vignoles P, Dardé ML (2010) Genotyping of Toxoplasma gondii isolates with 15 microsatellite markers in a single multiplex PCR assay. J Clin Microbiol 48:4641–4645
Ambroise-Thomas P, Petersen E (2000) Congenital toxoplasmosis. Scientific background, clinical management and control. Springer-Verlag, France
Dunn D, Wallon M, Peyron F, Petersen E, Peckham C, Gilbert R (1999) Mother-to-child transmission of toxoplasmosis: risk estimates for clinical counselling. Lancet 353:1829–1833
Ajzenberg D (2012) High burden of congenital toxoplasmosis in the United States: the strain hypothesis? Clin Infect Dis 54:1606–1607
Costa JM, Dardé ML, Assouline B, Vidaud M, Bretagne S (1997) Microsatellite in the beta-tubulin gene of Toxoplasma gondii as a new genetic marker for use in direct screening of amniotic fluids. J Clin Microbiol 35:2542–2545
Pardini L, Carral LA, Bernstein M, Gos ML, Olejnik P, Unzaga JM et al (2014) First isolation and molecular characterization of Toxoplasma gondii from a human placenta in Argentina. Parasitol Int 63:470–472
Marković M, Ivović V, Stajner T, Djokić V, Klun I, Bobić B et al (2014) Evidence for genetic diversity of Toxoplasma gondii in selected intermediate hosts in Serbia. Comp Immunol Microbiol Infect Dis 37:173–179
Messaritakis I, Detsika M, Koliou M, Sifakis S, Antoniou M (2008) Prevalent genotypes of Toxoplasma gondii in pregnant women and patients from Crete and Cyprus. Am J Trop Med Hyg 79:205–209
Rico-Torres CP, Figueroa-Damián R, López-Candiani C, Macías-Avilés HA, Cedillo-Peláez C, Cañedo-Solares I et al (2012) Molecular diagnosis and genotyping of cases of perinatal toxoplasmosis in Mexico. Pediatr Infect Dis J 31:411–413
Aspinall TV, Guy EC, Roberts KE, Joynson DH, Hyde JE, Sims PF (2003) Molecular evidence for multiple Toxoplasma gondii infections in individual patients in England and Wales: public health implications. Int J Parasitol 33:97–103
Fuentes I, Rubio JM, Ramírez C, Alvar J (2001) Genotypic characterization of Toxoplasma gondii strains associated with human toxoplasmosis in Spain: direct analysis from clinical samples. J Clin Microbiol 39:1566–1570
Cneude F, Deliege R, Barbier C, Barbier C, Durand-Joly I, Bourlet A et al (2003) Septic shock due to congenital disseminated toxoplasmosis? Arch Pediatr 10:326–328
Gilbert RE, Freeman K, Lago EG, Bahia-Oliveira LM, Tan HK, Wallon M et al (2008) Ocular sequelae of congenital toxoplasmosis in Brazil compared with Europe. PLoS Negl Trop Dis 2:e277
Elbez-Rubinstein A, Ajzenberg D, Dardé ML, Cohen R, Dumètre A, Yera H et al (2009) Congenital toxoplasmosis and reinfection during pregnancy: case report, strain characterization, experimental model of reinfection, and review. J Infect Dis 199:280–285
Delhaes L, Ajzenberg D, Sicot B, Bourgeot P, Dardé ML, Dei-Cas E et al (2010) Severe congenital toxoplasmosis due to a Toxoplasma gondii strain with an atypical genotype: case report and review. Prenat Diagn 30:902–905
Kieffer F, Rigourd V, Ikounga P, Bessieres B, Magny JF, Thulliez P (2011) Disseminated congenital Toxoplasma infection with a type II strain. Pediatr Infect Dis J 30:813–815
Nowakowska D, Colón I, Remington JS, Grigg M, Golab E, Wilczynski J et al (2006) Genotyping of Toxoplasma gondii by multiplex PCR and peptide-based serological testing of samples from infants in Poland diagnosed with congenital toxoplasmosis. J Clin Microbiol 44:1382–1389
Costache CA, Colosi HA, Blaga L, Györke A, Paştiu AI, Colosi IA et al (2013) First isolation and genetic characterization of a Toxoplasma gondii strain from a symptomatic human case of congenital toxoplasmosis in Romania. Parasite 20:11
Djurković-Djaković O, Klun I, Khan A, Nikolić A, Knezević-Usaj S, Bobić B et al (2006) A human origin type II strain of Toxoplasma gondii causing severe encephalitis in mice. Microbes Infect 8:2206–2212
Asgari Q, Fekri M, Monabati A, Kalantary M, Mohammadpour I, Motazedian MH et al (2013) Molecular genotyping of Toxoplasma gondii in human spontaneous aborted fetuses in Shiraz, Southern Iran. Iran J Public Health 42:620–625
Sarkari B, Abdolahi Khabisi S (2015) Severe congenital toxoplasmosis: a case report and strain characterization. Case Rep Infect Dis 2015:851085
Gallego C, Castaño JC, Giraldo A, Ajzenberg D, Dardé ML, Gómez JE (2004) Molecular and biological characterization of the CIBMUQ/HDC strain, a reference strain for Colombian Toxoplasma gondii. Biomedica 24:282–290
Gallego C, Saavedra-Matiz C, Gómez-Marín JE (2006) Direct genotyping of animal and human isolates of Toxoplasma gondii from Colombia (South America). Acta Trop 97:161–167
Demar M, Ajzenberg D, Maubon D, Djossou F, Panchoe D, Punwasi W et al (2007) Fatal outbreak of human toxoplasmosis along the Maroni River: epidemiological, clinical, and parasitological aspects. Clin Infect Dis 45:e88–e95
Vidigal PV, Santos DV, Castro FC, Couto JC, Vitor RW, Brasileiro Filho G (2002) Prenatal toxoplasmosis diagnosis from amniotic fluid by PCR. Rev Soc Bras Med Trop 35:1–6
Ferreira Ade M, Vitor RW, Gazzinelli RT, Melo MN (2006) Genetic analysis of natural recombinant Brazilian Toxoplasma gondii strains by multilocus PCR-RFLP. Infect Genet Evol 6:22–31
Silva LA, Andrade RO, Carneiro AC, Vitor RW (2014) Overlapping Toxoplasma gondii genotypes circulating in domestic animals and humans in Southeastern Brazil. PLoS One 9:e90237
Abdel-Hameed DM, Hassanein OM (2008) Genotyping of Toxoplasma gondii strains from female patients with toxoplasmosis. J Egypt Soc Parasitol 38:511–520
Boughattas S, Ben-Abdallah R, Siala E, Siala E, Souissi O, Aoun K et al (2010) Direct genotypic characterization of Toxoplasma gondii strains associated with congenital toxoplasmosis in Tunisia (North Africa). Am J Trop Med Hyg 82:1041–1046
Boughattas S, Abdallah RB, Siala E, Aoun K, Bouratbine A (2011) An atypical strain associated with congenital toxoplasmosis in Tunisia. New Microbiol 34:413–416
Boughattas S, Ben-Abdallah R, Siala E, Souissi O, Maatoug R, Aoun K et al (2011) Case of fatal congenital toxoplasmosis associated with I/III recombinant genotype. Trop Biomed 28:615–619
Yera H, Ajzenberg D, Lesle F, Eyrolle-Guignot D, Besnard M, Baud A et al (2014) New description of Toxoplasma gondii genotypes from French Polynesia. Acta Trop 134:10–12
Flegr J (2013) Influence of latent Toxoplasma infection on human personality, physiology and morphology: pros and cons of the Toxoplasma-human model in studying the manipulation hypothesis. J Exp Biol 216:127–133
Denkers EY, Gazzinelli RT (1998) Regulation and function of T-cell mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev 11:569–588
Correa D, Cañedo-Solares I, Ortiz-Alegría LB, Caballero-Ortega H, Rico-Torres CP (2007) Congenital and acquired toxoplasmosis: diversity and role of antibodies in different compartments of the host. Parasite Immunol 29:651–660
Gazzinelli RT, Mendonça-Neto R, Lilue J, Howard J, Sher A (2014) Innate resistance against Toxoplasma gondii: an evolutionary tale of mice, cats, and men. Cell Host Microbe 15:132–138
Meira CS, Pereira-Chioccola VL, Vidal JE, de Mattos CC, Motoie G, Costa-Silva TA et al (2014) Cerebral and ocular toxoplasmosis related with IFN-γ, TNF-α, and IL-10 levels. Front Microbiol 5:492
Galván Ramírez ML, Castillo-de-León Y, Espinoza-Oliva M, Bojorques-Ramos MC, Rodríguez-Pérez LR, Bernal Redondo R et al (2006) Acute infection of Toxoplasma gondii and Cytomegalovirus reactivation in a pediatric patient receiving liver transplant. Transpl Infect Dis 8:233–236
Israelski DM, Chmiel JS, Poggensee L, Phair JP, Remington JS (1993) Prevalence of Toxoplasma infection in a cohort of homosexual men at risk of AIDS and toxoplasmic encephalitis. J Acquir Immune Defic Syndr 6:414–418
Khan A, Jordan C, Muccioli C, Vallochi AL, Rizzo LV, Belfort R Jr (2006) Genetic divergence of Toxoplasma gondii strains associated with ocular toxoplasmosis, Brazil. Emerg Infect Dis 12:942–949
McLeod R, Dowel M (2000) Basic inmmunology: the fetus and the newborn. In: Ambroise-Thomas P, Petersen E (eds) Congenital toxoplasmosis. Scientific backgound, clinical management and control. Springer, France, pp 37–68
Correa D, Caballero-Ortega H, Rico-Torres CP, Cañedo-Solares I, Ortiz-Alegría LB, Becerra-Torres E et al (2007) Immunobiology of congenital toxoplasmosis. In: Terrazas LI (ed) Advances in the immunobiology of parasitic diseases. Research Signpost, India, pp 199–224
Ortiz-Alegría LB, Caballero-Ortega H, Cañedo-Solares I, Rico-Torres CP, Sahagún-Ruiz A, Medina-Escutia ME et al (2010) Congenital toxoplasmosis: candidate host immune genes relevant for vertical transmission and pathogenesis. Genes Immun 11:363–373
Romand S, Chosson M, Franck J, Wallon M, Kieffer F, Kaiser K et al (2004) Usefulness of quantitative polymerase chain reaction in amniotic fluid as early prognostic marker of fetal infection with Toxoplasma gondii. Am J Obstet Gynecol 190:797–802
Desmonts G, Couvreur J (1974) Congenital toxoplasmosis. A prospective study of 378 pregnancies. N Engl J Med 290:1110–1116
Guerina NG, Hsu HW, Meissner HC, Maguire JH, Lynfield R, Stechenberg B et al (1994) Neonatal serologic screening and early treatment for congenital Toxoplasma gondii infection. The New England Regional Toxoplasma Working Group. N Engl J Med 330:1858–1863
Ajzenberg D (2015) 1995–2015: it is time to celebrate 20 years of (intensive) genotyping of Toxoplasma gondii strains. Future Microbiol 10:689–691
Zenner L, Foulet A, Caudrelier Y, Darcy F, Gosselin B, Capron A et al (1999) Infection with Toxoplasma gondii RH and Prugniaud strains in mice, rats and nude rats: kinetics of infection in blood and tissues related to pathology in acute and chronic infection. Pathol Res Pract 195:475–485
Barragan A, Sibley LD (2003) Migration of Toxoplasma gondii across biological barriers. Trends Microbiol 11:426–430
Akbar H, Dimier-Poisson I, Moiré N (2015) Role of CD4+ Foxp3+ regulatory T cells in protection induced by a live attenuated, replicating type I vaccine strain of Toxoplasma gondii. Infect Immun 83:3601–3611
Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kühn R et al (1996) In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol 157:798–805
Mordue DG, Monroy F, La Regina M, Dinarello CA, Sibley LD (2001) Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J Immunol 167:4574–4584
Robert-Gangneux F, Murat JB, Fricker-Hidalgo H, Brenier-Pinchart MP, Gangneux JP, Pelloux H (2011) The placenta: a main role in congenital toxoplasmosis? Trends Parasitol 27:530–536
Xiao J, Garcia-Lloret M, Winkler-Lowen B, Miller R, Simpson K, Guilbert LJ (1997) ICAM-1-mediated adhesion of peripheral blood monocytes to the maternal surface of placental syncytiotrophoblasts: implications for placental villitis. Am J Pathol 150:1845–1860
Ferro EA, Mineo JR, Ietta F, Bechi N, Romagnoli R, Silva DA et al (2008) Macrophage migration inhibitory factor is up-regulated in human first-trimester placenta stimulated by soluble antigen of Toxoplasma gondii, resulting in increased monocyte adhesion on villous explants. Am J Pathol 172:50–58
Barragán A, Brossier F, Sibley LD (2005) Transepithelial migration of Toxoplasma gondii involves an interaction of intercellular adhesion molecule 1 (ICAM-1) with the parasite adhesin MIC2. Cell Microbiol 7:561–568
Melo MB, Jensen KD, Saeij JP (2011) Toxoplasma gondii effectors are master regulators of the inflammatory response. Trends Parasitol 27:487–495
Behnke MS, Khan A, Wootton JC, Dubey JP, Tang K, Sibley LD (2011) Virulence differences in Toxoplasma mediated by amplification of a family of polymorphic pseudokinases. Proc Natl Acad Sci USA 108:9631–9636
Rosowski EE, Lu D, Julien L, Rodda L, Gaiser RA, Jensen KD et al (2011) Strain-specific activation of the NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J Exp Med 208:195–212
McLeod R, Boyer KM, Lee D, Mui E, Wroblewski K, Karrison T et al (2012) Prematurity and severity are associated with Toxoplasma gondii alleles (NCCCTS, 1981–2009). Clin Infect Dis 54:1595–1605
Caballero-Ortega H, Ortíz-Alegría LB, Rico-Torres CP, Cedillo-Peláez C, Cañedo-Solares I, Besné-Mérida A et al (2014) Toxoplasmosis. In: Correa-Beltrán MD, Figueroa-Damián R (eds) Infecciones Congénitas y Perinatales, 1st edn. Médica Panamericana, México, pp 167–173
Kong JT, Grigg ME, Uyetake L, Parmley S, Boothroyd JC (2003) Serotyping of Toxoplasma gondii infections in humans using synthetic peptides. J Infect Dis 187:1484–1495
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was partially supported by grant 139721 from CONACyT, México.
Conflict of interest
The authors declare no conflict of interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Rico-Torres, C.P., Vargas-Villavicencio, J.A. & Correa, D. Is Toxoplasma gondii type related to clinical outcome in human congenital infection? Systematic and critical review. Eur J Clin Microbiol Infect Dis 35, 1079–1088 (2016). https://doi.org/10.1007/s10096-016-2656-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-016-2656-2