Abstract
The objectives of this study were to determine the incidence of enteric pathogens causing acute gastroenteritis (AGE) among hospitalized children in a large Italian hospital, to measure the incidence of coinfections, and to compare the clinical characteristics of those infected with one versus multiple agents. A prospective study was conducted from March 2010 to April 2011 at the Bambino Gesù Pediatric Hospital in Rome, Italy. All patients between 1 month and 16 years of age admitted to the Pediatric Department with a diagnosis of AGE were eligible for enrollment. Two stool samples for each patient were tested for gastrointestinal pathogens. We summarized the clinical severity of episodes, describing the duration of diarrhea, duration and frequency of vomiting, fever, and severity of dehydration. All the patients underwent medical evaluation with estimation of dehydration. One or more etiological agents were detected in 151 out of 232 patients (65.1 %), while we did not detect any etiological agent in 81 (34.9 %). Rotavirus was detected in 96 (63.6 %), adenovirus in 17 (11.2 %), norovirus in 7 (4.6 %), toxin-producing Clostridium difficile in 23 (15.2 %), Salmonella spp. in 15 (9.9 %, B group in 12/15 and D group in 3/15), C. perfringens in 12 (7.9 %), Campylobacter spp. in 6 (4 %), and verotoxigenic Escherichia coli (VTEC) in 2 (1.3 %). In 27 children out of 151 (17.9 %), we found evidence of coinfection. Coinfection with rotavirus and toxin-producing C. difficile was the most common (63 %). Children with coinfection had a more severe clinical presentation and had a higher probability to be severely dehydrated, independently of age and living community type.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Community-acquired acute gastroenteritis (AGE) is responsible for significant morbidity and mortality among infants and children worldwide [1]. AGE of infectious nature can be caused by a variety of microorganisms. Rotaviruses are generally considered to be the major etiological agents of severe infantile diarrhea worldwide, and are responsible for over 500,000 deaths/year in children younger than 5 years of age [2].
Frequently, AGE in children under 2 years of age requires hospitalization. Nevertheless, since most gastroenteritis is self-limiting and requires no specific therapy, a proven etiology is frequently missed. However, the etiological diagnosis remains a crucial tool for monitoring epidemiological trends, timely identifying emergent pathogens, and providing a basis for appraising the socio-economic impact of the less common coinfections.
Coinfections in AGE have been reported in previous European studies, but the available data are limited and not homogeneous [3–8].
In Italy, the surveillance of AGE is included in the official infectious disease surveillance program (SIMI) and in the laboratory-based surveillance network for enteric pathogens (Enter-net Italia). The Enter-net Italia surveillance system collects data on Salmonella, verotoxigenic Escherichia coli (VTEC), and Campylobacter strains isolated from human infections. Salmonella is the most frequently isolated bacteria in enteric infections, while VTEC is only sporadically reported [9].
Regarding viral infections, during three consecutive years (2007–2009), rotaviruses were identified on 2,697 specimens of stools collected from children, according to data from the surveillance network RotaNet-Italia. Most of the strains belonged to genotypes G1, followed by G9, G4, and G2 [10].
In a retrospective survey conducted in Italy between 2008 and 2009, the annual incidence rate of AGE was 1.08 episodes/person-year. Interestingly, the largest variation in the incidence of AGE was observed in the pediatric age. Children, in fact, represent the population group with the highest demand for medical care and medication, and have the highest impact in terms of indirect social costs due to the loss of school or work days by the affected subjects or their caregivers [11].
A recent study conducted on a population of Italian children hospitalized for AGE reported a coinfection in 3.5 % of the analyzed samples: norovirus–rotavirus (2.6 %), norovirus–adenovirus (0.6 %), rotavirus–adenovirus (0.2 %), and norovirus–enterovirus (0.2 %) [12].
To our knowledge, only a few other studies have reported robust information regarding coinfections in children hospitalized for AGE, in terms of prevalence, distribution, and clinical features. In fact, the association of coinfection with clinical manifestations and duration of hospitalization is controversial according to the published literature [3].
We conducted this study in order to determine the incidence of enteric pathogens causing AGE among hospitalized children in a large Italian pediatric hospital. The study aimed to measure the incidence of coinfections with viral or bacterial agents, and to compare the clinical characteristics of patients infected with one versus multiple agents.
Materials and methods
Study population
A prospective study was conducted from March 2010 to April 2011 at the Bambino Gesù Children’s Hospital (OPBG) in Rome, Italy. The OPBG is a large hospital which serves a large catchment area, with 9,000 admissions and 47,000 consultations at the Emergency Department per year.
All patients between 1 month and 16 years of age admitted to the Pediatric Department with a diagnosis of AGE were eligible for enrollment. We defined AGE as a decrease in the consistency of stools (loose or liquid) and/or an increase in the frequency of evacuations (typically, ≥3 in 24 h), with or without fever or vomiting [13].
In our hospital, the admission criteria for AGE are severe dehydration, incoercible vomiting, hypovolemic shock, non-responsive oral rehydration, age <3 months, and poor family compliance. The criteria for discharge are: significant reduction of the number of diarrhea episodes, tolerance of oral rehydration, and weight gain.
Most children admitted with a diagnosis of AGE require intravenous rehydration and careful observation, until they can be switched to oral rehydration.
The exclusion criteria included: previous history of diarrhea lasting more than 2 weeks, diarrhea caused by drugs or other substances, intestinal malabsorption, inflammatory bowel disease, food allergy, immune deficiency, cystic fibrosis, abdominal surgery within 4 weeks before hospitalization, and nosocomial infections.
A questionnaire was administered by two physician-investigators to obtain information on age, sex, demographic and family data, community type (urban or rural), previous rotavirus vaccine, school attendance, presenting symptoms, and duration of illness before admission.
We summarized the clinical severity of episodes, describing the duration of diarrhea, stool frequency, duration and frequency of vomiting, presence of fever, and presence and severity of dehydration. All patients underwent medical evaluation with estimation of dehydration, using the Gorelick score. This score is based on ten individual clinical findings: decreased skin elasticity, capillary refill >2 s, general appearance, absent tears, abnormal breathing, dry mucous membranes, sunken eyes, abnormal radial pulse, tachycardia, and decreased urine output [14].
Informed consent was obtained for all subjects. The study was approved by the Bambino Gesù Children’s Hospital ethical committee.
Laboratory methods
Two stool samples for each patient were sent to the laboratory and directly tested for bacterial and viral gastrointestinal pathogens.
The bacterial species investigated were: Salmonella spp., Shigella spp., Vibrio spp., Yersinia spp., Aeromonas spp., enterohemorrhagic Escherichia coli (EHEC), including Escherichia coli O157:H7, Campylobacter spp., Clostridium perfringens, and Clostridium difficile. The bacteriologic examination consisted of culture on selective media for each bacterial species and, after 24 h incubation in selenite broth (Copan, Brescia, Italy), for Salmonella spp. Final identification was performed for aerobic bacteria by the Vitek2 system (bioMérieux, Marcy l’Etoile, France), for Campylobacter spp. by the API Campy system (bioMérieux), and for anaerobic bacteria by the RapID™ System (Remel, Lenexa, KS, USA). An immunochromatographic test (ImmunoCard STAT!® EHEC, Meridian Bioscience, Inc., Cincinnati, OH, USA) was used for the detection of EHEC, after incubation overnight in brain heart infusion broth (bioMérieux). C. difficile toxin B strains were detected using the GeneXpert C. difficile real-time PCR (Cepheid, Sunnyvale, CA, USA) on characteristic colonies grown on C. difficile agar selective medium (bioMérieux).
The enteric viruses investigated were rotavirus, adenovirus, and norovirus. Immunochromatographic assays were used for the direct detection of viral antigens.
Rotavirus and adenovirus tests were based on membrane technology with colloidal gold particles sensitized with antibodies directed against, respectively, VP6 rotavirus antigen and specific proteins of human adenovirus group (Rota-Strip, Adeno-Strip; Coris BioConcept, Gembloux, Belgium). The RIDA QUICK Norovirus detection kit (R-Biopharm AG, Darmstadt, Germany) was used for the rapid antigen detection of GI/GII norovirus. All tests were carried out according to the manufacturers’ instructions.
Statistical analysis
Data were collected by using an electronic support specifically prepared for the study. All statistical calculations were carried out using SPSS for Windows version 13.0 (SPSS, Inc., Chicago, IL, USA). The significance of differences among the study groups was tested using the Chi-square test or Fisher’s exact test for differences in proportions and the Mann–Whitney test for differences in continuous variables. We used logistic regression models to assess the association between the presence of coinfection and clinical symptoms adjusted for factors associated with a p-value < 0.20 on univariate analysis. A p-value < 0.05 was considered to be statistically significant.
Results
Among 275 children meeting the eligibility criteria, 23 patients had a diagnosis of non-infectious gastrointestinal disease and were excluded. The most frequent diagnoses in this subgroup were urinary tract infections (5), inflammatory bowel disease (1), febrile seizures (2), celiac disease (3), thrombocytopenia (1), flu (5), appendicitis (1), and recurrent abdominal pain (5). Among the remaining 252 patients, in 20, we were not able to collect stool samples.
We found evidence of infection in 151 children out of 232 (65.1 %). The characteristics of the studied population are shown in Table 1. The median age of children with monoinfection was higher compared with that of children with coinfection, although the difference was not statistically significant. Children with coinfection did not differ from those with monoinfection with regard to school or day care center attendance, type of community, and having received rotavirus vaccine.
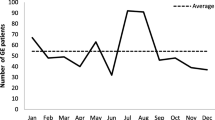
Table 2 shows the patients’ age distribution for each detected organism. The vast majority of etiological agents was represented by rotavirus, which was the most frequently isolated in the stools of children below 6 years of age. Adenovirus was frequently found in children under 2 years of age, while Salmonella spp. was the most frequent in children older than 6 years of age. Regarding seasonality, a peak of AGE was observed between March and April, when circulation of rotavirus infection was at its highest.
One or more etiological agents were detected in 151 out of 232 patients (65.1 %), while we did not detect any etiological agent in 81 children (34.9 %). Rotavirus was detected in 96 (63.6 %), adenovirus in 17 (11.2 %), norovirus in 7 (4.6 %), toxin-producing C. difficile in 23 (15.2 %), Salmonella spp. in 15 (9.9 %, B group in 12/15 and D group in 3/15), C. perfringens in 12 (7.9 %), Campylobacter spp. in 6 (4 %), and VTEC in 2 (1.3 %). One of the VTEC infections was associated to VTEC O157H7. Shigella, Yersinia, Vibrio, and Aeromonas were not detected.
In 27 children out of 151 (17.9 %), we found evidence of coinfection.
Table 3 shows the distribution of microorganisms detected in children with mono- and coinfection. In children with monoinfection, rotavirus was the most frequently isolated microorganism (75 cases, 60.5 %), while Salmonella accounted for 13 cases (10.5 %). Among children with coinfection, rotavirus was found in 77.8 % of cases, and toxin-producing C. difficile was found in 70.4 %.
The association of different microorganisms in coinfections and their frequency is reported in Table 4. Coinfection with rotavirus and toxin-producing C. difficile was the most common, and occurred in 17/27 cases (63 %). Coinfection with adenovirus and another pathogen was observed in 5/27 cases (18.5 %).
We compared the clinical picture of children with monoinfection with those with coinfection, and the results are summarized in Table 5. We applied logistic regression models to explore how coinfection affected the severity of clinical presentation, adjusting for potential confounders. Children with coinfection had a higher probability to be severely dehydrated, to present with fever ≥38 °C, to have a higher number of diarrhea episodes, and a longer duration of vomiting and diarrhea. However, patients did not differ in their length of hospitalization by mono- or coinfection.
A total of 31 children out of 151 (20.5 %) had received an antibiotic before the onset of the diarrhea. Of these 31 patients, 11 (35,4 %) were diagnosed with a C. difficile infection. We did not observe any difference between patients with monoinfection and coinfection with regard to previous antibiotic treatment.
On the other hand, during hospitalization, only two children received an antibiotic therapy, one for a monoinfection by Campylobacter and one for a coinfection by toxin-producing C. difficile and rotavirus.
Discussion
In our study, we found evidence of infection in the large majority of AGE cases. Moreover, children with evidence of coinfection had a more severe clinical presentation, independently of age and living community type.
As expected, rotavirus was the most frequent etiology, which is consistent with previous reports [15, 16]. Adenovirus and norovirus frequency was also in line with previous observations [17–20]. Furthermore, in the present study, norovirus infection occurred less frequently than in other studies [21–23]. The different figures observed in other countries might be explained by different age groups, seasonal variations at the time of sampling, and by the detection methods used. However, all studies, including ours, consistently showed that rotavirus was the most common etiological agent. It must be underscored that, although rotavirus vaccines were licensed in Italy in 2006, vaccination against rotavirus is not included in the Italian official recommendations for childhood immunization, and immunization coverage is still very low [24]. Indeed, only two children participating in this study had received rotavirus immunization.
We found a high frequency of C. difficile-associated gastroenteritis. The epidemiology of C. difficile disease in children has not been well characterized. However, recent studies have shown an increase in C. difficile disease among children in both community and hospital settings. Only a few epidemiologic studies evaluating risk factors for C. difficile disease have been conducted on pediatric patients. Infants represent a controversial group, in which carriage is often asymptomatic. The convention is to regard the presence of C. difficile in stools in children less than 2 years of age to be colonization; although severe disease does occur, most cases in this age group are asymptomatic. The reasons suggested for this include the lack of resistance to colonization, by which the immature gastrointestinal tract contains an incomplete intestinal flora that is unable to exclude C. difficile. This may explain the high frequency of carriage [25]. In our population, we observed 40 patients with non-toxin-producing C. difficile (26.5 %).
Recent studies have shown an increase in the diagnoses of C. difficile infections among infants aged less than 1 year [26, 27]. The major risk factors for C. difficile infection include older age, hospitalization, and exposure to antibiotics. In our study, we observed that 11 patients out of 31 (35.4 %) with a previous exposure to antibiotics had a C. difficile infection. Further studies are needed in order to better determine the epidemiology of C. difficile in children.
In our study, coinfections were observed in 17.9 % of cases. Coinfections with rotavirus and C. difficile were the most frequent. Previously published studies identified coinfections in 4.4 to 29 % of the pathogen-positive stool samples in different European countries, using classic or molecular techniques [5]. Other authors report that no specific clinical sign of gastroenteritis is significantly related to the presence of coinfections. However, the presence of mixed-agent infections has previously been associated with the development of necrotizing enterocolitis in premature infants [28, 29]. In a previous study conducted by Kim et al., additional pathogens were identified from the stool samples of three infants with C. difficile AGE: two rotavirus and one norovirus [30]. These patients had a severe C. difficile disease.
The authors of two case reports on young children with concurrent norovirus and C. difficile infection hypothesized that norovirus may affect epithelial homeostasis of the intestinal mucosa, therefore, exacerbating the effects of the toxins produced by C. difficile [31].
Statistical comparison of monoinfection with coinfection is complex because most of the mixed-infection groups are small. In our study, the clinical picture of children with coinfection was more severe for all clinical signs taken into examination, particularly dehydration. Our observations are consistent with other studies [4, 32].
Viral and bacterial intestinal pathogens could affect either the same or different regions of the gut, and their effects could be enhanced [33]. Currently, our knowledge on the exact pathology of human gut infections is limited, and it is useful to report studies which examine both viruses and bacteria. The results of our study emphasize the clinical importance of mixed infections as a cause of AGE with severe clinical presentation in children. The possibility of dual infection should be investigated more often [34].
Moreover, we did not find any direct or confounding effect of age and living community type on clinical symptoms in children with AGE and, therefore, it is likely that the observed association with the detection of multiple infectious agents was not biased.
Our study was conducted on the total population of children admitted for AGE in a single ward during an entire year of observation, and microbiological determination on stool samples was performed on almost all patients. Moreover, a large battery of laboratory tests was used in order to have a representative epidemiology of enteric pathogen circulation in this patient setting. Methods for the detection of enteric bacteria included the use of enrichment steps, selective culture media, and biochemical identification and serotyping, while the detection of enteric viruses was based on direct antigen detection in feces. In this study, the use of an in vitro rapid antigen detection on immunochromatographic strips was chosen instead of other traditional or molecular methods, such as enzyme immunoassay (EIA), cell culture, and reverse transcription polymerase chain reaction (RT-PCR), because it is easy to perform, not time consuming, and is reliable [35, 36].
Moreover, this method, when compared to other standard assays in a cohort of children, resulted in sensitivities of 98.5 % and 99.7 % and specificities of 96.2 % and 98 % for rotavirus and adenovirus, respectively [36–39]. The RIDA QUICK immunochromatographic testing for norovirus sensitivity is reported to have a different performance with respect to the GI or GII genogroups, ranging from 42.1 % to 76 %, while the maximum specificity (100.0 %) was tested in both genogroups.
Systematic detection of the main enteric pathogens associated with gastroenteritis in children allowed us to observe a relatively high percentage of coinfections (17.9 %). Dual infections raise the question as to whether a single pathogen is responsible for the illness or whether several pathogens act in synergy. Further studies must be performed in order to obtain a better understanding of these infections.
Children with coinfection represent a subgroup of patients with AGE who need increased assistance during hospitalization. Therefore, their identification with appropriate diagnostic tests is important in order to predict their clinical course.
Immunization programs against rotavirus are reasonably justified in our setting, given the burden of this infection in terms of hospitalizations.
References
Kosek M, Bern C, Guerrant RL (2003) The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ 81:197–204
Parashar UD, Gibson CJ, Bresee JS, Glass RI (2006) Rotavirus and severe childhood diarrhea. Emerg Infect Dis 12:304–306
Marie-Cardine A, Gourlain K, Mouterde O, Castignolles N, Hellot MF, Mallet E, Buffet-Janvresse C (2002) Epidemiology of acute viral gastroenteritis in children hospitalized in Rouen, France. Clin Infect Dis 34:1170–1178
Román E, Wilhelmi I, Colomina J, Villar J, Cilleruelo ML, Nebreda V, Del Alamo M, Sánchez-Fauquier A (2003) Acute viral gastroenteritis: proportion and clinical relevance of multiple infections in Spanish children. J Med Microbiol 52:435–440
Levidiotou S, Gartzonika C, Papaventsis D, Christaki C, Priavali E, Zotos N, Kapsali E, Vrioni G (2009) Viral agents of acute gastroenteritis in hospitalized children in Greece. Clin Microbiol Infect 15:596–598
Guerrero ML, Noel JS, Mitchell DK, Calva JJ, Morrow AL, Martínez J, Rosales G, Velázquez FR, Monroe SS, Glass RI, Pickering LK, Ruiz-Palacios GM (1998) A prospective study of astrovirus diarrhea of infancy in Mexico City. Pediatr Infect Dis J 17:723–727
Simpson R, Aliyu S, Iturriza-Gómara M, Desselberger U, Gray J (2003) Infantile viral gastroenteritis: on the way to closing the diagnostic gap. J Med Virol 70:258–262
Tran A, Talmud D, Lejeune B, Jovenin N, Renois F, Payan C, Leveque N, Andreoletti L (2010) Prevalence of rotavirus, adenovirus, norovirus, and astrovirus infections and coinfections among hospitalized children in northern France. J Clin Microbiol 48:1943–1946
Dionisi AM, Filetici E, Ocwzarek S, Arena S, Benedetti I, Lucarelli C, Luzzi I, Scavia G, Minelli F, Ciaravino G, Marziano ML, Caprioli A (2011) Enter-net international surveillance network. Report 2007–2009. Not Ist Super Sanitá 24:3–10
Ruggeri FM, Delogu R, Petouchoff T, Tcheremenskaia O, De Petris S, Fiore L; RotaNet-Italy Study Group (2011) Molecular characterization of rotavirus strains from children with diarrhea in Italy, 2007–2009. J Med Virol 83:1657–1668
Scavia G, Baldinelli F, Busani L, Caprioli A (2012) The burden of self-reported acute gastrointestinal illness in Italy: a retrospective survey, 2008–2009. Epidemiol Infect 140:1193–1206
Medici MC, Tummolo F, Albonetti V, Abelli LA, Chezzi C, Calderaro A (2012) Molecular detection and epidemiology of astrovirus, bocavirus, and sapovirus in Italian children admitted to hospital with acute gastroenteritis, 2008–2009. J Med Virol 84:643–650
Guarino A, Albano F, Ashkenazi S, Gendrel D, Hoekstra JH, Shamir R, Szajewska H; European Society for Paediatric Gastroenterology, Hepatology, and Nutrition; European Society for Paediatric Infectious Diseases (2008) European Society for Paediatric Gastroenterology, Hepatology, and Nutrition/European Society for Paediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe. J Pediatr Gastroenterol Nutr 46:S81–S122
Gorelick MH, Shaw KN, Murphy KO (1997) Validity and reliability of clinical signs in the diagnosis of dehydration in children. Pediatrics 99:E6
Giaquinto C, van Damme P; REVEAL Study Group (2010) Age distribution of paediatric rotavirus gastroenteritis cases in Europe: the REVEAL study. Scand J Infect Dis 42:142–147
Forster J, Guarino A, Parez N, Moraga F, Román E, Mory O, Tozzi AE, de Aguileta AL, Wahn U, Graham C, Berner R, Ninan T, Barberousse C, Meyer N, Soriano-Gabarró M; and the Rotavirus Study Group (2009) Hospital-based surveillance to estimate the burden of rotavirus gastroenteritis among European children younger than 5 years of age. Pediatrics 123:e393–e400
Finamore E, Vitiello M, Kampanaraki A, Rao M, Galdiero M, Galdiero E, Bevilacqua P, Gallo MA, Galdiero M (2011) G2 as an emerging rotavirus strain in pediatric gastroenteritis in southern Italy. Infection 39:113–119
Rosenfeldt V, Vesikari T, Pang XL, Zeng SQ, Tvede M, Paerregaard A (2005) Viral etiology and incidence of acute gastroenteritis in young children attending day-care centers. Pediatr Infect Dis J 24:962–965
Nitiema LW, Nordgren J, Ouermi D, Dianou D, Traore AS, Svensson L, Simpore J (2011) Burden of rotavirus and other enteropathogens among children with diarrhea in Burkina Faso. Int J Infect Dis 15:e646–e652
Rosenfeldt V, Vesikari T, Pang XL, Zeng SQ, Tvede M, Paerregaard A (2005) Viral etiology and incidence of acute gastroenteritis in young children attending day-care centers. Pediatr Infect Dis J 24:962–965
Junquera CG, de Baranda CS, Mialdea OG, Serrano EB, Sánchez-Fauquier A (2009) Prevalence and clinical characteristics of norovirus gastroenteritis among hospitalized children in Spain. Pediatr Infect Dis J 28:604–607
Gonzalez-Galan V, Sánchez-Fauqier A, Obando I, Montero V, Fernandez M, Torres MJ, Neth O, Aznar-Martin J (2011) High prevalence of community-acquired norovirus gastroenteritis among hospitalized children: a prospective study. Clin Microbiol Infect 17:1895–1899
Yori PP, Schwab K, Gilman RH, Nappier S, Portocarrero DV, Black RE, Olortegui MP, Hall ER, Moe C, Leon J, Cama VA, Kosek M (2009) Norovirus highly prevalent cause of endemic acute diarrhea in children in the Peruvian Amazon. Pediatric Infect Dis J 28:844–847
Giaquinto C, Jackson AE, Vesikari T (2012) Report of the second European expert meeting on rotavirus vaccination. Vaccine 30:2237–2244
Enoch DA, Butler MJ, Pai S, Aliyu SH, Karas JA (2011) Clostridium difficile in children: colonisation and disease. J Infect 63:105–113
Benson L, Song X, Campos J, Singh N (2007) Changing epidemiology of Clostridium difficile-associated disease in children. Infect Control Hosp Epidemiol 28:1233–1235
Zilberberg MD, Shorr AF, Kollef MH (2008) Increase in Clostridium difficile-related hospitalizations among infants in the United States, 2000–2005. Pediatr Infect Dis J 27:1111–1113
Bagci S, Eis-Hübinger AM, Franz AR, Bierbaum G, Heep A, Schildgen O, Bartmann P, Kupfer B, Mueller A (2008) Detection of astrovirus in premature infants with necrotizing enterocolitis. Pediatr Infect Dis J 27:347–350
Hunter CJ, Upperman JS, Ford HR, Camerini V (2008) Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC). Pediatr Res 63:117–123
Kim J, Shaklee JF, Smathers S, Prasad P, Asti L, Zoltanski J, Dul M, Nerandzic M, Coffin SE, Toltzis P, Zaoutis T (2012) Risk factors and outcomes associated with severe Clostridium difficile infection in children. Pediatr Infect Dis J 31:134–138
Lukkarinen H, Eerola E, Ruohola A, Vainionpää R, Jalava J, Kotila S, Ruuskanen O (2009) Clostridium difficile ribotype 027-associated disease in children with norovirus infection. Pediatr Infect Dis J 28:847–848
Koh H, Baek SY, Shin JI, Chung KS, Jee YM (2008) Coinfection of viral agents in Korean children with acute watery diarrhea. J Korean Med Sci 23:937–940
Di Biase AM, Petrone G, Conte MP, Seganti L, Ammendolia MG, Tinari A, Iosi F, Marchetti M, Superti F (2000) Infection of human enterocyte-like cells with rotavirus enhances invasiveness of Yersinia enterocolitica and Y. pseudotuberculosis. J Med Microbiol 49:897–904
Taylor MB, Marx FE, Grabow WO (1997) Rotavirus, astrovirus and adenovirus associated with an outbreak of gastroenteritis in a South African child care centre. Epidemiol Infect 119:227–230
Smith CK, McNeal MM, Meyer NR, Haase S, Dekker CL (2011) Rotavirus shedding in premature infants following first immunization. Vaccine 29:8141–8146
Wilhelmi I, Colomina J, Martín-Rodrigo D, Roman E, Sánchez-Fauquier A (2001) New immunochromatographic method for rapid detection of rotaviruses in stool samples compared with standard enzyme immunoassay and latex agglutination techniques. Eur J Clin Microbiol Infect Dis 20:741–743
Ouermi D, Karou D, Ilboudo D, Nadembega WMC, Pietra V, Belem A, Simpore J, Kabre G, Pignatelli S, Sawadogo L (2007) Prevalence of rotavirus, adenovirus and enteric parasites among pediatric patients attending Saint Camille Medical Centre in Ouagadougou. Pak J Biol Sci 10:4266–4270
Viehoff R, Van Beers D, De Foor M, Col D, Venuti M, Paulart F, Leclipteux Th (1998) Comparison of a rapid immunochromatographic diagnostic test for Adenovirus detection. In: Proceedings of the 1998 European Society for Clinical Virology meeting: Progress in Clinical Virology IV, Hamburg, Germany, August/September 1998
Battaglioli G, Nazarian EJ, Lamson D, Musser KA, St George K (2012) Evaluation of the RIDAQuick norovirus immunochromatographic test kit. J Clin Virol 53:262–264
Competing interests
The authors declare that they have no competing interests.
Author contributions
All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valentini, D., Vittucci, A.C., Grandin, A. et al. Coinfection in acute gastroenteritis predicts a more severe clinical course in children. Eur J Clin Microbiol Infect Dis 32, 909–915 (2013). https://doi.org/10.1007/s10096-013-1825-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-013-1825-9