Abstract
The present study focused on the antibacterial and biofilm inhibitory potential of 4-epi-pimaric acid isolated from aerial parts (stem and leaves) of Aralia cachemirica L. (Araliaceae) against oral cavity pathogens. 4-epi-Pimaric acid exhibited minimum inhibitory concentration (MIC) in the range of 4–16 μg/ml and minimum bactericidal concentration (MBC) two- to four-folds higher than MIC. There was significant inhibition in the biofilm formation by Streptococcus mutans on the saliva coated surface (P < 0.05), and confocal microscopy revealed that 4-epi-pimaric acid inhibited the clumping and attachment of S. mutans. At 8 × MIC concentration, it significantly prevented the pH drop and reduced S. mutans biofilms (P < 0.05). Increased propidium iodide staining and leakage of 260- and 280-nm absorbing material by 4-epi-pimaric acid treated cells of S. mutans suggested that it probably causes disruption of the cytoplasmic membrane structure. It also exhibited significant suppression of TNF-α expression in human neutrophils, suggestive of its anti-inflammatory activity. Furthermore, the compound was found to be significantly safe (IC50 >100 μg/ml) in the MTT assay on AML-12 cell lines. In conclusion, 4-epi-pimaric acid showed promising antibacterial, anti-biofilm and anti-inflammatory potency and this compound can be exploited for therapeutic application in oral microbial infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The two most common human diseases associated with the oral cavity infections are dental caries and periodontitis. Although, neither of these diseases are generally considered life threatening, but both of them are significantly painful and costly to manage [1]. However, there are recent reports that suggest potential role of periodontal infections in more serious systemic diseases including cardiovascular disease, respiratory infections, diabetes and low-birth weight complications [2, 3].
Oral biofilms formation is an important event associated with the initiation of most common infections in the oral cavity such as caries, gingivitis, and periodontal diseases [4]. Streptococcus mutans has been implicated as one of the principal etiological agents in the pathogenesis of dental caries in human, in which short chain carboxylic acids released as its fermentation by-products demineralize the enamel and lead to cavitation in the tooth. It is a key contributor to the formation of biofilms associated with dental caries disease [5–7]. The biofilms of S. mutans are also involved in infective endocarditis, a serious disease with a mortality rate of up to 50% despite antibiotic treatment [2]. Colonization of enamel surfaces by the cariogenic bacteria such as S. mutans and Actinomyces viscosus is thought to be initiated by attachment to a saliva-derived conditioning film, the acquired enamel pellicle (AEP) [8]. The AEP is formed largely by adsorption of many heterogeneous salivary proteins [9] onto dental enamel and promotes the adhesion of S. mutans by specific and nonspecific mechanisms [10]. This is followed by multiplication and exopolysaccharide formation by the bacteria to form a biofilm. The acids released by the adhered bacteria decalcify the minerals in the enamel, leading to decavitation of the tooth [1, 7]. Therefore, inhibition of overgrowth and biofilm formation of S. mutans is one of the preventive strategies of dental caries.
A renewed interest in natural substances has focused attention on plants rich in bioactive compounds well known for their antimicrobial properties [11, 12]. There are many reports that show natural molecules as good antibacterial agents active against oral pathogens like Streptococcus mutans, Actinomyces viscosus, Streptococcus sanguis, Fusobacterium nucleatum, Prevotella intermedia, Haemophilus actinomycetemcomitans, and Porphyromonas gingivalis [13–17].
Aralia cachemirica is an important species of the plant family Araliaceae, consisting of about 68 species, and the genus is native to Asia and America, with most species occurring in mountain woodlands [18, 19], and in India the species occurs at a height of 8000 ft (Himalayan region). In folklore, the genus Aralia is reported to have usage in treating rheumatism, sores, burns, itchy skin, ulcers and skin problems such as eczema [20, 21]. A. cachemirica remains unexplored for its chemistry and biological studies except for the alcoholic extract of root which is known to possess hypoglycemic activity in rats [22], and the essential oil known to have oxygenated and non-oxygenated monoterpenes as its major constituents [23]. In our recent study, antibacterial activity in the methanolic extract of the aerial parts of A. cachemerica, attributed mainly due to the presence of pimarane type diterpenes, was observed [24]. These diterpenoid compounds isolated from Viguiera arenaria have also been shown to exhibit antibacterial activity against selected panel of bacteria [25]. The present work describes the detailed antibacterial study of 4-epi-pimaric acid isolated from A. cachemirica against a panel of oral cavity pathogens.
Materials and methods
Microorganisms and culture conditions
The bacteria used in this study included Streptococcus mutans ATCC 25175, Enterococcus faecalis ATCC 29212, Enterococcus faecium ATCC 8042, Staphylococcus aureus ATCC 29213, Streptococcus epidermidis ATCC 12228, Actinomyces viscosus ATCC 15987, Streptococcus sanguis ATCC 10556, Porphyromonas gingivalis ATCC 33277, and Prevotella intermedia ATCC 25611. The reference strains were purchased from ATCC (American Type Culture Collection, Manassas, VA, USA). The clinical isolates were isolated from the swab samples and plaque samples provided by Corps Dental Hospital, Jammu, India. Collection and isolation of clinical isolates is described in our previous study [15]. Cultures of S. mutans, A. viscosus and S. sanguis were maintained on brain–heart infusion agar (BHI; Difco Laboratories, Detroit, MI, USA) at 37°C in a 5% CO2 atmosphere. E. faecalis and E. faecium were maintained on trypticase soy agar (Difco Laboratories) at 37°C. For the growth of P. gingivalis, tryptic soy broth-yeast extract medium (Difco Laboratories) supplemented with cysteine hydrochloride (0.05%) (Himedia, Laboratories Mumbai, India), menadione (0.02 mg/ml), haemin (5 mg/ml) (Himedia) and potassium nitrate (0.02%) was used. P. intermedia was grown on Wilkins-Chalgren agar (WC; Difco Laboratories). Both of these bacterial cultures were incubated anaerobically (90% N2, 5% H2 and 5% CO2) at 37°C up to 48 h in anaerobic jars (Anoxomat; Mart, Lichtenvoorde, The Netherlands).
Isolation, purification and characterization of 4-epi-pimaric acid from A. cachemirica
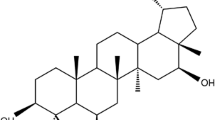
The plant material collected from the Ladakh region of Jammu and Kashmir (India) was identified as A. cachemirica by the plant taxonomist of our institute. The identified specimen was deposited in the herbarium of our institute (accession No. 21697). Shade dried aerial parts (stem and leaves) of the plant were powdered (800 g), soaked in methanol (3.0 L) and the contents stirred at 250 rpm for 24 h and then filtered. The marc was reconstituted in methanol (2.5 L) and extracted again as described above. The process was repeated again. The combined filtrates were concentrated on rotavapor at <50°C under reduced pressure to give a dark brown gummy extract (extractive value 16%), out of which 50 g of the extract was taken in a mixture of methanol:water (9:1) and defatted with hexane (3 × 100 ml). The defatted material was then extracted with chloroform by solvent-solvent extraction method (3 × 100 ml), followed by extraction with ethyl acetate (3 × 100 ml) and the extracts concentrated. The antibacterial activities of chloroform, ethyl acetate and the residual extracts were determined against S. mutans. The MIC of chloroform, ethyl acetate and the residual extracts were 64, >256 and >256 μg/ml against S. mutans, respectively. The chloroform extract showing the lowest MIC was subjected to flash chromatography (silica gel column, 200–400 mesh) and was eluted with n-hexane followed by a mixture of n-hexane:ethyl acetate (19:1). The fractions obtained by elution with n-hexane:ethyl acetate (19:1) having the same TLC pattern were pooled and concentrated to furnish a crystalline solid. The crystalline solid was further purified by crystallization from a acetone:hexane mixture to afford a colourless compound, mp 162–164°C, identified as 4-epi-pimaric acid (Fig. 1) by spectral analysis. The compound showed >95% purity as determined by HPLC (RP-18 column, 215 nm wavelength, PDA detector) using acetonitrile:water gradient as mobile phase (Fig. 2).
MIC and MBC of 4-epi-pimaric acid against oral cavity pathogens
MIC of the 4-epi-pimaric acid was determined as per the guidelines of the Clinical and Laboratory Standard Institute (CLSI; M11-A7) [26]. All the oral cavity bacteria used in this study were grown to stationary phase as described above. Bacterial suspensions were prepared by suspending 24 h grown culture in brucella broth (Difco Laboratories) (for anaerobic bacteria) and sterile normal saline (for aerobic bacteria). MIC was performed in the respective growth media and conditions for bacteria as mentioned above. Two-fold serial dilutions of 4-epi-pimaric acid were prepared in the 100-μl volume of respective growth medium in 96-well U-bottom microtitre plates (Tarson, Mumbai, India). The final concentrations of 4-epi-pimaric acid ranged from 0.12 to 128 μg/ml. The turbidity of bacterial suspensions was adjusted to 0.5 McFarland (≈ 1.5 × 108 CFU/ml), which was further diluted in the respective growth medium and a 100-μl volume of this diluted inoculum was added to each well of the plate, resulting in a final inoculum of 5 × 106 CFU/ml for anaerobic bacteria and 5 × 105 CFU/ml for the aerobic bacterial species. After incubation at 37°C for 48 h, the plates were read visually, and the minimum concentration of the compound showing no turbidity was recorded as the MIC. The minimum bactericidal concentration (MBC) was determined by spreading a 100-μl volume on the respective growth media agar plate, and the bacterial cells were enumerated after incubation at 37°C for 48 h. MBC was defined as the lowest concentration of 4-epi-pimaric acid at which more than 99.9% of the cells were killed compared with a non-treated control [27].
Post antibacterial effect (PAE)
The PAE of 4-epi-pimaric acid was measured by methods described by Craig and Gudmundsson with slight modification [28]. 4-epi-Pimaric acid was added at MIC, 2 × MIC and 4 × MIC to BHI broth containing ≈ 106 CFU of each S. mutans and A. viscosus per ml. After exposure to the 4-epi-pimaric acid for 5 min, samples were diluted to 1:1,000 in BHI broth to effectively remove the compound. Aliquots were removed from the samples taken every hour until visual cloudiness was noted. PAE was defined as T − C, where T is the time required for the count in the test culture to increase 1 log10 above the count observed immediately after dilution and C is the time required for the count in the untreated control to increase 1 log10 above the count observed immediately after dilution.
Collection of saliva
Saliva was stimulated by chewing a piece of paraffin and collected over ice from four healthy individuals who refrained from eating and tooth brushing for a minimum of 2 h prior to donation. The pooled saliva was cooled on ice and then clarified by centrifugation at 4000 g for 20 min at 4°C [29]. The supernatant was then filtered through a 0.22-μm pore filter (Millipore, Bangalore, India) and frozen at −20°C until use.
Biofilm susceptibility assays
The biofilms of S. mutans ATCC 25175 were prepared in 96-well flat-bottom polystyrene microtiter plates (Tarson), using a previously described protocol of Wei et al., with few modifications [30]. The clarified saliva (100 μl) was added to 96-well flat bottomed microtiter plates with 100 μl of coating buffer (20 mmol of carbonate buffer, pH 9.3), and then incubated for 2 h at 4°C. The saliva-buffer mixture was then decanted and wells were washed twice with Tris-HCl sodium chloride and calcium chloride buffer (10 mmol Tris–HCl, 150 mmol NaCl, 5 mmol CaCl2, pH 7.6). The suspension of overnight grown S. mutans was adjusted to an optical density at 610 nm (OD610) of 0.7 (≈1 × 109 CFU/ml). One hundred microliters BHI broth supplemented with 2% sucrose (w/v; BHIS) containing 2 × concentration of sub MIC of 4-epi-pimaric acid was added to the wells of a 96-well flat-bottom polystyrene tissue culture plate. The fresh BHIS broth (40 μl) was added to each well, followed by the addition of 60 μl of the above-mentioned suspension to each well of the plate. This resulted in the final inoculum of 6 × 107 CFU/ml in each well. After incubation for 24 h at 37°C, media and unattached cells were decanted from the microtiter plates. The remaining planktonic cells were removed by gentle rinsing with phosphate buffer saline (10 mM, pH 7.2). The biofilm was fixed with methanol for 15 min and then air dried at room temperature. It was then stained with 0.1% (w/v) crystal violet (Sigma Chemical Co., St Louis, MO, USA) for 5 min and rinsed thoroughly with water until the control wells appeared colourless. The amount of biofilm was quantified by the addition of 200 μl of 95% ethanol to each crystal violet-stained well. The plate was rocked at room temperature for 30 min and the absorbance at 595 nm (A595) was determined using a microplate reader (Multiskan Spectrum; Thermo Electron, Vantaa, Finland). The effect of 4-epi-pimaric acid (4 μg/ml) was studied at 4, 12, 20 and 24 h exposure and the biofilms were analysed by the above-stated protocol. The percentage of adherent cells on the microtiter plate was calculated using the equation (A595 of biofilm treated with 4-epi-pimaric acid/A595 of non-treated control) × 100. Culture without 4-epi-pimaric acid was used as the non-treated control.
Biofilms and confocal microscopy
Confocal microscopy analysis of biofilm, cultivated on glass cover slips (n = 3) coated with clarified and filter sterilized saliva was carried out as described by Islam et al. [31] with few modifications. Briefly, nine-well microtiter plate seeded with glass cover slips (Nunc, Rochester, NY, USA) were incubated for 2 h at 4°C with 3 ml of saliva in 3 ml of 20 mmol/L carbonate buffer, pH 9.3. The cover slips were then washed with sterile saline and seeded in nine-well microtiter plates containing 3 ml of BHI broth with 2% sucrose and 4 μg/ml of 4-epi-pimaric acid. The wells were inoculated with 100 μl of mid-exponential grown culture of S. mutans and then the plates were incubated at 37°C for 24 h in 5% CO2 atmosphere. The cover slips were then removed and gently washed with potassium phosphate buffer (PBS; 10 mM, pH 7.1). The specimens were stained for 1 h with propidium iodide (0.2 mg/ml) (Sigma) PBS containing 0.1% sodium citrate, 0.1 mg/ml RNase and 0.3% brij-58 (Sigma). The cover slips were then removed, washed with PBS, and non-invasive confocal analysis of fully hydrated biofilms was performed using Olympus Fluoview, FV-1000 CLSM (Valley Point Office Tower, Singapore) fitted with a water immersion dipping objective lens (60×) and a He–Ne laser. The excitation wavelength was 543 nm. A scan speed of 400 lines/s was used to ensure minimum dislocation due to the movement of cells. The biofilm images were adapted to the 8 bit range of the system. An PLA PON 60 × Oil NA: 1.42 objective was used with additional zoom of × 2, resulting in 512 × 512 images with a pixel size of 0.12 mm. Each stack of an experiment was examined and the threshold value that best fitted all image stacks of a trial was chosen.
Effect of 4-epi-pimaric acid on the pH of broth, S. mutans growth and its adhesion to glass surface
Glass surface adherence assay of S. mutans was performed by the method described previously [15] with slight modifications. Actively grown culture of S. mutans 25175 (≈1× 106 CFU/ml) was incubated in a 5% CO2 atmosphere for 24 h at 37°C at an angle of 30° in a glass tube containing 10 ml of BHI broth with 2% sucrose (wt/vol) (Himedia). After incubation, loosely attached cells were decanted. The S. mutans biofilms were washed and resuspended in 10 ml of PBS (10 mM pH 7.1) containing 64 μg/ml (8 × MIC) of 4-epi-pimaric acid and incubated at 37°C for 5 min. The mixture was gently removed and rinsed twice with 10 ml of PBS. The 4-epi-pimaric acid treated biofilms were resuspended in 10 ml of fresh BHI broth containing 2% (wt/vol) sucrose, followed by incubation at 37°C. After incubation for 0, 3, 6, 12, 18 and 24 h, the acidogenicity of the biofilm was measured with a pH meter, and OD of broth was also taken at 610 nm. After 24 h of incubation, the fluid containing free cells of S. mutans ATCC 25175 was gently removed, and biofilms were resuspended in 10 ml of sterile water and homogenized using five 30-s ultrasonic bursts, and the turbidity was measured at 610 nm. The percentage of biofilms reduction on the glass surface was calculated using the equation (A595 of biofilm treated with 4-epi-pimaric acid/A595 of non-treated control) × 100. Culture without 4-epi-pimaric acid was used as the non-treated control.
Cell viability assay
Cell viability of S. mutans was determined by serial dilution method [13] with little modification. Bacterial suspensions were prepared by suspending actively growing cultures of S. mutans and A. viscosus in sterile normal saline, and turbidity was adjusted to an optical density at 610 nm (OD610) of 0.7 (≈1 × 109 CFU/ml). One millilitre of this bacterial suspension was added to 9 ml of BHI broth with 4-epi-pimaric acid (64 μg/ml) and without 4-epi-pimaric acid (untreated control). Samples were taken after 60 and 90 min of incubation; 100 μl of sample were serially diluted in the sterile normal saline, and then 20 μl of these dilutions were spotted on to the BHI agar plate. These plates were incubated in the 5% CO2 atmosphere at 37°C for 48 h, and the viable cell number reported as colony-forming units (CFU) per ml. The results of S. mutans viability were expressed as percent of control (without 4-epi-pimaric acid).
Propidium iodide uptake
The membrane disruption caused by the action of 4-epi-pimaric acid was assessed by propidium iodide uptake assay by flow cytometry analysis [15]. The bacterial suspension of S. mutans ATCC 25175 (1 × 109 CFU/ml) was prepared in sodium phosphate buffer (50 mM, pH 7.1) and treated with 8 × MIC of 4-epi-pimaric acid (64 μg/ml) at 37°C for 60 min. Following incubation, 50-μl aliquots were transferred to fluorescence-activated cell sorting (FACS) tubes containing sodium phosphate buffer (50 mM, pH 7.1). These tubes were stored on ice, and stained with propidium iodide (10 μg/ml) for 10 min. The cells were subjected to FACS analysis on a flow cytometer (BD-LSR; Becton Dickinson, Biosciences, CA). The percentage of propidium iodide-stained cells was determined using Cell Quest Pro software (Becton Dickinson).
Leakage of 260 and 280 nm absorbing material
The release of 260- and 280-nm absorbing material from cell suspension was determined via a spectrophotometrical method [13]. Cell suspensions of S. mutans were prepared as for propidium iodide uptake assay. 4-epi-Pimaric acid was added at 64 μg/ml (8 × MIC) in 1 ml of the above bacterial suspension (≈1 × 109 CFU/ml). After incubation at 37°C for 60 and 90 min, cell samples were withdrawn and filtered through a 0.22-μm filter (Millipore) and the absorbance of the filtrate estimated at 260 and 280 nm using a spectrophotometer. Background leakage rate from cells (without 4-epi-pimaric acid) was negligible. The total 260- and 280-nm absorbing material released from cell suspensions was measured after incubation in 100 μg/ml of lysozyme (Sigma) at 37°C for 60 and 90 min, followed by sonication. The extent of leakage of 260- and 280-nm material was expressed as a percentage of values measured in cell filtrate from cell suspensions treated with cell-lytic enzymes.
Anti-inflammatory activity
The estimation of intracellular tumor necrosis factor alpha (TNF-α) expression in a gated population of neutrophils was determined according to a method described previously [15]. Neutrophils were isolated from human blood and transferred to FACS tubes (Becton Dickinson). Lipopolysaccharide (LPS) derived from Escherichia coli (Sigma) was added at a concentration of 10 μg/ml to stimulate the cells. 4-epi-Pimaric acid was added at a concentration of 1, 5 and 10 μg/ml and incubated for 3 h at 37°C. Controls included unstimulated cells (naıve control) and LPS-stimulated cells (LPS control). Further processing was done by the addition of FACS permeabilizing solution (Becton Dickinson), followed by the addition of phycoerythrin (PE)-labeled anti-human TNF-α (Becton Dickinson). After incubation in the dark, samples were washed, resuspended in PBS (pH 7.4) and acquired directly on the flow cytometer (BD LSR). A fluorescence trigger was set on the PE (FL1) parameter of the gated neutrophil populations (10,000 events). Rolipram (100 μg/ml) as standard inhibitor of TNF-α was used as internal control in this study. Fluorescence compensation, data analysis, and data presentation were performed using Cell Quest Pro software (Becton Dickinson).
Cytotoxicity assay
4-epi-pimaric acid was evaluated for cytotoxic effect on the murine cell line (AML-12; ATCC) using the MTT assay, in a 96-well-plate format [32]. Cells were incubated in the presence of 4-epi-pimaric acid (1–100 μg/ml) in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal calf serum (FCS) for 24 h at 37°C in a CO2 incubator. After the completion of incubation 3-(4, 5-dimethylthiazol- 2-yl)-2, 5-diphenyltetrazolium bromide (MTT) was added and cells were further incubated for 3 h at 37°C in a CO2 incubator. Formation of formazan salt by mitochondrial dehydrogenases was determined by an ELISA reader at 565 nm. The percentage cytotoxicity was calculated with respect to the untreated cells.
Statistical analysis
The values were calculated as the mean of individual experiments in triplicate and compared with those of the control groups. Differences between two mean values were calculated by Student’s t-test. A one-way analysis of variance (ANOVA) was performed for comparison of multiple means followed by post Bonferroni test. The chosen level of significance for all statistical tests was P <0.05.
Results
Identification of the compound
The phytomolecule isolated from A. cachemirica after crystallization afforded a colourless crystalline compound, which in the MS spectrum (LCMS) showed a molecular ion peak at m/z (M+–1) 301, compatible with the molecular formula C20H30O2; it exhibited laevorotation [α]D = −110˚ (C 0.75 CHCl3) and in the IR spectrum, showed an IR band at 1692 cm-1, characteristic for –CO (COOH), besides other bands at 3345, 2951, 2826, 1451 and 918 cm-1. In the 1H NMR spectrum, the signals for H–14, H–15, H–16a and H–16b olefinic protons were observed at δ 5.15 (brs), 5.72 (1 H, dd, J = 16.80, 10.77 Hz), 4.92 (1 H, dd, J = 17.01, 1.82 Hz) and 4.94 (1 H, dd, J = 10.54, 1.79 Hz), respectively. Signals for H–17, H–18 and H–20 methyls were observed as singlets at δ 1.00, 1.26 and 0.65, respectively. In 13C NMR, signals for carbons were observed at δ 39.19 (C–1), 19.56 (C–2), 38.56 (C–3), 44.02 (C–4), 56.07 (C–5), 24.09 (C–6), 36.40 (C–7), 137.94 (C–8), 50.50 (C–9), 39.19 (C–10), 19.21 (C–11), 35.77 (C–12), 37.94 (C–13), 127.96 (C–14), 147.17 (C–15), 112.67 (C–16), 29.33 (C–17), 29.10 (C–18) 184.13 (C–19) and 13.79 (C–20), respectively. On the basis of the above data, the compound was identified as 4-epi-pimaric acid (Fig. 1), which is in full agreement with the spectral data reported in the literature for 4-epi-pimaric acid [33], one of the four naturally occurring diastereoisomers of pimaric acid [34].
MIC and MBC of 4-epi-pimaric acid
The antimicrobial activity of 4-epi-pimaric acid (isolated through activity guided fractionation of chloroform extract) against oral cavity pathogens is shown in Table 1. The compound exhibited potent antibacterial activity, with MIC ranging from 4 to 16 μg/ml against all the tested isolates. The broad spectrum antibacterial activity of 4-epi-pimaric acid included Gram-positive cariogenic and noncariogenic bacteria, such as S. mutans and A. viscosus, as well as Gram negative anaerobic periodontal and supragingival pathogens, such as P. gingivalis, and P. intermedia. The MBC of the 4-epi-pimaric acid was two to four times higher than the MIC values, indicating its bactericidal effect against all tested oral cavity pathogens. The antibacterial activity of 4-epi-pimaric acid was also tested on the clinical isolates and its activity (MICs and MBCs) was found in a similar range with the ATCC strain (data not shown).
Post antibacterial effects (PAEs)
Keeping in mind the potential use of 4-epi-pimaric acid as an oral care agent where the contact time of the agent is limited to a few minutes, the PAE method was slightly modified, and the bacterial cells were exposed to 4-epi-pimaric acid for 5 min only. At MIC concentration, 4-epi-pimaric acid exhibited a PAE of 2.5 ± 0.1 h for S. mutans, whereas at 2 × MIC and 4 × MIC, the PAE was 3.5 ± 0.2 and 5.3 ± 0.1 h for S. mutans, respectively, which is notably long for such a brief exposure.
Biofilm susceptibility
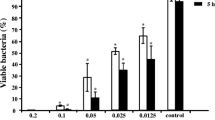
To determine the anti-biofilm activity of the 4-epi-pimaric acid, its effect on biofilm formation of S. mutans on saliva-coated flat bottom 96-well microtiter plates was observed. The effect of 4-epi-pimaric acid was tested at 4, 12, 20 and 24 h to visualize whether it could adversely affect S. mutans biofilms in each phase of biofilm growth: 4 h, adherent phase; 12 h, active accumulated phase; 20 h, initial plateau accumulated phase; and 24 h, plateau accumulated phase [35]. At 4-h exposure, the sub MIC concentration of 4-epi-pimaric acid exhibited an insignificant inhibitory effect on the growth of biofilm as the percentage of adherent cells was similar to untreated control (P > 0.05). But at 12 h, the percentage of adherent cells in the 4-epi-pimaric acid treated group was 77% less than that in the control group (P < 0.05) (Fig. 3). The adherent cells were further reduced at 20 and 24 h of S. mutans biofilm growth. Thus, biofilm formation was inhibited by 4-epi-pimaric acid during the active accumulated phase, initial plateau accumulated phase and plateau accumulated phase.
Effect of 4-epi-pimaric acid on the biofilm at sub MIC concentration (4 μg/ml) and at varied phases of growth of S. mutans ATCC 25175. 4-epi-Pimaric acid significantly inhibited bioifilm formation at varied growth phases (12, 20 and 24 h) of S. mutans compared to control (P < 0.05). Data represent the mean ± SD of three independent experiments with triplicate for each. *P < 0.05 (Student’s t test)
Confocal laser scanning microscopy of biofilm
S. mutans biofilm formation on a saliva-coated glass cover slip in the absence and presence of 4-epi-pimaric acid was performed to examine its effect on biofilm architecture by confocal laser scanning microscopy. Figure 4 shows the biofilm formation of S. mutans in the absence (Fig. 4a) and in the presence (Fig. 4b) of 4-epi-pimaric acid (4 μg/ml). The cells appeared red against the black background due to staining of DNA by propidium iodide. Bacterial cells not exposed to 4-epi-pimaric acid showed aggregates, whereas the aggregates formation was less in the presence of 4 μg/ml of 4-epi-pimaric acid.
Antimicrobial activity against adherent S. mutans in biofilms on glass surface
The adherence of the cells to the glass surface was evident in the form of a pellicle at the interface of the liquid and glass surface when S. mutans ATCC 25175 was grown in BHI broth containing 2% sucrose (wt/vol) for 24 h. This assay was used to determine the effect of 4-epi-pimaric acid on the preformed biofilms with brief exposure of 5 min. Change in the OD610 and pH of 4-epi-pimaric acid-treated biofilms was compared with the untreated control (Figs. 5 and 6). The biofilms generated by S. mutans ATCC 25175, treated with 4-epi-pimaric acid at 64 μg/ml grew slowly, and S. mutans growth in biofilms was significantly inhibited (Fig. 5) (P < 0.05). There was also significant reduction in the S. mutans biofilms (59%) attached to glass surface in the presence of 4-epi-pimaric acid when compared to untreated control (P < 0.05). The pH of the untreated control decreased rapidly from pH 6.98 to pH 4.6 after 9 h of incubation, in presence of sucrose (Fig. 6). Brief exposure of S. mutans biofilm to 4-epi-pimaric acid at 64 μg/ml significantly prevented the acidification of the broth and maintained pH to 6.61 till 12 h (P < 0.05).
Effect of 4-epi-pimaric acid on the decrease in pH of broth in adherent cells of S. mutans ATCC 25175 in the presence of 2% sucrose. The biofilms of S. mutans was exposed to 4-epi-pimaric acid for 5 min and then fresh medium was added. The pH of broth was measured with a pH meter at different time intervals. 4-epi-Pimaric acid significantly prevented the pH drop of broth containing 2% sucrose till 12 h when compared to untreated control (P < 0.05). Data represent the mean and standard deviations (±SD) of three different experiments performed in triplicate. *P < 0.05 (Student’s t test)
Inhibitory effect of 4-epi-pimaric acid on S. mutans biofilm on glass surface in the presence of sucrose. The biofilm of S. mutans was exposed to 4-epi-pimaric acid for 5 min and then fresh medium was added. The OD at 610 nm of samples was taken at different time intervals. 4-epi-Pimaric acid significantly prevented the growth of S. mutans in adherent cells when compared to untreated control (P < 0.05). Values are mean (±SD) from three independent determinations. *P < 0.05 (Student’s t test)
Effects of 4-epi-pimaric acid on membrane integrity
The effects of 4-epi-pimaric acid on the microbial viability, propidium iodide uptake and release of intracellular material were measured for S. mutans ATCC 25175. Sixty and 90 minutes exposure of 4-epi-pimaric acid at 64 μg/ml resulted in 61% and 51% decrease in the cell viability of S. mutans, respectively, with respect to the untreated cells (P < 0.05) (Fig. 7). The flowcytometric analysis of the treated cells revealed enhanced uptake of propidium iodide 30% and 35% in 60 and 90 min, respectively, for S. mutans (Fig. 7). The amount of 260- and 280-nm absorbing material in S. mutans cell filtrate (relative to the total released upon complete cell lysis) was 20% and 15%, respectively, in 60 min exposure of 4-epi-pimaric acid, which was significantly higher than the untreated control (P < 0.05) (Fig. 8). Further increase in exposure time to 90 min did not result in significant increase in the percentage of 260- and 280-nm absorbing material in S. mutans (24% and 18%, respectively).
Effect of 4-epi-pimaric acid (64 μg/ml) on cell viability and uptake of propidium iodide in S. mutans ATCC 25175 cells. 4-epi-Pimaric acid significantly reduced the cell viability and also increased uptake in S. mutans cells (P < 0.05). Values are means (± standard errors) from three independent determinations. *P < 0.05 (Student’s t test)
Effect of 4-epi-pimaric acid on the leakage of 260- and 280-nm absorbing material in S. mutans ATCC 25175 cells. Control group (treated with lytic enzymes and considered as 100% leakage) and treated with 4-epi-pimaric acid at 64 μg/ml for 90 and 120 min. No compound added served as untreated control. Percent leakage of 260- and 280-nm absorbing materials was significantly higher in S. mutans cells treated with 4-epi-pimaric acid when compared to untreated control (P < 0.05). Data represent the mean and standard deviations (±SD) of three different experiments performed in triplicate. *P < 0.05 (Student’s t test)
Anti-inflammatory activity
The anti-inflammatory activity of 4-epi-pimaric acid was determined by the estimation of the percentage of TNF-α in a gated population of neutrophils. In order to rule out the cytotoxic effects, 4-epi-pimaric acid was tested up to a maximum concentration of 10 μg/ml, which did not demonstrate any cytotoxicity in the MTT [3-(4,5-dimethylthiazol- 2-yl) 2,5-diphenyl tetrazolium bromide] assay (data not shown). In naive cells TNF-α was expressed in 1.4% of the gated population of neutrophils, which was increased twofold (approximately) in the LPS-stimulated cells. There was inhibition in TNF-α expression when these LPS-stimulated cells were exposed to 4-epi-pimaric acid at graded concentrations of 1, 5, and 10 μg/ml. All the tested concentrations of 4-epi-pimaric acid reduced the TNF-α expression level to below the naıve and the rolipram-inhibited control levels (Fig. 9).
Effect of 4-epi-pimaric acid on intracellular TNF-α expression. The graph shows the percentage of TNF-α-expressing cells in the gated population of neutrophils. TNF-α-expressing cells are labelled with PE-anti-human TNF-α antibody. Naıve control basal level of TNF-α expression, LPS control TNF-α expression after LPS stimulation. Values are means (± standard errors) from three independent determinations. *P < 0.001 (Student’s t test)
Cytotoxicity assay
The cytotoxic effect of 4-epi-pimaric acid was studied on human foreskin fibroblast (HFF) cells by MTT assay. The compound exhibited no cytotoxic effect (IC50 >100 μg/ml). The ratio of IC50 value to MIC provided an acceptable safety index >10.
Discussion
4-epi-Pimaric acid isolated from methanolic extract of aerial parts of A. cachemirica was tested for its antibacterial effect against oral cavity pathogens. It was found to be bactericidal against all the cariogenic as well as periodontal pathogens tested in this study. It also exhibited an impressive increase in the PAEs even at brief exposure of 4-epi-pimaric acid against S. mutans ATCC 25175.
The genus Aralia has been subjected to numerous chemical and pharmacological studies due to its use in traditional medicine to treat gastritis, rheumatic arthritis, inflammation, nephritis and diabetes mellitus [23, 36]. The essential oil of A. cachemirica is rich in monoterpenes and oxygenated monoterpenes [23]. A series of pimarane type diterpenoids has been isolated from Aralia cordata, exhibiting anti-inflammatory activity by the inhibition of COX-1 activity [37]. In our preliminary study, 4-epi-pimaric acid was found to possess the antibacterial activity against S. aureus [24] and recently Porto et al. [25] have shown the antibacterial activity of 4-epi-pimaric acid against a few oral cavity pathogens. Taking clue from our earlier study, we performed a detailed anti-bacterial activity investigation of 4-epi-pimaric acid including its biofilm inhibitory potential and anti-microbial mode of action.
S. mutans resides in oral cavity and depends on a “biofilm life-style” for survival and persistence in its natural ecosystem, dental plaque [38]. Biofilm formation by S. mutans grown in BHI media usually shows three accumulation phases: (i) the adherent phase (0–4 h), (ii) the active accumulated phase (4–20 h), and (iii) the slow or plateau accumulated phase (after 20 h) [35]. In this study, we categorized S. mutans biofilm formation into four accumulation phases: 4 h, adherent phase; 12 h, active accumulated phase; 20 h, beginning plateau accumulated phase and 24 h, plateau accumulated phase. It was observed that 4-h exposure of 4-epi-pimaric acid did not inhibit primary adherence of cells to the polystyrene plates, thereby reflecting its inhibitory role in biofilm development rather than primary attachment to the surface. The cells showed reduced adherence after 12, 20 and 24 h, implying that the 4-epi-pimaric acid is inhibitory at the active accumulation and plateau phases (Fig. 3). These stages of accumulation are marked by the active synthesis of glucan from sucrose via catalytic activity of glucosyltransferases (GTFs; EC 2.4.1.5), which are streptococcal extracellular and intracellular enzymes of 140 to 175 KDa involved in hydrolyzing sucrose to its fructose and sucrose moieties, and then polymerizes the resulting glucose moieties into glucans [39]. This is further supported by an ongoing study in our laboratory which revealed that 4-epi-pimaric acid inhibited the activity of cell-associated GTFs and extracellular GTFs isolated from S. mutans (unpublished data). These findings indicate that 4-epi-pimaric acid inhibits glucan synthesis. Further, we are evaluating its specific role in the inhibition of different GTFs (GTF B, GTF C and GTF D) of S. mutans. The biofilm formation was maximally inhibited by 4-epi-pimaric acid at plateau phase and hence was preferred for microscopic evaluation. The CLSM images depict the architecture of cells in a biofilm. The cells in the untreated control were embedded in a polysaccharide matrix that stimulates cell clustering. Undoubtedly, the biofilm architecture was disturbed in the presence of sub MIC concentration of 4-epi-pimaric acid (4 μg/ml) and reduced the aggregates of S. mutans (Fig. 4). The observation that the formation of S. mutans biofilm can be significantly reduced by 4-epi-pimaric acid at sub-inhibitory concentrations is of great significance, considering the fact that most of the antibiotics such as tetracycline, amoxicillin and clindamycin require 500- to 1000-fold greater concentrations to inhibit or kill bacteria in biofilm than those required to inhibit or kill planktonically grown strains [40]. Another important finding reported in this study was that the 4-epi-pimaric acid effectively prevented the acidification of the suspension and also inhibited S. mutans growth in biofilms, after a brief exposure (5 min), thus indicating its use in oral application.
In order to determine the impact of 4-epi-pimaric acid on the cytoplasmic membrane of S. mutans ATCC 25175, we used bacterial viability, propidium iodide uptake assay and leakage of 260- and 280-nm absorbing material. Cell viability counts revealed the extent to which treated cells were able to survive and reproduce to form colonies when removed from the presence of 4-epi-pimaric acid and re-cultured in a nutrient medium. The flowcytometric analysis of the treated cells revealed enhanced uptake of propidium iodide (Fig. 7) and leakage of 260- and 280-nm absorbing material from these cells (Fig. 8). The amount of 260- and 280-nm absorbing material in S. mutans cell filtrate (relative to the total release upon complete lysis of the control cells) was less extensive than the propidium iodide uptake. This difference may be attributed to the large size of the macromolecular cytosolic constituents. Thus the results of cell viability, propidium iodide uptake and the leakage of 260- and 280-nm absorbing material assays, suggested that 4-epi-pimaric acid probably works through the permeabilization of microbial membrane. This antimicrobial mode of action of 4-epi-pimaric acid is similar to that of other broad-spectrum, membrane-active disinfectants, such as tea tree oil [13], bakuchiol [17], chlorhexidine, hexachlorophene, phenethyl alcohol, tetracyclines, polymyxin, α-pinene, lemongrass oil and terpene alcohols (farnesol, nerolidol and plaunotol) [41].
Pro-inflammatory responses resulting from de novo or increased cytokine synthesis are a frequent outcome of streptococcal interactions with a range of host cells. These responses can significantly influence the progression of disease by affecting a number of processes including immune cell infiltration, tissue inflammation, and tissue damage [38]. The potent anti-inflammatory activity of 4-epi-pimaric acid was evident by its potential to inhibit the expression of the proinflammatory cytokine TNF-α. Earlier reported studies have demonstrated that 4-epi-pimaric acid isolated from the methylene chloride fraction of the root of Aralia cordata inhibited COX-1 activity [36] and also inhibited induction of inflammatory mediators by blocking NF-κB activation and mitogen-activated protein kinase pathways [42], thereby indicating its anti-inflamatory potential. This add-on activity of 4-epi-pimaric acid will be of significant importance in suppressing the inflammatory processes that occur in the tissues surrounding the teeth in response to bacterial accumulations (dental plaque) on the teeth.
In summary, this study presented a convincing antibacterial, anti-biofilm and anti-inflammatory activity of 4-epi-pimaric acid isolated from A. cachemirica. The reported folklore use of this plant supported by our data on the cytotoxicity profile of 4-epi-pimaric acid warrants in vivo efficacy studies, aiming at its potential use as oral care agent against dental caries and other oral cavity diseases.
References
Islam B, Khan SN, Khan AU (2007) Dental caries: from infection to prevention. Med Sci Monit 13:196–203
Nakano K, Nomura R, Nemoto H, Mukai T, Yoshioka H, Shudo Y, Hata H, Toda K, Taniguchi K, Amano A, Ooshima T (2007) Detection of novel serotype k Streptococcus mutans in infective endocarditis patients. J Med Microbiol 56:1413–1415
Li X, Kolltveit KM, Tronstad L, Olsen I (2000) Systemic diseases caused by oral infection. Clin Microbiol Rev 13:547–558
Kolenbrander PE (2000) Oral microbial communities: biofilms, interactions, and genetic systems. Annu Rev Microbiol 54:413–437
Hamada S, Slade HD (1980) Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev 44:331–384
Kuramitsu HK (1993) Virulence factors of mutans streptococci: role of molecular genetics. Crit Rev Oral Biol Med 4:159–176
Loesche WJ (1986) Role of Streptococcus mutans in human dental decay. Microbiol Rev 50:353–380
Gibbons RJ, Qureshi JV (1976) Microbial aspects of dental caries. In: Washington, DC: information retrieval. Al-Hashimi, I. Levine, M.J (1989) Characterization of in vivo salivary-derived enamel pellicle. Arch Oral Biol 34:289–295
Mogi M, Hiraoka BY, Harada M (1986) Analysis and identification of human parotid salivary proteins by micro two-dimensional electrophoresis and Western blot techniques. Arch Oral Biol 31:337–339
Busscher HJ, Cowan MM, Van der Mei HC (1992) On the relative importance of specific and non-specific approaches to oral microbial adhesion. FEMS Microbiol Rev 8:199–209
Wang X, Yao X, Zhu Z, Tang T, Dai K, Sadovskaya I, Flahaut S, Jabbouri S (2009) Effect of berberine on Staphylococcus epidermidis biofilm formation. Int J Antimicrob Agents 34:60–66
Koo H, Jeon JG (2009) Naturally occurring molecules as alternative therapeutic agents against cariogenic biofilms. Adv Dent Res 21:63–68
Cox SD, Mann CM, Markham JL, Bell HC, Gustafson JE, Warmington JR, Wyllie SG (2000) The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tree oil). J Appl Microbiol 88:170–175
Morgan TD, Beezer AE, Mitchell JC, Bunch AW (2001) A microcalorimetric comparison of the anti-Streptococcus mutans efficacy of plant extracts and antimicrobial agent in oral hygiene formulations. J Appl Microbiol 90:53–58
Sharma S, Khan IA, Ali I, Ali F, Kumar M, Kumar A, Johri RK, Abdullah ST, Bani S, Pandey A, Suri KA, Gupta BD, Satti NK, Dutt P, Qazi GN (2009) Evaluation of the antimicrobial, antioxidant, and anti-inflammatory activities of hydroxychavicol for its potential use as an oral care agent. Antimicrob Agents Chemother 53:216–222
Slots J, Rams TE (1990) Antibiotics in periodontal therapy: advantages and disadvantages. Oral Microbiol Immunol 17:479–493
Reddy MV, Thota N, Sangwan PL, Malhotra P, Ali F, Khan IA, Chimni SS, Koul S (2010) Novel bisstyryl derivatives of bakuchiol: targeting oral cavity pathogens. Eur J Med Chem 45:3125–3134
Frodin DG, Govaerts R (2003) World checklist and bibliography of Araliaceae. Kew, Royal Botanic Gardens, p 444
Wen J (2004) Systematics and biogeography of Aralia L. sect. Dimorphanthus (Miq.) Miq. (Araliaceae). Cathaya 15:1–187
Bown D (1995) Encyclopaedia of herbs and their uses. Dorling Kindersley, London
Weiner MA (1980) Earth medicine, earth Food. Ballantine, New York
Bhat ZA, Ansari SH, Mukhtar HM, Naved T, Khan NA, Chashoo IA (2005) Anti hyperglycemic activity of the alcoholic extract of Aralia cachemirica Decne roots. J Nat Rem 5:160–164
Verma RS, Padalia RC, Yadav A, Chauhan A (2010) Essential oil composition of Aralia cachemirica from Uttarakhand, India. Rec Nat Prod 4:163–166
Sangwan PL, Koul S, Khan IA, Raja AF, Qazi GN (2008) Antibacterial constituent of Aralia cachemiric. Proceedings of Asian Symposium on Medicinal Plants, Spices and other Natural Products (ASOMPS) XIII, Abstract 15, p 107, IICT. Hyderabad, India
Porto TS, Rangel R, Furtado NAJC, de Carvalho TC, Martins CHG, Veneziani RCS, Costa FBD, Vinholis AHC, Cunha WR, Heleno VCG, Ambrosio SR (2009) Pimarane-type diterpenes: antimicrobial activity against oral pathogens. Molecules 14:191–199
Clinical and Laboratory Standards Institute (CLSI) (2007) Methods for antimicrobial susceptibility testing of anaerobic bacteria, 7th edn. CLSI document M11-A7 (ISBN 1–56238–626–3). Clinical and Laboratory Standards Institute, Wayne, Pennsylvania USA
Eliopoulus GM, Moellering RCJ (1996) Antimicrobial combinations. In: Lorian V (ed) Antibiotics in laboratory medicine, 4th edn. The Williams & Wilkins Co., Baltimore, MD, pp 330–396
Craig WA, Gudmundsson S (1996) Postantibiotic effect. In: Lorian V (ed) Antibiotics in laboratory medicine, 4th edn. Williams and Wilkins Co, Baltimore, MD, pp 296–299
Shellis RP, Addy M, Rees GD (2005) In vitro studies on the effect of sodium tripolyphosphate on the interactions of stain and salivary protein with hydroxyapatite. J Dent 33:313–324
Wei GX, Campagna AN, Bokek LA (2006) Effect of MUC7 peptides on the growth of bacteria and on Streptococcus mutans biofilm. J Antimicrob Chemother 57:1100–1109
Islam B, Khan SN, Haque I, Alam M, Mushfiq M, Khan AU (2008) Novel anti-adherence activity of mulberry leaves: inhibition of Streptococcus mutans biofilm by 1-deoxynojirimycin isolated from Morus alba. J Antimicrob Chemother 62:751–757
Dorsey WC, Tchounwou PB, Sutton D (2004) Mitogenic and cytotoxic effects of pentachlorophenol to AML 12 mouse hepatocytes. Int J Environ Res Public Health 1:100–105
Wenkert E, Afonso A, Beak P, Carney RWJ, Jeffs P, McChesney W (1965) The proton magnetic resonance spectral characteristics of tricyclic diterpenic substances. J Org Chem 30:713–722
Sy L-K, Brown GD (1998) Abietane Diterpenes from Illicium angustisepalum. J Nat Prod 61:907–912
Li YH, Hanna MN, Svensater G, Ellen PR, Cvitkovitch DG (2001) Cell density modulates acid adaptation in Streptococcus mutans: implication for survival in biofilm. J Bacteriol 183:6875–6884
Chung YS, Choi YH, Lee SJ, Choi SA, Lee JH, Kim H, Hong EK (2005) Water extract of Aralia elata prevents cataractogenesis in-vitro and in-vivo. J Ethnopharmacol 101:49–54
Dang NH, Zhang X, Zheng M, Son KH, Chang HW, Kim HP, Bae K, Kang SS (2005) Inhibitory constituents against cyclooxygenases from Aralia cordata Thunb. Arch Pharm Res 28:28–33
Marsh PD (2000) Oral ecology and its impact on oral microbial diversity. In: Kuramitsu HK, Ellen RP (eds) Oral bacterial ecology: the molecular basis. Horizon Scientific Press, Bymondham, Norfolk, United Kingdom, pp 11–65
Nobbs AH, Lamont RJ, Jenkinson HF (2009) Streptococcus adherence and colonization. Microbiol Mol Biol Rev 73:407–450
Sedlacek MJ, Walker C (2007) Antibiotic resistance in an in vitro subgingival biofilm model. Oral Microbiol Immunol 22:333–339
Carson CF, Mee BJ, Riley TV (2002) Mechanism of action of Melaleuca alternifolia (Tea Tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob Agents Chemother 46:1914–1920
Kang O-H, Chae H-S, Choi J-G, Oh Y-C, Lee Y-S, Kim J-H, Seung M-J, Jang H-J, Bae K-H, Lee J-H, Shin D-W, Kwon DY (2008) ent-pimara-8(14), 15-dien-19-oic acid isolated from the roots of Aralia cordata inhibits induction of inflammatory mediators by blocking NF-κB activation and mitogen-activated protein kinase pathways. Eur J Pharmacol 601:179–185
Acknowledgments
We gratefully acknowledge the Corps Dental Hospital, Jammu, India for providing clinical samples. This work was supported by a grant from Colgate-Palmolive Company, New Jersey, USA, under the grant no. SSP0415.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ali, F., Sangwan, P.L., Koul, S. et al. 4-epi-Pimaric acid: a phytomolecule as a potent antibacterial and anti-biofilm agent for oral cavity pathogens. Eur J Clin Microbiol Infect Dis 31, 149–159 (2012). https://doi.org/10.1007/s10096-011-1287-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-011-1287-x