Abstract
Vancomycin lock solution (LS) is recommended for the conservative treatment of subcutaneous injection port (SIP)-related infections, but may be associated with failure. We used an in vitro dynamic model of biofilm formation in an SIP, based on a continuous flow circulating via a real SIP, to assess the effectiveness of vancomycin (5 mg/ml), daptomycin (5 mg/ml) and ethanol 40 % LS in eradicating a pre-established Staphylococcus epidermidis biofilm. Heparin, Ringer’s lactate and enoxaparin sodium LS were used as controls. The logarithmic reductions of colony-forming units (CFU) were compared by Student’s t-test. After 24 h of exposure, the vancomycin LS did not exert a greater bactericidal effect than the heparin LS control (mean logarithmic reduction: 2.27 ± 0.58 vs. 1.34 ± 0.22, respectively, p = 0.3). The mean logarithmic reduction was greater with daptomycin LS (5.45 ± 0.14 vs. 0.39 ± 0.12, p < 0.01) and ethanol LS (6.79 ± 1.03 vs. 1.43 ± 0.54, p = 0.02). Bacterial revival after exposure to 24 h of LS was assessed. The mean viable bacteria count was significantly higher for vancomycin LS (9.36 ± 0.10 log10CFU) and daptomycin LS (9.16 ± 0.02 log10CFU) than for ethanol LS (2.95 ± 1.65 log10CFU). Ethanol appeared to be the most attractive option to treat SIP-related infection, but its poor ability to entirely disrupt the biofilm structure may require its use in association with a dispersal agent to avoid renewal of the biofilm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Subcutaneous injection ports (SIPs) are composed of a subcutaneously inserted injection port made of titanium or plastic that is linked to a central venous catheter made of silicone rubber or polyurethane [1]. They can be used to take blood samples and to administer drugs, blood products or parenteral nutrition [1]. The main advantages over external central venous catheters include a better quality of life for the patient owing to unrestricted mobility and reduced susceptibility to infection [2]. SIPs are used worldwide, mainly in the treatment of patients with cancer or chronic diseases requiring permanent central venous access.
SIP-related infections are less frequent than with other central venous lines, having an estimated incidence of 0.016 to 1 infections per 1,000 device days [3]. The main microorganism isolated from infected SIPs is coagulase-negative staphylococci (CoNS), especially in patients receiving long-term parenteral nutrition [4, 5]. However, Gram-negative bacteria, Staphylococcus aureus, fungi, enterococci or any other bacteria may also be involved [5]. The rate of SIP-related infection is higher in terminally ill patients with advanced cancer and in paediatric haematology–oncology patients [6, 7].
There are two options for the management of non-complicated SIP-related infections due to CoNS: removal or salvage therapy with retention of the device. When the device is left in place, SIP CoNS-related infections are more difficult to sterilise than with other external central venous catheters and are associated with a greater likelihood of recurrent bacteraemia [6, 8, 9]. This may be due to the ability of CoNS to produce a biofilm formed by bacteria in stationary phase embedded in an extracellular matrix. The biofilm protects bacteria against the immune system and antibiotics, and constitutes a depository that may release bacteria into the bloodstream [10]. It is thought to be the leading cause of the persistence of CoNS infection and of recurrent CoNS bacteraemia [8, 9].
Retention should be combined with antibiotic-lock therapy and, in cases of bloodstream infection, with systemic antibiotic therapy [11]. The antibiotic-lock technique consists of filling the chamber and the catheter with an antimicrobial agent at a concentration high enough to reach 100 to 1,000 times its minimal inhibitory concentration (MIC). When the SIP is not used, the solution dwells for a given period of time according to the characteristics of the antimicrobials (generally 24 h) [12–14]. For CoNS SIP-related infections, the antibiotic lock solution (LS) recommended is vancomycin, at a concentration of 5 mg/ml, for 7–14 days, with renewal of the solution every 24–48 h [11]. Vancomycin LS reaches very high concentrations, 500 to 1,000 times greater than the MIC, and is considered to be effective in killing sessile bacteria embedded in the biofilm [11]. However, when the device is left in place, SIP-related infections are more difficult to sterilise than with other external central venous catheters and are associated with a greater likelihood of recurrent bacteraemia, especially with CoNS [2, 8, 9, 15].
The ability of vancomycin to eradicate S. epidermidis biofilm has been studied in several in vitro models of experimental catheter-related infection, but with conflicting results. Some authors suggested that vancomycin, even at high doses, is unable to eradicate bacterial colonisation from catheters [16, 17], while others found that vancomycin is effective in eradicating S. epidermidis biofilm [18, 19]. There is only one randomised placebo-controlled trial that has compared lock therapy (mainly with vancomycin) and placebo, in 40 patients with SIP-related infections [20]. The authors reported a failure rate of 33 % in the lock therapy group, with 3 patients out of 21 relapsing during follow-up [20]. Retrospective studies confirmed the potential interest of ALT, but the real effectiveness and the length of ALT treatment remain uncertain [4, 21].
Because the regular increase in vancomycin MICs among CoNS isolates will make this antibiotic less effective against such bacteria in the near future, new ALT compounds with greater and swifter effectiveness should be studied in order to reduce the length of ALT treatment, the risk of antimicrobial resistance and the rate of failure [19, 22–24]. In this study, we used a dynamic model of SIP-related infection adapted from a previously described method [23] to assess the activity of vancomycin, daptomycin and ethanol on a pre-established CoNS biofilm in an SIP.
Materials and methods
Microorganism
An S. epidermidis (CIP 21.25, Collection Institut Pasteur, Paris, France) biofilm was produced over 72 h inside an SIP (port made of silicon rubber with an inner volume of 0.6 ml and a polyurethane catheter 61 cm long with an inner diameter of 1.6 mm, X-port isp, Bard Access Systems, Salt Lake City, UT, USA). S. epidermidis 21.25 exhibits decreased susceptibility to teicoplanin (MIC: 8 mg/L) but remains susceptible to daptomycin (MIC: 0.38 mg/L) and vancomycin (MIC: 2 mg/L).
Method of biofilm formation
Our model of biofilm production was adapted to the SIP from an experimental model described for endoscopes [25, 26]. The SIP was aseptically connected to sterile polyvinylchloride (PVC) tubes (Nalgène, Illkirch, France) to form a loop that was supplied with tryptone soya broth culture medium (TSB, CM0129, Oxoid, Cambridge, United Kingdom). The system was activated by two pumps (Watson Marlow 205S, La Queue Lez Yvelines, France): one provided a continuous flow of TSB medium in the system and the other a homogeneous diffusion of the TSB medium and of the S. epidermidis suspension in the loop (Fig. 1). Pump 1 was turned on at a speed of 23 ml/min to fill the system with TSB medium and then stopped. The circuit was inoculated at one time point with 20 ml of a suspension containing about 108 S. epidermidis per ml. Pump 2 was turned on at a speed of 15 ml/min for 4 h to allow the dissemination and the first adhesion of bacteria in the loop. The continuous flow was then turned on at a speed of 2 ml/min and the speed of pump 2 was increased to 23 ml/min. The system was run for 72 h to allow biofilm formation.
Lock solutions
Vancomycin LS was composed of vancomycin at a concentration of 5 mg/ml (Vancocin® 250 mg, Mylan®, Saint Priest, France) and heparin at a concentration of 2,500 IU/ml (Héparine Choay® 25,000 IU/5 mL, Sanofi-Aventis, Paris, France) diluted in 0.9 % NaCl (Versylène®, Fresenius, Sèvres, France) [11]. Daptomycin LS was composed of daptomycin at a concentration of 5 mg/ml (Cubicin® 500 mg, Novartis, Horsham, United Kingdom) diluted in Ringer’s lactate (Macoflex® 250 ml, MacoPharma, Mouveaux, France, containing 80 μg/ml calcium [Ca2+]) [19, 22]. Ethanol LS was composed of ethanol 40 % and enoxaparin sodium at a concentration of 400 IU/ml (Lovenox® 4,000 IU anti-Xa/0, 4 ml, Sanofi-Aventis, Paris, France) diluted in 0.9 % NaCl. Enoxaparin is an anticoagulant, which mainly inhibits factor Xa by activating antithrombin III. Thus, enoxaparin decreases thrombin formation and, ultimately, prevents clot formation. This combination of ethanol/enoxaparin showed stable antithrombotic and antimicrobial activities and led to no structural degradation of the catheter surfaces (patent submission number: 1000136848; unpublished data). Control LS were heparin sodium at a concentration of 2,500 IU/ml and Ringer’s lactate and enoxaparin sodium at a concentration of 400 IU/ml. Three millilitres of each LS were instilled into the injection port to fill up the SIP. The catheter was then clamped, placed in a sterile drape and incubated at 37 °C for 24 h. In a first set of experiments, we assessed the activity of the three LS (vancomycin, daptomycin and ethanol) dwelled for 24 h on a 72-h bacterial biofilm. Three SIPs were tested in each trial: one used as a control, one filled with the antimicrobial LS (antibiotic or ethanol) and one filled with the control LS. Each trial was performed in triplicate. In a second set of experiments, we assessed the ability of the bacteria to reform a biofilm after 24 h of exposure to antimicrobial LS. The treated SIPs were reconnected to the system for 72 h. No additional bacterial inoculum was added to the system. Each trial was performed in triplicate.
Recovery of treated S. epidermidis from the SIP
The viable bacteria in LS were recovered under a laminar flow hood without centrifugation. The injection port and the catheter were then separated and processed separately. The treated biofilm was recovered by a previously described mechanical technique [25, 26]. The bacteria were recovered from the injection port by three successive flushes of 3 ml of Letheen Broth® (VWR Prolabo, Fontenay-sous-Bois, France). Between each flush, the injection port was vortexed (Vortex-Genie, shake 8, Scientific Industries Inc., Bohemia, NY, USA) for 5 min, ultra-sonicated at 125 W (Branson 2210, Bransonic, Danbury, CT, USA) for 5 min and then vortexed again for 5 min. After removal of the septum, the injection port was immersed in 10 mL of Letheen Broth® and scraped with a scalpel before a session of vortex–sonication–vortex. Bacterial recovery from the catheter was performed by three successive flushes (10 ml, 3 ml and 3 ml) of Letheen Broth®. Between each flush, the catheter was subjected to vortex–sonication–vortex. It was then cut into several 1-cm portions that were immersed in 10 ml of Letheen Broth® and subjected to vortex–sonication–vortex. The solutions recovered from the lock, the injection port and the catheter were diluted up to 10−7 and plated on Tryptone soya plates (TSA bioMérieux, Lyon, France) that were incubated at 37 °C for 24 h. The bacterial count from the lock solution was added to the bacterial count found in the catheter and the injection port. The quantity of biofilm present in the SIP or in the catheter was given in each trial as log10CFU (colony-forming units) per SIP or as CFU per SIP.
Scanning electron microscopy (SEM) of the injection port
Samples were fixed in 2 % glutaraldehyde in cacodylate buffer (0.2 M at pH 7.2) for 1 h. The septum was then aseptically removed and the injection port filled with the fixative for 24 h at 4 °C. Samples were mounted on a metallic support with an adhesive carbon tab and then sputter-coated with gold-palladium (JFC-1300; Jeol, Tokyo, Japan). Microscopic analysis was made using a scanning electron microscope (JSM-6060LV; Jeol) in high-vacuum mode.
Statistics
We calculated the logarithmic reduction for each trial between the SIP used as the control and the antimicrobial LS (vancomycin, daptomycin and ethanol) or the control LS (heparin, Ringer’s lactate and enoxaparin sodium). Comparisons between groups were made by Student’s t-test with a significance level set at 0.05. Statistical analysis was done with SPSS software (version 10.1 for Windows; SPSS, Inc., Chicago, IL, USA).
Results
SIP experimental biofilm model
The strain of S. epidermidis (CIP 21.25, Collection Institut Pasteur, Paris, France) was chosen because it showed commonly observed susceptibility results and was able to produce a biofilm on a plastic support (data not shown). The injection of bacterial inoculum at one time point was chosen to simulate the one-time contamination of the SIP by skin-related bacteria, such as that which may occur during a failure of skin antisepsis.
We then assessed the reproducibility of the biofilm formation in nine SIPs. The mean viable bacteria count was 8.35 log10CFU per injection port (range 7.56–9.52), 7.83 log10CFU per catheter (range 6.76–8.68) and 8.51 log10CFU per SIP (range 7.88–9.57). Low standard deviations were found for the injection port, catheter and SIP (0.19, 0.21 and 0.17, respectively). There was no significant difference between the total bacterial recovery rates in the injection port and the catheter (p = 0.1).
Activity of antimicrobial LS on a 72-h-old S. epidermidis biofilm
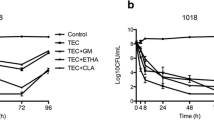
Table 1 and Figure 2 show the mean viable bacteria count and the mean logarithmic reductions in CFU of 72-h-old S. epidermidis biofilms after a 24-h exposure to antimicrobial LS (vancomycin, daptomycin or ethanol) and to control LS (heparin in Ringer’s lactate and enoxaparin sodium).
Mean logarithmic reduction after antimicrobial lock. Δ: calculated difference between the SIP used as the control and the antimicrobial lock (vancomycin, daptomycin and ethanol) or the control lock (heparin, Ringer’s lactate and enoxaparin sodium). Comparisons between groups were performed by Student’s t-test with a significance level set at 0.05. p-value of mean logarithmic reductions: vancomycin lock solution (LS) versus heparin LS, p = 0.3; daptomycin LS versus Ringer’s lactate LS, p < 0.01; ethanol LS versus enoxaparin LS, p = 0.02; vancomycin LS versus daptomycin LS, p = 0.02; vancomycin LS versus ethanol LS, p = 0.02; daptomycin LS versus ethanol LS, p = 0.4. The box-and-whisker plots show the mean (+), the median (horizontal bar inside the box), the 25th/75th percentile and the extreme values as bars at the extremities of the whiskers
The vancomycin LS did not exert a greater bactericidal effect than its heparin control LS (mean logarithmic reduction 2.27 ± 0.58 vs. 1.34 ± 0.22, respectively, p = 0.3). In contrast, the mean logarithmic reduction was statistically greater than that of the control for daptomycin LS (5.45 ± 0.14 vs. 0.39 ± 0.12, p < 0.01) and ethanol LS (6.79 ± 1.03 vs. 1.43 ± 0.54, p = 0.02). Compared to that of vancomycin LS, the mean logarithmic reduction was greater after daptomycin LS exposure (p = 0.02) and after ethanol LS exposure (p = 0.02). No significant difference was found between daptomycin and ethanol LS (p = 0.4).
The biofilm was well visualised by SEM of the injection ports in the control (Fig. 3d–f) and after vancomycin LS exposure (Fig. 3g–i). Despite the activity, we also observed a biofilm after treatment with daptomycin LS (Fig. 3j–l) and with ethanol LS (Fig. 3m–o). Figure 3a–c show a non-infected injection port.
Biofilm reformation after 24 h of exposure to lock solution
We assessed bacterial revival after 24 h of exposure to antimicrobial LS followed by 72 h of further incubation in our biofilm development system without additional bacterial inoculum (Fig. 4). The mean viable bacteria count was high in assays performed with vancomycin LS (9.36 ± 0.10 log10CFU/SIP) and with daptomycin LS (9.16 ± 0.02 log10CFU/SIP). The mean bacteria count of ethanol LS was significantly lower than that of vancomycin LS (2.95 ± 1.65 log10CFU/SIP vs. 9.36 ± 0.10 log10CFU/SIP, p = 0.018).
Mean bacterial count in control SIP immediately after antimicrobial lock solution and bacterial revival after 24 h of exposure to antimicrobial LS followed by 72 h in the biofilm development system. SIPs exposed to an antimicrobial LS (vancomycin, daptomycin or ethanol) were reconnected to the biofilm development system for a 72-h period without additional bacterial inoculum. White bars bacterial count of control SIP; grey bars bacterial count immediately after antimicrobial lock solution; black bars bacterial regrowth after 72 h. Comparisons between groups after 72 h of regrowth were performed by Student’s t-test with a significance level set at 0.05. p-values of the mean bacterial count of the SIPs: vancomycin LS versus daptomycin LS (p = 0.13); vancomycin LS versus ethanol LS (p = 0.018)
Discussion
We used an in vitro dynamic model of biofilm formation in an SIP, based on a continuous flow circulating via a real SIP. The model was adapted from a previously described technique [25, 26]. It was able to study not only biofilm formation in the catheter but also in the port, which offers a large surface for the bacteria to adhere to [8, 9]. Quantification of the biofilm was determined by enumeration of the CFU, the only method suitable for the solutions recovered from the SIP. The biofilm obtained was reproducible. We used the model to assess the effectiveness of three different LS with a dwell time of 24 h to eradicate a 72-h-old, S. epidermidis-related biofilm adherent to the SIP. Vancomycin had very limited activity against bacteria embedded in SIP-adherent biofilm. The mean logarithmic reduction of CFU after vancomycin LS exposure was not statistically different from that of its control, allowing the biofilm to reform after discontinuation of treatment. Thus, our results show that vancomycin is poorly able to kill bacteria protected by their sessile state, despite a concentration 2,500 times greater than the MIC. This lack of efficacy is consistent with observations in certain in vitro and clinical studies, in which vancomycin LS therapy was associated with a high failure rate [16, 17, 20]. In contrast, several in vitro studies showed vancomycin ALT to be effective but with different models, which makes it difficult to compare results [18, 27]. None of these studies used a port linked to a catheter. In a rat model of staphylococcal central venous infection, Van Praagh et al. showed that 3 days of vancomycin ALT and daptomycin ALT were equally effective in eradicating S. epidermidis biofilms [19]. However, the authors combined systemic antibiotic therapy with ALT and no port was included in the experiment. Chauhan et al. recently developed a promising rat model of SIP-related infection but did not evaluate the effectiveness of vancomycin ALT [28]. Daptomycin is known for its ability to penetrate the biofilm [19]. In addition, it was more effective in killing bacteria than vancomycin in our model. However, despite the fact that its concentration reached 13,000 times the MIC, 24-h daptomycin LS did not completely eradicate the bacteria from the SIP. This could facilitate recurrences or favour subsequent distinct infections with other bacteria, which may use the structure of the pre-existing biofilm (mainly the matrix) to adhere to the port and catheter [9]. In addition, the wide use of daptomycin LS poses the risk of staphylococcal resistance, and daptomycin is ineffective against Gram-negative bacteria, which are also frequently responsible for SIP-related infections.
Ethanol was the most effective solution in this model and was able to kill most of the bacteria adhering to the SIP. This may explain why bacterial regrowth after ethanol exposure was weak in comparison with exposure to daptomycin and vancomycin. However, total eradication was not achieved, possibly owing to limited diffusion of the ethanol solution within the biofilm [29]. In addition, low ethanol concentration may promote biofilm formation [30]. Thus, although ethanol LS seems to be the most effective LS in our model, failure or distinct infections following ethanol lock treatment may occur. In future studies, another agent that is able to disperse the biofilm could be added to the classical treatment, which combines LS with systemic antibiotic therapy. Several candidates for dispersal solutions have recently emerged and could be tested, concomitantly or sequentially, with the antimicrobial lock solution [10, 31].
Compared to in vivo studies of SIP-related infections, our model has certain limits. In vivo SIPs are submitted to multiple factors such as fibrin and coagulation debris that may favour the establishment of the biofilm and its persistence [8]. The model does not take into account external infections. Lastly, the dwelling time in our study was only 24 h instead of 7–14 days, as is standard in clinical practice (the LS being renewed every 24–48 h) [11]. We chose 24 h because more manipulations of the SIP, even under a laminar hood, would have run the risk of contamination. We also believe that, in the future, LS should aim to be rapidly effective, for the convenience of patients, to reduce costs and to limit manipulation of the SIP.
In conclusion, vancomycin LS, which is recommended for the conservative treatment of SIP, was poorly effective in eradicating S. epidermidis biofilm in our model. Ethanol and daptomycin lock solutions were significantly more effective, but daptomycin was unable to inhibit biofilm reformation after the discontinuation of lock treatment. Ethanol appeared to be the most attractive option to treat SIP-related infection.
References
Walser EM (2012) Venous access ports: indications, implantation technique, follow-up, and complications. Cardiovasc Intervent Radiol 35:751–764
Maki DG, Kluger DM, Crnich CJ (2006) The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc 81:1159–1171
Crisinel M, Mahy S, Ortega-Debalon P, Buisson M, Favre JP, Chavanet P, Piroth L (2009) Incidence, prévalence et facteurs de risque de survenue d’une première complication infectieuse sur chambres à cathéter implantables. Méd Mal Infect 39:252–258
Opilla M (2008) Epidemiology of bloodstream infection associated with parenteral nutrition. Am J Infect Control 36:S173.e5–S173.e8
Simon A, Ammann RA, Bode U, Fleischhack G, Wenchel HM, Schwamborn D, Gravou C, Schlegel PG, Rutkowski S, Dannenberg C, Körholz D, Laws HJ, Kramer MH (2008) Healthcare-associated infections in pediatric cancer patients: results of a prospective surveillance study from university hospitals in Germany and Switzerland. BMC Infect Dis 8:70
Adler A, Yaniv I, Solter E, Freud E, Samra Z, Stein J, Fisher S, Levy I (2006) Catheter-associated bloodstream infections in pediatric hematology–oncology patients: factors associated with catheter removal and recurrence. J Pediatr Hematol Oncol 28:23–28
Chang YF, Lo AC, Tsai CH, Lee PY, Sun S, Chang TH, Chen CC, Chang YS, Chen JR (2011) Higher complication risk of totally implantable venous access port systems in patients with advanced cancer—a single institution retrospective analysis. Palliat Med (in press)
Flynn PM, Willis B, Gaur AH, Shenep JL (2003) Catheter design influences recurrence of catheter-related bloodstream infection in children with cancer. J Clin Oncol 21:3520–3525
Raad I, Kassar R, Ghannam D, Chaftari AM, Hachem R, Jiang Y (2009) Management of the catheter in documented catheter-related coagulase-negative staphylococcal bacteremia: remove or retain? Clin Infect Dis 49:1187–1194
Donlan RM (2011) Biofilm elimination on intravascular catheters: important considerations for the infectious disease practitioner. Clin Infect Dis 52:1038–1045
Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK (2009) Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 49(1):1–45
Del Pozo JL, Alonso M, Serrera A, Hernaez S, Aguinaga A, Leiva J (2009) Effectiveness of the antibiotic lock therapy for the treatment of port-related enterococci, Gram-negative, or Gram-positive bacilli bloodstream infections. Diagn Microbiol Infect Dis 63:208–212
Fernandez-Hidalgo N, Almirante B, Calleja R, Ruiz I, Planes AM, Rodriguez D, Pigrau C, Pahissa A (2006) Antibiotic-lock therapy for long-term intravascular catheter-related bacteraemia: results of an open, non-comparative study. J Antimicrob Chemother 57:1172–1180
Messing B, Peitra-Cohen S, Debure A, Beliah M, Bernier JJ (1988) Antibiotic-lock technique: a new approach to optimal therapy for catheter-related sepsis in home-parenteral nutrition patients. JPEN J Parenter Enter Nutr 12:185–189
Guedon C, Nouvellon M, Lalaude O, Lerebours E (2002) Efficacy of antibiotic-lock technique with teicoplanin in Staphylococcus epidermidis catheter-related sepsis during long-term parenteral nutrition. JPEN J Parenter Enter Nutr 26:109–113
Giacometti A, Cirioni O, Ghiselli R, Orlando F, Mocchegiani F, Silvestri C, Licci A, De Fusco M, Provinciali M, Saba V, Scalise G (2005) Comparative efficacies of quinupristin–dalfopristin, linezolid, vancomycin, and ciprofloxacin in treatment, using the antibiotic-lock technique, of experimental catheter-related infection due to Staphylococcus aureus. Antimicrob Agents Chemother 49:4042–4045
Wiederhold NP, Coyle EA, Raad II, Prince RA, Lewis RE (2005) Antibacterial activity of linezolid and vancomycin in an in vitro pharmacodynamic model of gram-positive catheter-related bacteraemia. J Antimicrob Chemother 55:792–795
Lee JY, Ko KS, Peck KR, Oh WS, Song JH (2006) In vitro evaluation of the antibiotic lock technique (ALT) for the treatment of catheter-related infections caused by staphylococci. J Antimicrob Chemother 57(6):1110–1115
Van Praagh AD, Li T, Zhang S, Arya A, Chen L, Zhang XX, Bertolami S, Mortin LI (2011) Daptomycin antibiotic lock therapy in a rat model of staphylococcal central venous catheter biofilm infections. Antimicrob Agents Chemother 55(9):4081–4089
Rijnders BJ, Van Wijngaerden E, Vandecasteele SJ, Stas M, Peetermans WE (2005) Treatment of long-term intravascular catheter-related bacteraemia with antibiotic lock: randomized, placebo-controlled trial. J Antimicrob Chemother 55:90–94
Fortún J, Grill F, Martín-Dávila P, Blázquez J, Tato M, Sánchez-Corral J, García-San Miguel L, Moreno S (2006) Treatment of long-term intravascular catheter-related bacteraemia with antibiotic-lock therapy. J Antimicrob Chemother 58(4):816–821
LaPlante KL, Mermel LA (2007) In vitro activity of daptomycin and vancomycin lock solutions on staphylococcal biofilms in a central venous catheter model. Nephrol Dial Transplant 22:2239–2246
Qu Y, Istivan TS, Daley AJ, Rouch DA, Deighton MA (2009) Comparison of various antimicrobial agents as catheter lock solutions: preference for ethanol in eradication of coagulase-negative staphylococcal biofilms. J Med Microbiol 58:442–450
Stevens DL (2006) The role of vancomycin in the treatment paradigm. Clin Infect Dis 42(Suppl 1):S51–S57
Aumeran C, Thibert E, Chapelle FA, Hennequin C, Lesens O, Traoré O (2012) Assessment on experimental bacterial biofilms and in clinical practice of the efficacy of sampling solutions for microbiological testing of endoscopes. J Clin Microbiol 50:938–942
Pineau L, Roques C, Luc J, Michel G (1997) Automatic washer disinfector for flexible endoscopes: a new evaluation process. Endoscopy 29:372–379
Curtin J, Cormican M, Fleming G, Keelehan J, Colleran E (2003) Linezolid compared with eperezolid, vancomycin, and gentamicin in an in vitro model of antimicrobial lock therapy for Staphylococcus epidermidis central venous catheter-related biofilm infections. Antimicrob Agents Chemother 47(10):3145–3148
Chauhan A, Lebeaux D, Decante B, Kriegel I, Escande MC, Ghigo JM, Beloin C (2012) A rat model of central venous catheter to study establishment of long-term bacterial biofilm and related acute and chronic infections. PLoS One 7(5):e37281, Epub 2012 May 16
Balestrino D, Souweine B, Charbonnel N, Lautrette A, Aumeran C, Traoré O, Forestier C (2009) Eradication of microorganisms embedded in biofilm by an ethanol-based catheter lock solution. Nephrol Dial Transplant 24:3204–3209
Chaieb K, Zmantar T, Souiden Y, Mahdouani K, Bakhrouf A (2011) XTT assay for evaluating the effect of alcohols, hydrogen peroxide and benzalkonium chloride on biofilm formation of Staphylococcus epidermidis. Microb Pathog 50:1–5
Cazander G, van de Veerdonk MC, Vandenbroucke-Grauls CMJE, Schreurs MWJ, Jukema GN (2010) Maggot excretions inhibit biofilm formation on biomaterials. Clin Orthop Relat Res 468:2789–2796
Acknowledgements
Damien Balestrino, Nicolas Charbonnel [Clermont Université, UMR CNRS 6023 Laboratoire Microorganismes: Génome Environnement (LMGE), Université d’Auvergne, CHU Clermont Ferrand, 63003 Clermont-Ferrand, France], Henri Laurichesse, Jean Beytout, Violaine Corbin (Service des Maladies Infectieuses, Pôle REUNNHIR, CHU Clermont-Ferrand, Clermont-Ferrand, France), Bertrand Souweine, Alexandre Lautrette, Natacha Mrozek (Réanimation Médicale, Pôle REUNNHIR, CHU Clermont-Ferrand, Clermont-Ferrand, France), Christelle Blavignac (CICS platform, Clermont University, electron microscopy).
This research was supported, in part, by an unrestricted, investigator-initiated grant from Cubist Pharmaceuticals, Inc., 65 Hayden Avenue, Lexington, MA 02421, USA. We thank Bard Access Systems, Salt Lake City, UT, USA, for providing the titanium central venous pressure port (TICVP) and Jeffrey Watts for reviewing the manuscript (Université Clermont 1, Faculté de Médecine de Clermont Ferrand, 63003 Clermont-Ferrand, France).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Aumeran, C., Guyot, P., Boisnoir, M. et al. Activity of ethanol and daptomycin lock on biofilm generated by an in vitro dynamic model using real subcutaneous injection ports. Eur J Clin Microbiol Infect Dis 32, 199–206 (2013). https://doi.org/10.1007/s10096-012-1732-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-012-1732-5