Abstract
Although Clostridium difficile is a major cause of antibiotic-associated diarrhoea in adults, the incidence and severity of C. difficile infection (CDI) in children is unclear. One complicating factor in assessing the role of CDI in children is the possibility of co-infection with other gastrointestinal pathogens. In this review, we summarise the literature concerning C. difficile co-infections in young children, in an attempt to discuss the rate of co-infections and their potential role in the severity of CDI clinical presentation. We identified 31 studies where co-infections were analysed, comprising 1,718 patients with positive C. difficile tests. The pooled percentage of reported co-infections was 20.7 % (range 0–100 %). Viral co-infections were most commonly reported (46 %), with bacteria and parasites accounting for 14.9 % and 0.01 % of cases, respectively. However, the panel of co-infections tested for varied considerably among studies and 38 % of stated co-infections did not have a pathogen reported. Substantial variation in how and when tests for gastrointestinal co-infections are carried out, small sample sizes and a lack of clear CDI case definitions preclude meaningful conclusions on the true rate of co-infections in this patient population. This review suggests that co-infections may be common in children with diarrhoea who tested positive for C. difficile. Given a lack of CDI case definitions, especially in young children under the age of 5 years, a broad panel of pathogens should be tested for to exclude other microbiological causes. However, the summarised poor quality of the available literature on this subject highlights a need for further studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clostridium difficile is a Gram-positive, anaerobic, spore-forming bacillus that capitalises on disruption of the normal intestinal microbiota to colonise the large intestine, causing disease symptoms through the action of its toxins [1–3]. C. difficile infection (CDI) is associated with significant morbidity and mortality in adults [2], but the incidence and severity of CDI in neonates and infants is currently unclear. Diarrhoeal illness is very common in young children in whom high carriage rates of C. difficile are reported [4]. Alongside a lack of clear CDI case definitions in those children under the age of 3 years [5], detection of C. difficile is commonly interpreted as asymptomatic colonisation and not the causative agent for diarrhoea. However, there have been reports of a potential pathogenic role of C. difficile in this patient population, as occurs in adults [4, 6, 7]. A recent study suggested that the use of adult markers of disease severity are not useful in guiding the management of CDI in children ≤16 years of age, which makes it difficult to design and interpret clinical studies [8].
A key complicating factor in assessing the pathophysiology of C. difficile in children is that detection of C. difficile in children with diarrhoea can be indicative of colonisation only, and co-infection with another gastrointestinal pathogen can be the true cause of the disease. Studies have shown that rates of positive C. difficile tests are similar in stools of asymptomatic young children and children with diarrhoea [9, 10]. Consequently, it has been suggested that, if C. difficile is detected in children under 3 years of age, alternative causative agents for diarrhoea should be sought [11, 12]. Currently, the precise rate of co-infections in C. difficile-positive children, and the role co-infections play in disease severity, is not known. In this article, we review the literature in an attempt to discuss the prevalence of co-infection with C. difficile and other pathogens in children under 18 years of age with diarrhoea, the effect of co-infections on CDI severity and variations in diagnostic testing practises.
Literature search criteria
PubMed and EMBASE were searched for all citations with C. difficile and children using the search string (Clostridium difficile OR C. difficile OR difficile) AND (child OR child* OR infan* OR neonat* OR baby OR babies OR pediatric OR paediatric OR adolescen*). The search was limited to articles, and articles cited therein, published from January 1st 1980 until the date of search on December 13th 2013. Studies were excluded if they were not published in English, German, French or Dutch; if no investigations for co-infection were performed; if the study did not contain original data; if the paediatric population could not be separated from adult patients; or if the study population was smaller than 50 patients (to reduce the potential bias induced when taking into account case reports or case series).
Studies included in the analysis
A total of 1,333 hits were obtained in the literature search, which were then screened based on title and abstract. Articles were excluded for the following reasons: not in English/German/French/Dutch (n = 131), no mention of co-infection (n = 603), review without original data (n = 161), the paediatric population could not be separated from the adult patients (n = 135) and study population of <50 patients (n = 221). The full texts of the remaining 82 articles were screened and 51 were excluded because no data were available on co-infections. Thirty-one studies were included, incorporating a total of 10,201 patients meeting individual study inclusion criteria, of which 1,718 patients had a positive C. difficile test and diarrhoea (Table 1). Studies included children aged 0–2 years (n = 4), 0–5 years (n = 2), 0–12 years (n = 3) and 0–18 years (n = 22). The majority of studies were from North America (n = 12; 6,184 cases) [13–24] and Europe (n = 12; 2,467 cases) [8, 25–35], with other studies from Asia (n = 4; 1,762 cases) [36–39], Australia (n = 2; 148 cases) [40, 41] and South America (n = 1; 210 cases) [42]. Ten studies included only community-onset patients [13, 14, 25, 27, 28, 31, 32, 34, 35, 38], three included only hospital patients [20, 30, 40], 12 included both hospital and community [8, 15, 16, 19, 21–24, 26, 29, 33, 42] and six studies did not report the place of onset [17, 18, 36, 37, 39, 41]. Co-morbidities cited in the studies included cancer, transplantation, immunosuppression, inflammatory bowel disease and bone marrow transplantation. No studies described the inclusion of patients during an outbreak of gastrointestinal disease.
Rate of C. difficile co-infection with other gastrointestinal pathogens
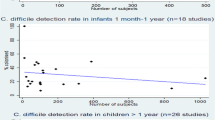
Of the 10,201 patients included in all the studies, a total of 1,708 (16.1 %) C. difficile-positive tests were obtained from patients with diarrhoea, using a variety of diagnostic methodologies, which are summarised in Table 1. In this group, a total of 355 co-infections were reported (pooled percentage 20.8 %). Reported co-infection rates varied between 0 and 100 %, with seven studies reporting a co-infection rate of ≥50 % among C. difficile-positive patients (Fig. 1).
The frequencies of co-infection in each study are described in Table 1. We found that the panel of co-infecting pathogens tested for varied substantially among the 31 included studies. Only four systematically tested for viruses, bacteria and parasites in all cases [23, 30, 33, 37]. The study by Oğuz et al. of 100 children (aged 0–13 years) with diarrhoea isolated a co-infecting pathogen in 25 % (6/24) of those with a positive C. difficile test, of which rotavirus was isolated in four cases and Entamoeba histolytica in two [30]. Shastri et al. screened stool samples from 267 children (aged 0–16 years) based on symptoms of vomiting, diarrhoea or feeding intolerance, and identified an astrovirus co-infection in 10 % (4/40) of those with a positive C. difficile test [23]. The study by Uhnoo et al. analysed stool samples from 616 children (aged 0–14 years) and identified a co-infecting pathogen in 45.3 % (39/86) of C. difficile-positive patients (rotavirus, n = 19; adenovirus, n = 12; calicivirus, n = 1; Y. enterocolitica, n = 2; C. jejuni, n = 3; enteropathogenic E. coli, n = 1; S. typhimurium + C. jejuni + E. coli, n = 1) [33]. Albert et al. analysed stool samples from 814 children with diarrhoea (aged 0–5 years), noting a co-infection in 53.8 % (7/13) of those with a positive C. difficile test (rotavirus, n = 2; C. jejuni, n = 1, enteropathogenic E. coli, n = 1, enterotoxigenic E. coli, n = 1, Aeromonas spp., n = 1; Shigella spp., n = 1) [37]. Twenty studies tested for bacterial co-infection in all samples [13, 18, 19, 23, 24, 27–41], 13 tested for viral pathogens (of which five tested for rotavirus only) [16, 18, 19, 23, 27–31, 33–35, 37] and six tested for parasites [23, 30, 32, 33, 37, 40]. In ten studies, not all samples were tested for co-infection or no data were reported on the number of tested samples [8, 14, 15, 17, 20–22, 25, 26, 42].
The number of reported co-infections by pathogen is described in Table 2. We also observed that, where co-infections were found, details of the co-infecting pathogen were often not reported. Of the 355 children in whom a co-infection was found, in 133 patients (37.5 %), no specific organism was reported. Of the remaining 222 children, viruses accounted for most reported co-infections in C. difficile-positive children with diarrhoea (74 %, n = 164), including rotavirus (59 %, n = 97), adenovirus (20 %, n = 32), norovirus (10 %, n = 17), astrovirus (5 %, n = 9), sapovirus (3 %, n = 5) and others (2 %, n = 4). Bacteria accounted for 53 cases (24 %), including E. coli (32 %, n = 17), Salmonella spp. (21 %, n = 11), Campylobacter spp. (21 %, n = 11), Yersinia spp. (11 %, n = 6) and others (15 %, n = 8); co-infection with parasites was only reported in five cases (2 %).
Difference in disease severity with or without co-infection
Four studies assessed the impact of C. difficile co-infection with other gastrointestinal pathogens on clinical presentation [16, 26, 32, 34], but a clear correlation between a co-infecting organism and the presence of C. difficile on disease severity was not identified, and each study used different clinical markers of severity. The study by El Feghaly et al. [16] assessed the role of viral co-infections only in patients with a positive C. difficile test (aged 0–16 years), concluding that patient groups with (15/62; 24.2 %) and without (47/62; 75.8 %) viral co-infections were clinically indistinguishable, with a median time to resolution of diarrhoea on CDI therapy of 3 days, regardless of the viral co-infection status. Dulęba et al. [26] reported co-infection in 6/22 (27.3 %) children with severe CDI (defined by two or more of the following: fever ≥38.5 °C, white blood cell count ≥15,000/mm3, elevated age-adjusted serum creatinine, albumin <2.5 g/dl) and 9/42 (21.4 %) children with non-severe CDI (age range 0–16 years), concluding that co-infection was not a significant risk factor for severe disease (p = 0.83). There was no significant difference in the incidence of severe CDI among all age groups. Tvede et al. [32] reported that 17.9 % (15/84) of CDI cases had a co-infection with another pathogenic bacterial species, but excluded viral co-infection. However, symptoms and duration of diarrhoea did not differ from those with CDI alone. A recent study by Valentini et al. [34] suggested that C. difficile viral co-infections in children might influence the severity of clinical presentation. The study found a co-infection in 83.3 % (19/23) of C. difficile-positive patients (age range 0–16 years). Detection of C. difficile and rotaviruses were the most common (63 % of patients with any co-infection). Children with a co-infection in general had a more severe clinical presentation and had a higher probability of being severely dehydrated than those with mono-infection, independent of age and living in urban or rural areas. This analysis compared any co-infection with any mono-infection and was not specific for C. difficile co-infection.
Testing methods for C. difficile
Most studies in this analysis used the decision to test for C. difficile as an inclusion criterion. There are many different approaches that can be used in the laboratory to test for the presence of C. difficile. However, the best standard laboratory test or combination of tests has not yet been fully established in children, although UK guidelines for adult disease incorporate a two-step approach of a screening test followed by a confirmatory test [43]. Commonly used tests include: (i) the detection of C. difficile products such as toxin A and/or B, glutamate dehydrogenase (GDH) or cell culture cytotoxicity; (ii) culture of toxigenic C. difficile; and (iii) polymerase chain reaction (PCR) amplification of 16S RNA, toxin genes or GDH. The studies included in this analysis utilised a variety of methodologies for the diagnosis of CDI (summarised in Table 1), including C. difficile culture, in vitro cell culture cytotoxicity, presence of toxin A/B based on PCR amplification and/or enzyme immunoassay, or a combination of these methods. Of the 31 studies in our analysis, 16 included testing for C. difficile and identification of free toxin when diagnosing CDI, ten tested for toxin only, while five of the studies defined CDI only as the presence of C. difficile culture (Table 1). In these five studies, the presence of toxigenic C. difficile was not confirmed, and to eliminate any potential bias in our analysis resulting from the inclusion of studies where non-toxigenic C. difficile may have been identified, we repeated the analysis after removing these studies. Of the remaining 8,882 patients from the 26 studies, 1,449 positive C. difficile tests were reported and co-infections were noted in 327 cases (22.6 %). This co-infection rate was similar to our observed pooled rate from all 31 studies.
Discussion
The findings of this review suggest that co-infection with C. difficile and other gastrointestinal pathogens is common in children with diarrhoea, with a pooled rate of reported co-infection of 20.7 % in C. difficile-positive children. However, although co-infection is an important factor in understanding and managing C. difficile in children, the literature is very limited. The majority of studies included in our initial literature search did not test for co-infections. In those that did, the panel of co-infections tested for varied considerably. Taking this into account, along with the fact that a causative organism is found in only 23.2–67 % of children with diarrhoea [44, 45], this review highlights an under-appreciation of co-infections in children, and the true rate could be substantially higher than the reported pooled rate. Unfortunately, the studies in our analysis often consisted of small cohorts (with a mean of 55 C. difficile-positive samples), were not stratified by age groups or risk factors, and outcomes such as survival, length of hospital stay and incidence of complications were not discussed in depth, preventing a more meaningful interpretation of the data. Consequently, larger multi-centre studies that systematically analyse co-infection would be beneficial to better understand the role of co-infection in the pathophysiology and prevalence of C. difficile in this patient population.
The impact of C. difficile in children with diarrhoea is often debated. Although severe CDI is reported to occur, most cases in this group tend to be asymptomatic and the current convention is to consider the presence of C. difficile in stools of patients under 2 years old as colonisation. Indeed, a recent expert panel meeting concluded that there is currently no accepted case definition of CDI in infants [5]. As a result, stool samples of children are not routinely sent for C. difficile testing [12] and guidelines suggest that an alternative aetiology should be considered in young paediatric patients with diarrhoea. We found that, when co-infections were tested for in children with a positive C. difficile test, a variety of pathogens were encountered (Table 2). This pattern is not specific to children with a positive C. difficile test and can be expected in any child with diarrhoea [44]. Unfortunately, only four studies systematically tested for the presence of viruses, bacteria and parasites, and the specific pathogen was not reported in over one-third of co-infections, which limits the reliability of the data and underlines the need for future studies.
It seems reasonable to assume that isolated co-infecting viruses, bacteria and parasites are a sufficient explanation for the presence of diarrhoea, in which case the detected C. difficile reflects an asymptomatic colonisation in infants and neonates. Alternatively, C. difficile in children could be the primary cause of diarrhoea, or contributing to an additive or synergistic clinical effect with another pathogen. The included studies were not conclusive regarding the impact of co-infection on CDI severity. The study by Valentini et al. noted a high rate of C. difficile co-infections [34] and more severe clinical presentation with higher probability of dehydration was observed in the group with any co-infection compared with those with a mono-infection. However, this and other studies did not observe a correlation between C. difficile co-infection and disease severity [16, 26, 32]. Due to the small sample sizes in the included studies, and, in particular, the small numbers of co-infected patients, statistical analysis of the data is problematic. The markers of disease severity also varied for each study, which makes any conclusion on the effects of co-infection unreliable. Further studies comprising larger patient cohorts and consistent clinical markers are necessary to identify any link between C. difficile co-infections and the severity of clinical presentation.
The current UK CDI management guidelines, suitable for adults and older children, recommend that testing for toxigenic C. difficile should involve a positive first test for C. difficile (e.g. GDH), followed by identification of free toxin in faeces to diagnose symptomatic CDI [46]. Of the studies in our analysis, only 16 included testing for C. difficile and toxin, while five could not differentiate toxigenic and non-toxigenic C. difficile. The differences in diagnostic approaches that we observed are unsurprising given that the dates of study publication span more than three decades. Broad use of multiple-step algorithms for diagnosing CDI in future studies may allow for an improved understanding of whether C. difficile is the causative agent of diarrhoea in cases of co-infections. However, the sensitivity and specificity of the different tests in paediatric populations, and the issue of positive predictive value in infants where the colonisation rate is high, remain to be established.
Concluding remarks
Our analysis shows that, if a young child presents with diarrhoea and a stool sample is tested for Clostridium difficile and other gastrointestinal pathogens, co-infections are frequently found. However, deficiencies in the current literature preclude meaningful conclusions on the true rate of co-infection in this patient group and the age group where co-infection is clinically important. More robust future studies incorporating larger sample sizes, consistent case definitions and diagnostic testing for a broad panel of viral, bacterial and parasitic co-infections are necessary to improve our understanding and management of C. difficile in children.
References
Poxton IR, McCoubrey J, Blair G (2001) The pathogenicity of Clostridium difficile. Clin Microbiol Infect 7(8):421–427
Rupnik M, Wilcox MH, Gerding DN (2009) Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol 7(7):526–536
Crobach MJT, Dekkers OM, Wilcox MH, Kuijper E (2009) European Society of Clinical Microbiology and Infectious Diseases (ESCMID): data review and recommendations for diagnosing Clostridium difficile-infection (CDI). Clin Microbiol Infect 15:1053–1066
Enoch DA, Butler MJ, Pai S, Aliyu SH, Karas JA (2011) Clostridium difficile in children: colonisation and disease. J Infect 63:105–113
Faust SN, Wilcox MH, Banaszkiewicz A, Bouza E, Raymond J, Gerding DN (2015) Lack of evidence for an unmet need to treat Clostridium difficile infection in infants aged <2 years: expert recommendations on how to address this issue. Clin Infect Dis 60:912–918
Morris O, Tebruegge M, Pallett A, Green SM, Pearson AD, Tuck A, Clarke SC, Roderick P, Faust SN (2013) Clostridium difficile in children: a review of existing and recently uncovered evidence. Adv Exp Med Biol 764:57–72
Khanna S, Baddour LM, Huskins WC, Kammer PP, Faubion WA, Zinsmeister AR, Harmsen WS, Pardi DS (2013) The epidemiology of Clostridium difficile infection in children: a population-based study. Clin Infect Dis 56:1401–1406
Pai S, Aliyu SH, Enoch DA, Karas JA (2012) Five years experience of Clostridium difficile infection in children at a UK tertiary hospital: proposed criteria for diagnosis and management. PLoS One 7:e51728
Denno DM, Shaikh N, Stapp JR, Qin X, Hutter CM, Hoffman V, Mooney JC, Wood KM, Stevens HJ, Jones R, Tarr PI, Klein EJ (2012) Diarrhea etiology in a pediatric emergency department: a case control study. Clin Infect Dis 55:897–904
Vernacchio L, Vezina RM, Mitchell AA, Lesko SM, Plaut AG, Acheson DW (2006) Diarrhea in American infants and young children in the community setting: incidence, clinical presentation and microbiology. Pediatr Infect Dis J 25:2–7
Dubberke ER, Gerding DN, Classen D, Arias KM, Podgorny K, Anderson DJ, Burstin H, Calfee DP, Coffin SE, Fraser V, Griffin FA, Gross P, Kaye KS, Klompas M, Lo E, Marschall J, Mermel LA, Nicolle L, Pegues DA, Perl TM, Saint S, Salgado CD, Weinstein RA, Wise R, Yokoe DS (2008) Strategies to prevent Clostridium difficile infections in acute care hospitals. Infect Control Hosp Epidemiol 29(Suppl 1):S81–S92
Schutze GE, Willoughby RE; Committee on Infectious Diseases; American Academy of Pediatrics (2013) Clostridium difficile infection in infants and children. Pediatrics 131:196–200
Boenning DA, Fleisher GR, Campos JM, Hulkower CW, Quinlan RW (1982) Clostridium difficile in a pediatric outpatient population. Pediatr Infect Dis 1:336–338
Denno DM, Stapp JR, Boster DR, Qin X, Clausen CR, Del Beccaro KH, Swerdlow DL, Braden CR, Tarr PI (2005) Etiology of diarrhea in pediatric outpatient settings. Pediatr Infect Dis J 24:142–148
Deorari S, McConnell A, Tan KK, Jadavji N, Ma D, Church D, Katzko G, Gall DG, Jadavji T, Davies HD (1999) Differential yield of pathogens from stool testing of nosocomial versus community-acquired paediatric diarrhea. Can J Infect Dis 10:421–428
El Feghaly RE, Stauber JL, Tarr PI, Haslam DB (2013) Viral co-infections are common and are associated with higher bacterial burden in children with Clostridium difficile infection. J Pediatr Gastroenterol Nutr 57:813–816
Kim J, Shaklee JF, Smathers S, Prasad P, Asti L, Zoltanski J, Dul M, Nerandzic M, Coffin SE, Toltzis P, Zaoutis T (2012) Risk factors and outcomes associated with severe Clostridium difficile infection in children. Pediatr Infect Dis J 31:134–138
Klein EJ, Boster DR, Stapp JR, Wells JG, Qin X, Clausen CR, Swerdlow DL, Braden CR, Tarr PI (2006) Diarrhea etiology in a Children’s Hospital Emergency Department: a prospective cohort study. Clin Infect Dis 43:807–813
Kotloff KL, Wade JC, Morris JG Jr (1988) Lack of association between Clostridium difficile toxin and diarrhea in infants. Pediatr Infect Dis J 7:662–663
Langley JM, LeBlanc JC, Hanakowski M, Goloubeva O (2002) The role of Clostridium difficile and viruses as causes of nosocomial diarrhea in children. Infect Control Hosp Epidemiol 23:660–664
Rexach CE, Tang-Feldman YJ, Cantrell MC, Cohen SH (2006) Epidemiologic surveillance of Clostridium difficile diarrhea in a freestanding pediatric hospital and a pediatric hospital at a university medical center. Diagn Microbiol Infect Dis 56:109–114
Sandora TJ, Fung M, Flaherty K, Helsing L, Scanlon P, Potter-Bynoe G, Gidengil CA, Lee GM (2011) Epidemiology and risk factors for Clostridium difficile infection in children. Pediatr Infect Dis J 30:580–584
Shastri S, Doane AM, Gonzales J, Upadhyayula U, Bass DM (1998) Prevalence of astroviruses in a children’s hospital. J Clin Microbiol 36:2571–2574
Thompson CM Jr, Gilligan PH, Fisher MC, Long SS (1983) Clostridium difficile cytotoxin in a pediatric population. Am J Dis Child 137:271–274
Bauer MP, Veenendaal D, Verhoef L, Bloembergen P, van Dissel JT, Kuijper EJ (2009) Clinical and microbiological characteristics of community-onset Clostridium difficile infection in The Netherlands. Clin Microbiol Infect 15:1087–1092
Dulęba K, Pawłowska M, Wietlicka-Piszcz M (2014) Clostridium difficile infection in children hospitalized due to diarrhea. Eur J Clin Microbiol Infect Dis 33:201–209
Ellis ME, Mandal BK, Dunbar EM, Bundell KR (1984) Clostridium difficile and its cytotoxin in infants admitted to hospital with infectious gastroenteritis. Br Med J (Clin Res Ed) 288:524–526
Hjelt K, Nielson OH, Paerregaard A, Grauballe PC, Krasilnikoff PA (1987) Acute gastroenteritis in children attending day-care centres with special reference to rotavirus infections. II. Clinical manifestations. Acta Paediatr Scand 76:763–768
Nivenius K, Blomberg J, Hagander B, Mårdh PA, Schalén C (1987) Pediatric gastroenteritis in primary care and in hospitalized patients. Scand J Prim Health Care 5:41–45
Oğuz F, Uysal G, Daşdemir S, Oskovi H, Vidinlisan S (2001) The role of Clostridium difficile in childhood nosocomial diarrhea. Scand J Infect Dis 33:731–733
Rosenfeldt V, Vesikari T, Pang XL, Zeng SQ, Tvede M, Paerregaard A (2005) Viral etiology and incidence of acute gastroenteritis in young children attending day-care centers. Pediatr Infect Dis J 24:962–965
Tvede M, Schiøtz PO, Krasilnikoff PA (1990) Incidence of Clostridium difficile in hospitalized children. A prospective study. Acta Paediatr Scand 79:292–299
Uhnoo I, Wadell G, Svensson L, Olding-Stenkvist E, Ekwall E, Mölby R (1986) Aetiology and epidemiology of acute gastro-enteritis in Swedish children. J Infect 13:73–89
Valentini D, Vittucci AC, Grandin A, Tozzi AE, Russo C, Onori M, Menichella D, Bartuli A, Villani A (2013) Coinfection in acute gastroenteritis predicts a more severe clinical course in children. Eur J Clin Microbiol Infect Dis 32:909–915
Vesikari T, Isolauri E, Mäki M, Grönroos P (1984) Clostridium difficile in young children. Association with antibiotic usage. Acta Paediatr Scand 73:86–91
Ahmad SH, Kumar P, Fakhir S, Ahmad KN, Rattan A, Channa RS, Bajaj G (1993) Antibiotic associated colitis. Indian J Pediatr 60:591–594
Albert MJ, Faruque ASG, Faruque SM, Sack RB, Mahalanabis D (1999) Case–control study of enteropathogens associated with childhood diarrhea in Dhaka, Bangladesh. J Clin Microbiol 37(11):3458–3464
Kim KH, Suh IS, Kim JM, Kim CW, Cho YJ (1989) Etiology of childhood diarrhea in Korea. J Clin Microbiol 27:1192–1196
Niyogi SK, Bhattacharya SK, Dutta P, Naik TN, De SP, Sen D, Saha MR, Datta D, Nair GB, Mitra U, Bhattacharya M, Rasaily R, Pal SC (1991) Prevalence of Clostridium difficile in hospitalised patients with acute diarrhoea in Calcutta. J Diarrhoeal Dis Res 9:16–19
Burgner D, Siarakas S, Eagles G, McCarthy A, Bradbury R, Stevens M (1997) A prospective study of Clostridium difficile infection and colonization in pediatric oncology patients. Pediatr Infect Dis J 16:1131–1134
Kennedy E, Burke V, Pearman J, Robinson J, Gracey M (1991) Cytotoxic effects of children’s faeces: relation to diarrhoea due to Clostridium difficile and other enteric pathogens. Ann Trop Paediatr 11:107–112
Pinto LJF, Alcides APP, Ferreira EO, Avelar KES, Sabrá A, Domingues RMCP, Ferreira MCS (2003) Incidence and importance of Clostridium difficile in paediatric diarrhoea in Brazil. J Med Microbiol 52:1095–1099
Department of Health (2012) Updated guidance on the diagnosis and reporting of Clostridium difficile. Available online at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/215135/dh_133016.pdf. Accessed August 2014
Onori M, Coltella L, Mancinelli L, Argentieri M, Menichella D, Villani A, Grandin A, Valentini D, Raponi M, Russo C (2014) Evaluation of a multiplex PCR assay for simultaneous detection of bacterial and viral enteropathogens in stool samples of paediatric patients. Diagn Microbiol Infect Dis 79:149–154
Huhulescu S, Kiss R, Brettlecker M, Cerny RJ, Hess C, Wewalka G, Allerberger F (2009) Etiology of acute gastroenteritis in three sentinel general practices, Austria 2007. Infection 37(2):103–108
Debast SB, Bauer MP, Kuijper EJ, Allerberger F, Bouza E, Coia J, Cornely OA, Fitzpatrick F, Guery B, Wilcox MH, Nathwani D, Noren T, Olesen B, Rakoczi E, Welte T, Widmer A (2014) European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect 20:1–26
Acknowledgements
Medical writing support was provided by DAB on behalf of Astellas Pharma EMEA.
Conflict of interest
DAB and JAK are employees of Astellas Pharma EMEA. DAE has received funding from Astellas Pharma EMEA for attendance at a scientific congress. SNF received funding from Astellas, Cubist and Actelion to attend an international meeting of experts in 2013 to discuss generic issues related to conducting trials of C. difficile in infants and has attended advisory boards for vaccine and antimicrobial manufacturers. All honoraria are paid to the university or hospital, with no personal payment of any kind.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Graaf, H., Pai, S., Burns, D.A. et al. Co-infection as a confounder for the role of Clostridium difficile infection in children with diarrhoea: a summary of the literature. Eur J Clin Microbiol Infect Dis 34, 1281–1287 (2015). https://doi.org/10.1007/s10096-015-2367-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-015-2367-0