Abstract
Objectives
Little is known about efficacy and safety of ethanol lock therapy (ELT) to treat totally implantable venous access device (TIVAD) infections. The objective of this trial was to evaluate the effectiveness and safety profile of a local treatment with ELT without removal for TIVAD infection due to coagulase-negative staphylococci.
Methods
We performed a prospective, multicenter, double-blind, randomized clinical trial comparing the efficacy of 40% ELT versus vancomycin lock therapy (VLT) in TIVAD infections due to coagulase-negative staphylococci, complicated or not by bloodstream infection.
Results
Thirty-one patients were assigned to the ELT group and 30 to the VLT arm. Concomitant bacteremia was present in 41 patients (67.2%). Treatment success was 58.1 % (18 of 31) for the ELT arm and 46.7% (14 of 30) for the VLT arm (p = 0.37). The overall treatment success was 52.5% (32). The risk of treatment failure due to uncontrolled infections, superinfections, and mechanical complications did not differ significantly between participants receiving ELT (13 out of 31 [42%]) and those receiving VLT (16 out of 30 [53%]) with a hazard ratio of 0.70 (p = 0.343; 95% CI [0.34–1.46], Cox model). Catheter malfunctions were significantly more frequent in the ELT arm (11 patients versus 2 in the VLT group, p = 0.01).
Conclusions
We found an overall high rate of treatment failure that did not differ between the ELT arm and the VLT arm. TIVAD removal must be prioritized to prevent complications (uncontrolled infections, superinfections, and catheter malfunctions) except in exceptional situations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Totally implantable venous access devices (TIVADs) are widely used to provide long-term, central venous access for drugs, parenteral nutrition, or blood products in patients with chronic conditions, especially cancer [1]. The infection rate for TIVADs is estimated to range from 0.018 to 0.35 around events 0.1 per 1000 IVD-days in adult cancer [2] and is increased by high frequency of access to the device [3], total parenteral nutrition [4], hematologic malignancy [5], neutropenia [6], and hospitalization regimen [7]. Gram-positive bacteria, in particular coagulase-negative staphylococci (CoNS), are the most frequent causative microorganisms even if an increasing proportion of Gram-negative is observed in case of hematological malignancies [8, 9].
Recurrence after treatment in TIVAD infections is frequent because of biofilm production in the catheter and chamber, especially with CoNS [10, 11]. Biofilm reduces the efficacy of antibiotic therapy by increasing the antibiotic concentration needed to kill the bacteria [11, 12]. Removal of the TIVAD combined with systemic antibiotic treatment should be the rule if S. aureus, Pseudomonas aeruginosa, S. lugdunensis, or Candida is identified as the causative agent, or in case of tunneled catheter pocket infections or port abscess, tunnelitis, septic thrombophlebitis, secondary septic infections, and septic shock [13]. In the absence of any of the above criteria, TIVAD salvage can be attempted using lock therapy (LT) in combination with systemic antibiotic therapy [13]. LT consists in the administration for a given period of time (up to 24 h) of a high concentration of antimicrobial solution in the device when it is not in use and that is renewed for an extended period. To avoid thrombosis, the solution is combined with heparin [13]. The lock solution dwells inside the catheter and improves the killing of microorganisms in biofilms [14]. Vancomycin LT (VLT) is frequently used to treat CoNS TIVAD infections [8].
In one randomized, placebo-controlled study, LT combined with systemic antibiotic therapy was more effective than systemic antibiotic therapy alone but not to a level of significance [14]. Of the 44 patients with catheter-related bloodstream infection enrolled in the study, 27 had CoNS. Cure or relapse with the same strain occurred in 33% (7/21) of patients in the LT arm and in 57% (13/23) in the placebo arm (hazard ratio 0.55, p = 0.10). Because many observational uncontrolled studies have reported the efficacy of LT, its use is now an option to treat TIVAD infections [8, 13].
Ethanol lock therapy (ELT) is an interesting option because ethanol has excellent activity against microorganisms in biofilm [11, 15,16,17]. However, only two randomized trials have shown ELT to be significantly effective to prevent infection in long-term catheter use [18, 19], while in seven others, its efficacy was non-significant or nil [20,21,22,23,24,25,26]. Only two randomized studies have investigated the efficacy of ELT in combination with systemic antibiotic therapy to treat catheter-related bloodstream infection (CRBSI) [27, 28]. In the first study, which involved a pediatric population with cancer, infections were caused by Gram-positive bacteria in 48% of cases [27]. The rate of treatment failure was similar to that of ELT (44% of 48) and placebo (43% of 46, relative risk: 1·0, 95% CI 0·6–1·6, p = 0·98), while catheter occlusion requiring thrombolytic therapy was more common with ELT. However, the investigators used a high concentration of ethanol (70%) with a short dwelling period of 2–4 h for 5 days (treatment phase) and a prophylaxis phase of 24 weeks (up to 3 non-consecutive days per week). The second study compared the efficacy of 60% ELT (2 milliliters injected in both lines of the catheter at each hemodialysis) in combination with systemic antibiotic therapy and systemic antibiotic therapy alone in the treatment of hemodialysis-related jugular catheter infection [28]. Overall, 45% of blood cultures were negative and CoNS were the major causative agent. After 3 weeks of treatment, the infection was considered cleared in 90.6% of 29 patients with ELT and 56.2% of 18 patients in the control group (p = 0.002). There was no follow-up after the end of treatment and no side effect was observed [28].
The aim of our double-blind, randomized clinical trial was to show the greater efficacy of ELT in TIVAD infection due to coagulase-negative staphylococci compared to that of VLT in adults with or without bacteremia. The secondary objective was to study ELT tolerance.
Material and methods
Trial design
We performed a prospective, multicenter, double-blind randomized clinical trial comparing the efficacy of 40% ELT versus VLT in TIVAD infection due to CoNS, complicated or not by CRBSI. The investigator, the physician in charge of the patient, and the patients themselves were not aware of the nature of the LT administered.
Settings and periods of recruitment
The trial was conducted in seven French hospital centers from April 2015 to August 2019.
Eligibility criteria
Inclusion criteria were as follows: (a) ≥18 years old, (b) proven or likely TIVAD infection with or without CRBSI, (c) infection due to CoNS (excluding S. lugdunensis), (d) maintaining TIVAD was possible (no sign of local infections (pocket infection and tunnelitis), no sign of septic shock, no secondary septic location, no septic thrombophlebitis, no TIVAD dysfunction, and the TIVAD had been implanted for at least 15 days), (e) blood culture results were delivered within 48 hours, and (f) patients were affiliated to a health system. Patients were not eligible if they had any of the following criteria: (a) adult under guardianship, (b) pregnant or breastfeeding woman, (c) known ethanol allergy, (d) prosthetic heart valve, (e) TIVAD removal criteria at inclusion or nonfunctional TIVAD, and (f) history of infection of the current TIVAD.
TIVAD infection diagnosis
The diagnosis of proven TIVAD infection complicated by CRBSI was defined as follows: sign of infection with no differential diagnosis and blood cultures performed through the TIVAD and peripheral blood cultures positive to the same microorganism with a differential time to positivity of at least 2 hours [29]. The diagnosis of likely TIVAD infection without CRBSI was defined as follows: fever with or without chills in the absence of any other identifiable source of infection and at least two positive blood cultures performed through the TIVAD and negative peripheral blood cultures [29]. Patients with signs of local infections such as pocket infection or tunnelitis require quick removal of the TIVAD and were not included in the study. Patients with no sign of infection and positive blood cultures performed through the TIVAD were considered as TIVAD colonization and were not included in the study.
Intervention
After oral and written informed consent, patients were randomly assigned in a 1 : 1 ratio to receive either VLT (vancomycin 5 mg/mL and heparin at a concentration of 2500 IU/mL) or ELT (40% v/v ethanol and enoxaparin at a concentration of 400 IU/mL) using a randomization list predetermined by the trial biostatistician with Stata software, with permuted random sized blocks (4 and 6), stratified according to center and (presence or absence of CRBSI), and allocated using an interactive response technology system administered by the pharmacy of the sponsoring center (Clermont-Ferrand Hospital). In both groups, 4 mL of the lock solution was administered through a Huber needle after manual mixture in the ward unit by the nurse. The lock solution dwelled for 24 h in the lumen and was then aspirated and renewed on each intervention day for 10 days. In case of CRBSI, a systemic antibiotic course via a distinct peripheral venous line was prescribed by the patient’s practitioner under the supervision of an infectious disease physician [13]. In the absence of CRBSI, the LT was implemented without systemic antibiotic therapy. Clinical and tolerance data were collected at days 3 ± 1 and 15 ± 1 from the intervention. Two pairs of blood cultures, one through the TIVAD and one from a peripheral blood sample, were taken during the two visits after the TIVAD had been flushed with normal saline. After intervention, the clinical research associate had weekly phone contact with the subject until week 14 to assess the outcome. An additional visit was made by an infectious disease specialist if a complication was suspected. If the complication was confirmed, the TIVAD was removed and treatment was considered to have failed.
Follow-up
Patients were followed up until week 14 ± 4 days for patients with no failure and TIVAD still in place. Data were censored at the date of failure or death or removal of TIVAD because no longer used.
Primary outcome: overall treatment success
Overall treatment success was a composite criterion defined as follows: (a) TIVAD in place and absence of failure until the end of follow-up at week 14 ± 4 days or (b) absence of failure until the end of the use of TIVAD, i.e., death related to the underlying disease justifying TIVAD or the removal of TIVAD because no longer used. One or more of the following criteria during follow-up was considered as a failure: death attributable to the TIVAD infection, septic shock attributable to the TIVAD infection, persistent infection after intervention, thrombophlebitis occurring during the intervention, no venous return during the intervention, preventing the lock solution from being aspirated, endocarditis and/or septic embolism caused by the same pathogen, pocket infection or pocket abscess, positive peripheral blood cultures collected at day 3 ± 1 and at day 15 ± 1, TIVAD removal for suspicion of sepsis even if the TIVAD culture was sterile, subsequent TIVAD infection during follow-up leading to a new treatment (conservative or not) regardless of the pathogen (same one or different). Superinfection was defined by a subsequent TIVAD infection due to pathogen different from the one initially treated.
Secondary outcomes
Treatment success in the subgroups of proven TIVAD infection complicated by CRBSI and likely TIVAD infection without CRBSI
LT complications, tolerance, and side effects
Catheter malfunctions were defined as follows [30]: (1) withdrawal occlusion was defined as the loss of capacity to withdraw blood from the catheter without difficulty infusing through it (due to development of a fibrin sheath around the tip of the catheter or abutment of the catheter tip against the vein wall). Withdrawal occlusion was treated with saline infusion or injection of fibrinolytic drugs. (2) Occlusion of the catheter lumen was defined as the loss of capacity to infuse and withdraw the blood from the system (due to clotted blood or precipitation from the lock therapy). In that case, a treatment with a small dose of a fibrinolytic agent was attempted. (3) TIVAD-related venous thrombosis was suspected clinically in case of redness, swelling, and shoulder or retrosternal pain and was confirmed by Doppler ultrasonography. The treatment was TIVAD removal with anticoagulation.
Ethanol concentration was performed from samples collected 30 minutes after the first implementation of LT irrespective of its nature.
Side effects of LT occurring within 30 minutes after the LT administration (VLT or ELT) were notified by the nurse in charge and the investigator during study visits. The adverse effects recorded were arrhythmia, asthenia, somnolence, headache, dizziness, nausea, drunkenness, flushing, abdominal pain, urticaria, changes in personality, speech disorders, chest pain, sneezing, transitory dyspnea, persistent dyspnea, puffy cheeks, and allergy.
Statistics
Assuming a cure rate of 60% at 12 weeks or until death or TIVAD removal (with no infection), 86 patients per group were necessary to show a difference of about 20% between randomized groups with a two-sided type I error set at 5% and a statistical power of 0.8 (Freedman method for censored data) [14].
A planned interim analysis was performed when 60 patients were recruited. At that time, the study could be stopped by an independent adjudication committee on the grounds of the toxicity of the products tested or the greater efficacy of one of them or a difficulty of recruitment.
Data were recorded anonymously with REDCap software [31].
The primary outcome was estimated by the Kaplan–Meier method and then compared between randomization groups using Cox proportional hazard regression model. The proportional-hazard hypothesis was verified by Schoenfeld’s test and plotting residuals. The results were expressed as hazard ratio (HR) and 95% confidence interval. Owing to imbalance at randomization, multivariable analysis was performed with Cox regression to take into account possible confounding factors such as sex.
The binary outcomes were tested using an unadjusted chi-squared or Fisher exact tests as appropriate. Continuous variables were compared using Student t-test or Mann–Whitney test when the assumptions of t-test were not met. Homoscedasticity was analyzed by Fisher–Snedecor test.
Prespecified subgroup analysis was conducted according to the stratification variable of presence or absence of bacteremia. The interaction term was used in the Cox model to test for heterogeneity of effect between subgroups.
Data were analyzed in an intention-to-treat population. All analyses were generated with Stata software, version 15.0 (StataCorp, College Station, US). A two-sided p value of less than 0.05 was considered to indicate statistical significance. No correction for multiple testing was applied in the analysis of secondary outcomes or subgroup analysis. Because of the potential for type 1 error due to multiple comparisons, findings from analyses of secondary endpoints were interpreted as exploratory.
Research ethics approval
The study was approved by the CPP Sud-Est VI ethics committee n°AU 1120, the CNIL (French Data Protection Authority) n°1223379, and the ANSM (National Drug Agency) and registered under the ClinicalTrials.gov Identifier number NCT02411331.
Results
Patients
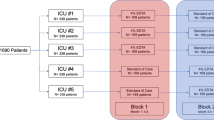
Sixty-one patients were included between April 2015 and August 2019. After the planned blinded interim analysis, the independent adjudication committee and the trial management committee agreed to stop the study because of the high rate of failure in both groups, the high rate of mechanical obstruction, and the low level of inclusions. Of the 61 patients, 31 were assigned to the ELT group and 30 to the VLT arm. No patients were lost to follow-up after randomization (Fig. 1).
Patient characteristics
Baseline characteristics of the patients are given in Table 1. Concomitant bacteremia was present in 41 patients (67.2%), 21 (67.7%) from the ELT group, and 20 (66.7%) in the VLT group. TIVAD infection was due to oxacillin-resistant CoNS in 52.5% of cases with an equal distribution between the two arms. In case of CRBSI, the systemic antibiotic regimen consisted in daptomycin for oxacillin-resistant CoNS and cefazolin for oxacillin susceptible CoNS.
Outcomes
Primary outcome
Treatment success was 58.1% (18 of 31) for the ELT arm and 46.7% (14 of 30) for the VLT arm (p = 0.37). Overall treatment success was obtained in 52.5% (32) of patients (Table 1). Fourteen (23%) patients died during follow-up. All deaths were not attributable to the TIVAD infection but related to the underlying disease. Seventeen patients (29%) required TIVAD removal during follow-up because of sepsis (8 in the ELT arm and 9 in the VLT arm). Primary outcome analyses showed that risk of treatment failure did not differ significantly between participants given ELT (13 out of 31 [42%]) and those given VLT (16 out of 30 [53%]) with a hazard ratio of 0.70 (p = 0.343, 95% CI [0.34–1.46], Cox model) (Fig. 2A). The causes of failure are given in Table 2. Superinfections represented the second cause of failure and were equally represented in ELT and VLT groups (Table 2).
A Primary outcome comparing ELT and VLT in 61 patients with proven or likely TIVAD due to CoNSs. B Primary outcome comparing ELT and VLT in 41 patients with bacteremic TIVAD due to CoNS infection. C Primary outcome comparing ELT and VLT in 20 patients with non-bacteremic TIVAD infection due to CoNSs
Secondary outcomes
Catheter malfunctions
Catheter malfunctions were significantly more frequent in the ELT arm (11 patients (35.5%) as against 2 (6.7%) in the VLT group, p = 0.01). We observed 3 occlusions of the catheter lumen needing replacement of TIVAD, all in the ELT arm, and 9 withdrawal occlusions requiring injection of urokinase (8 in the ELT group and 1 in the VLT group). TIVAD-related venous thrombosis was observed in 1 patient with VLT, who did not require removal of TIVAD.
Treatment success in the subgroups of proven TIVAD infection complicated by CRBSI and likely TIVAD infection without CRBSI
We did not find any significant difference in treatment success for patients with CRBSI versus no CRBSI (hazard ratio = 1.62, 95% CI [0.69–3.81], p = 0.262). Treatment success in patients with CRBSI was not statistically different between ELT and VLT groups (hazard ratio = 1.20, p = 0.665, 95% CI [0.52–2.79]) (Fig. 2B). In patients with no CRBSI, ELT was statistically associated with a better rate of treatment success (hazard ratio = 0.11, p = 0.045, 95% CI [0.13–0.96]) (Fig. 2C).
Ethanol concentration
Ethanol was not detected in any samples collected 30 minutes after the first implementation of LT.
Side effects of LT
Adverse effects (excluding mechanical complications) occurred in 15 patients (24.5%), 9 (29%) in the ELT group, and 6 (20%) in the VLT group (p = 0.41) and were mainly represented by asthenia and drowsiness.
Discussion
Several important conclusions can be drawn from this prospective randomized trial involving a homogenous group of patients with CoNS TIVAD infection. First, the overall rate of failure of LT was high (47.5%), irrespective of whether treatment was with vancomycin or ethanol. This finding implies that TIVAD removal should be the rule in case of infection except in specific populations. Second, the high rate of failure of the conservative treatment was due to the lack of control of the primary infection but also to superinfections, which complicated the primary infection, and catheter malfunctions (Table 2). Third, the overall mortality rate at 3 months was high (22.9%) in the study population with a majority having metastatic cancer and parenteral nutrition, which suggests that infection mainly occurs in patients with very advanced disease and poor prognosis. Patients with reduced life expectancy or in palliative care could benefit from maintenance of TIVAD with LT for quality-of-life reasons. In addition, situations in which there is no alternative other than TIVAD for central venous access because of a poor venous capital can be an exception to the rule of catheter removal [13]. Fourth, our study did not show that ELT in TIVAD infection due to CoNS was more effective than VLT in adults with or without bacteremia. Therefore, ELT with enoxaparin regimen as performed in our study cannot be routinely recommended for all CoNS TIVAD infection because of the increasing risk of catheter malfunction, especially occlusions. The use of ELT should be assessed on a case-by-case basis and reserved for exceptional situations. Fifth, there was no statistical difference overall in the cure rate between non-bacteremic and bacteremic TIVAD infections. However, in non-bacteremic patients, there was a trend for greater efficacy of ELT compared to VLT. This suggests that non-bacteremic-infected TIVADs should be removed when possible. If they are maintained, ELT could be an alternative to VLT in selected patients.
Several retrospective or uncontrolled observational studies have also been published on the combined treatment of CRBSI with systemic antibiotics and ELT. They suggested that ELT was an effective treatment of CRBSI [32]. However, there was great heterogeneity between the different studies regarding patient profile, type of infected catheter, ethanol concentration, dwelling time, and follow-up duration [32]. Of the two randomized studies, the one that showed a benefit of a 3-week 60% ELT for hemodialysis-related catheter infection did not provide outcome after the end of treatment [28]. Our results are in agreement with those of the second randomized study conducted by Wolf et al. in 2018, which concluded that ELT was not effective to prevent treatment failure in CRBSI among pediatric patients with cancer [25]. A recent meta-analysis on catheter salvage strategies in children reported high rates of successful catheter salvage at 30 days for infections with CoNS and a good efficacy of ethanol lock. However, most included studies were not RCTs and there was a high degree of heterogeneity among them [33].
The choice of the composition of the ethanol lock solution was determined by several factors: the impact of the ethanol concentration on the integrity of the catheter structure, the risk of precipitation with heparin, and the effectiveness on biofilm. High-concentrated ethanol may affect the structural stability and the mechanical properties of catheters [34,35,36], while a 40% v/v ethanol has little impact on catheter integrity [36]. Moreover, whereas an unfractionated heparin cannot be combined with 40% ethanol because of precipitation, low molecular weight heparins can be combined with 40% ethanol [37]. In a previous study, we showed that a 40% ethanol solution combined with enoxaparin was stable, had a low impact on polyurethane, and had a antibiofilm activity against the microorganisms commonly involved in catheter infections [17, 38]. Therefore, we estimated that a lock solution with 40% v/v ethanol and enoxaparin at a concentration of 400 IU/mL was a reasonable option to use in clinical practice. However, in our study, ELT was associated with thrombosis and catheter malfunctions. One possible explanation would be that the seepage of the lock solution into the systemic circulation after ethanol instillation with concomitant blood inflow into the catheter may promote plasma protein precipitation [39].
Our study has certain limitations. First, the patient sample size was small. Therefore, the lack of difference between groups is not conclusive and may be biased due to a lack of power. As pointed out by Rijnders et al. [14], patients with TIVAD infection are difficult to recruit for several reasons: (a) TIVAD infections are rare events that can be difficult to diagnose, (b) patients frequently have advanced cancer and can be reluctant to participate in a randomized clinical trial, and (c) in order to obtain a homogenous population, recruitment was restricted to patients with a single type of catheter (TIVAD) and a single type of bacteria (CoNS). Second, non-bacteremic TIVAD infections were included, which can be difficult to distinguish from catheter colonization. However, we used strict diagnosis criteria to rule out simple colonization and, as recommended by Rijnders et al., we considered it important to include patients with non-bacteremic TIVAD infection [14]. Even limited by the population size, our results tend to show that non-bacteremic TIVAD infections can have a similar prognosis to that of bacteremic TIVAD infections and that ELT could be more effective than VLT to treat such patients.
Our study also has strengths that are not found in previous reports. It was randomized and double-blind and we recruited a homogenous population with a prolonged follow-up of 3 months. It is the first study to compare two types of LT. Finally, adverse effects were carefully recorded throughout follow-up.
Conclusions
This randomized double-blind study found an overall high rate of treatment failure that did not differ between the two groups treated. Uncontrolled infections, superinfections, and mechanical complications were too frequent for maintenance of TIVAD to be recommended in case of infection except in specific situations such as palliative care or poor central venous access. For selected patients with non-bacteremic TIVAD infections, ELT was more effective than VLT.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to risk of individual privacy compromission but are available from the corresponding author on reasonable request
Abbreviations
- TIVADs:
-
totally implantable venous access devices
- ELT:
-
ethanol lock therapy
- VLT:
-
vancomycin lock therapy
- CRBSI:
-
catheter-related bloodstream infection
- CoNS:
-
coagulase-negative staphylococci
References
Walser EM (2012) Venous access ports: indications, implantation technique, follow-up, and complications. Cardiovasc Intervent Radiol 35:751–764. https://doi.org/10.1007/s00270-011-0271-2
Pinelli F, Cecero E, Degl’Innocenti D, Selmi V, Giua R, Villa G, Chelazzi C, Romagnoli S, Pittiruti M (2018) Infection of totally implantable venous access devices: a review of the literature. J Vasc Access 19:230–242. https://doi.org/10.1177/1129729818758999
Gapany C, Tercier S, Diezi M, Clement C, Lemay K, Joseph J-M (2011) Frequent accesses to totally implanted vascular ports in pediatric oncology patients are associated with higher infection rates. J Vasc Access 12:207–210. https://doi.org/10.5301/JVA.2011.6258
Dibb M, Lal S (2017) Home parenteral nutrition: vascular access and related complications. Nutr Clin Pract 32:769–776. https://doi.org/10.1177/0884533617734788
Wang TY, Lee KD, Chen PT, Chen MC, Chen YY, Huang CE, Kuan FC, Chen CC, Lu CH (2015) Incidence and risk factors for central venous access port-related infection in Chinese cancer patients. J Formos Med Assoc 114:1055–1060. https://doi.org/10.1016/j.jfma.2015.06.013
Shim J, Seo T-S, Song MG, Cha I-H, Kim JS, Choi CW, Seo JH, Oh SC (2014) Incidence and risk factors of infectious complications related to implantable venous-access ports. Korean J Radiol 15:494–500. https://doi.org/10.3348/kjr.2014.15.4.494
Zerati AE, Figueredo TR, de Moraes RD, da Cruz AM, da Motta-Leal Filho JM, Freire MP, Wolosker N, de Luccia N (2016) Risk factors for infectious and noninfectious complications of totally implantable venous catheters in cancer patients. J Vasc Surg Venous Lymphat Disord 4:200–205. https://doi.org/10.1016/j.jvsv.2015.10.008
Lebeaux D, Fernández-Hidalgo N, Chauhan A, Lee S, Ghigo J-M, Almirante B, Beloin C (2014) Management of infections related to totally implantable venous-access ports: challenges and perspectives. Lancet Infect Dis 14:146–159. https://doi.org/10.1016/S1473-3099(13)70266-4
Laporte-Amargos J, Sastre E, Bergas A, Pomares H, Paviglianiti A, Rodriguez-Arias M, Pallares N, Badia-Tejero AM, Pons-Oltra P, Carratalà J, Gudiol C (2023) Increasing gram-negative catheter-related bloodstream infection in cancer patients. Pathogens 12:228. https://doi.org/10.3390/pathogens12020228
Flynn PM, Willis B, Gaur AH, Shenep JL (2003) Catheter design influences recurrence of catheter-related bloodstream infection in children with cancer. J Clin Oncol 21:3520–3525. https://doi.org/10.1200/JCO.2003.03.012
Aumeran C, Guyot P, Boisnoir M, Robin-Hennequin C, Vidal M, Forestier C, Traore O, Lesens O, Clermont-Ferrand Biofilm Study Group (2013) Activity of ethanol and daptomycin lock on biofilm generated by an in vitro dynamic model using real subcutaneous injection ports. Eur J Clin Microbiol Infect Dis 32:199–206. https://doi.org/10.1007/s10096-012-1732-5
Morales M, Méndez-Alvarez S, Martín-López J-V, Marrero C, Freytes CO (2004) Biofilm: the microbial “bunker” for intravascular catheter-related infection. Support Care Cancer 12:701–707. https://doi.org/10.1007/s00520-004-0630-5
Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, Raad II, Rijnders BJA, Sherertz RJ, Warren DK (2009) Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 49:1–45. https://doi.org/10.1086/599376
Rijnders BJ, Van Wijngaerden E, Vandecasteele SJ, Stas M, Peetermans WE (2005) Treatment of long-term intravascular catheter-related bacteraemia with antibiotic lock: randomized, placebo-controlled trial. J Antimicrob Chemother 55:90–94. https://doi.org/10.1093/jac/dkh488
Raad I, Hanna H, Dvorak T, Chaiban G, Hachem R (2007) Optimal antimicrobial catheter lock solution, using different combinations of minocycline, EDTA, and 25-percent ethanol, rapidly eradicates organisms embedded in biofilm. Antimicrob Agents Chemother 51:78–83. https://doi.org/10.1128/AAC.00154-06
Chambers ST, Pithie A, Gallagher K, Liu T, Charles CJ, Seaward L (2007) Treatment of Staphylococcus epidermidis central vascular catheter infection with 70% ethanol locks: efficacy in a sheep model. J Antimicrob Chemother 59:779–782. https://doi.org/10.1093/jac/dkl542
Balestrino D, Souweine B, Charbonnel N, Lautrette A, Aumeran C, Traoré O, Forestier C (2009) Eradication of microorganisms embedded in biofilm by an ethanol-based catheter lock solution. Nephrol Dial Transplant 24:3204–3209. https://doi.org/10.1093/ndt/gfp187
Sofroniadou S, Revela I, Kouloubinis A, Makriniotou I, Zerbala S, Smirloglou D, Kalocheretis P, Drouzas A, Samonis G, Iatrou C (2017) Ethanol combined with heparin as a locking solution for the prevention of catheter related blood stream infections in hemodialysis patients: a prospective randomized study. Hemodial Int 21:498–506. https://doi.org/10.1111/hdi.12524
Schoot RA, van Ommen CH, Stijnen T, Tissing WJE, Michiels E, Abbink FCH, Raphael MF, Heij HA, Lieverst JA, Spanjaard L, Zwaan CM, Caron HN, van de Wetering MD (2015) Prevention of central venous catheter-associated bloodstream infections in paediatric oncology patients using 70% ethanol locks: a randomised controlled multi-centre trial. Eur J Cancer 51:2031–2038. https://doi.org/10.1016/j.ejca.2015.06.126
Salonen BR, Bonnes SL, Vallumsetla N, Varayil JE, Mundi MS, Hurt RT (2018) A prospective double blind randomized controlled study on the use of ethanol locks in HPN patients. Clin Nutr 37:1181–1185. https://doi.org/10.1016/j.clnu.2017.05.009
Souweine B, Lautrette A, Gruson D, Canet E, Klouche K, Argaud L, Bohe J, Garrouste-Orgeas M, Mariat C, Vincent F, Cayot S, Cointault O, Lepape A, Guelon D, Darmon M, Vesin A, Caillot N, Schwebel C, Boyer A et al (2015) Ethanol lock and risk of hemodialysis catheter infection in critically ill patients. A randomized controlled trial. Am J Respir Crit Care Med 191:1024–1032. https://doi.org/10.1164/rccm.201408-1431OC
Broom JK, Krishnasamy R, Hawley CM, Playford EG, Johnson DW (2012) A randomised controlled trial of Heparin versus EthAnol Lock THerapY for the prevention of Catheter Associated infecTion in Haemodialysis patients--the HEALTHY-CATH trial. BMC Nephrol 13:146. https://doi.org/10.1186/1471-2369-13-146
Pérez-Granda MJ, Barrio JM, Muñoz P, Hortal J, Rincón C, Rabadán PM, Pernia MS, Bouza E (2014) Ethanol lock therapy (E-Lock) in the prevention of catheter-related bloodstream infections (CR-BSI) after major heart surgery (MHS): a randomized clinical trial. PLoS One 9:e91838. https://doi.org/10.1371/journal.pone.0091838
Lopes BC, Borges PSGN, Gallindo RM, Tenório TBS, Machado LB, de Orange FA (2019) Ethanol lock therapy for the prevention of nontunneled catheter-related bloodstream infection in pediatric patients. JPEN J Parenter Enteral Nutr 43:1044–1052. https://doi.org/10.1002/jpen.1508
Worth LJ, Slavin MA, Heath S, Szer J, Grigg AP (2014) Ethanol versus heparin locks for the prevention of central venous catheter-associated bloodstream infections: a randomized trial in adult haematology patients with Hickman devices. J Hosp Infect 88:48–51. https://doi.org/10.1016/j.jhin.2014.06.007
Slobbe L, Doorduijn JK, Lugtenburg PJ, El Barzouhi A, Boersma E, van Leeuwen WB, Rijnders BJA (2010) Prevention of catheter-related bacteremia with a daily ethanol lock in patients with tunnelled catheters: a randomized, placebo-controlled trial. PLoS One 5:e10840. https://doi.org/10.1371/journal.pone.0010840
Wolf J, Connell TG, Allison KJ, Tang L, Richardson J, Branum K, Borello E, Rubnitz JE, Gaur AH, Hakim H, Su Y, Federico SM, Mechinaud F, Hayden RT, Monagle P, Worth LJ, Curtis N, Flynn PM (2018) Treatment and secondary prophylaxis with ethanol lock therapy for central line-associated bloodstream infection in paediatric cancer: a randomised, double-blind, controlled trial. Lancet Infect Dis 18:854–863. https://doi.org/10.1016/S1473-3099(18)30224-X
Khosroshahi HT, Mahdipur H, Parkhideh S, Basmenji S, Khalilzadeh M, Tozihi M (2015) The effectiveness of systemic antibiotic therapy with and without ethanol-locked solution in the treatment of hemodialysis-related catheter infection. Saudi J Kidney Dis Transpl 26:477–481. https://doi.org/10.4103/1319-2442.157315
Mermel LA, Farr BM, Sherertz RJ, Raad II, O’Grady N, Harris JS, Craven DE, Infectious Diseases Society of America, American College of Critical Care Medicine, Society for Healthcare Epidemiology of America (2001) Guidelines for the management of intravascular catheter-related infections. Clin Infect Dis 32:1249–1272. https://doi.org/10.1086/320001
Kurul S, Saip P, Aydin T (2002) Totally implantable venous-access ports: local problems and extravasation injury. Lancet Oncol 3:684–692. https://doi.org/10.1016/s1470-2045(02)00905-1
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN, REDCap Consortium (2019) The REDCap consortium: building an international community of software platform partners. J Biomed Inform 95:103208. https://doi.org/10.1016/j.jbi.2019.103208
Wolf J, Shenep JL, Clifford V, Curtis N, Flynn PM (2013) Ethanol lock therapy in pediatric hematology and oncology. Pediatr Blood Cancer 60:18–25. https://doi.org/10.1002/pbc.24249
Buonsenso D, Salerno G, Sodero G, Mariani F, Pisapia L, Gelormini C, Di Nardo M, Valentini P, Scoppettuolo G, Biasucci DG (2022) Catheter salvage strategies in children with central venous catheter-related or -associated bloodstream infections: a systematic review and meta-analysis. J Hosp Infect 125:1–20. https://doi.org/10.1016/j.jhin.2022.03.010
Mermel LA, Alang N (2014) Adverse effects associated with ethanol catheter lock solutions: a systematic review. J Antimicrob Chemother 69:2611–2619. https://doi.org/10.1093/jac/dku182
Guenu S, Heng A-E, Charbonné F, Galmier M-J, Charlès F, Deteix P, Souweine B, Lartigue C (2007) Mass spectrometry and scanning electron microscopy study of silicone tunneled dialysis catheter integrity after an exposure of 15 days to 60% ethanol solution. Rapid Commun Mass Spectrom 21:229–236. https://doi.org/10.1002/rcm.2837
Msakni N, Galmier M-J, Couret M-J, Szczepaniak C, Bouchon B, Souweine B, Lartigue C (2013) Complementary mass spectrometric approaches and scanning electron microscopy to study the structural stability of polyurethane tunneled dialysis catheters after exposure to ethanol solutions. Rapid Commun Mass Spectrom 27:2343–2354. https://doi.org/10.1002/rcm.6691
Calvet L, Piot M, Lartigue C, Souweine B, Tardy-Poncet B (2015) Anticoagulant properties of enoxaparin 400 IU/mL-40% ethanol catheter lock solution. Springerplus 4:746. https://doi.org/10.1186/s40064-015-1533-2
Balestrino D, Quintana M, Charbonnel N, Forestier C, Lartigue C, Souweine B (2016) Compatibility of injectable anticoagulant agents in ethanol; in vitro antibiofilm activity and impact on polyurethane catheters of enoxaparin 400 U/mL in 40% v/v ethanol. PLoS One 11:e0159475. https://doi.org/10.1371/journal.pone.0159475
Schilcher G, Schlagenhauf A, Schneditz D, Scharnagl H, Ribitsch W, Krause R, Rosenkranz AR, Stojakovic T, Horina JH (2013) Ethanol causes protein precipitation--new safety issues for catheter locking techniques. PLoS One 8:e84869. https://doi.org/10.1371/journal.pone.0084869
Acknowledgements
We thank our microbiologist colleagues Julien Delmas and Frédéric Robin for their technical assistance. We thank Dr. Natacha Mrozek, Dr. Magali Vidal, Dr. Clément Théis, and Delphine Martineau for their participation.
Funding
The research protocol was accepted for funding by an Inter-Regional Hospital Clinical Research Program (PHRC-IR Auvergne-Rhône-Alpes).
Author information
Authors and Affiliations
Contributions
O.L. contributed to the study conception and design. Data collection was performed by all the other authors. The first draft of the manuscript was written by O.L. and L.S., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the CPP Sud-Est VI ethics committee n°AU 1120, the CNIL (French Data Protection Authority) n°1223379, and the ANSM (National Drug Agency) and registered under the ClinicalTrials.gov Identifier number NCT02411331.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lesens, O., Forestier, E., Botelho-Nevers, E. et al. Comparing ethanol lock therapy versus vancomycin lock in a salvation strategy for totally implantable vascular access device infections due to coagulase-negative staphylococci (the ETHALOCK study): a prospective double-blind randomized clinical trial. Eur J Clin Microbiol Infect Dis 43, 223–232 (2024). https://doi.org/10.1007/s10096-023-04702-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-023-04702-w