Abstract
The purpose of this study was to evaluate the diagnostic accuracy and prognostic value of neutrophil CD64 expression for bacterial infection in febrile adult patients presenting to our hospital emergency department. We prospectively included 132 patients with fever ≥38ºC (≥100.4ºF) during the last 24 hours and we measured CD64 expression on neutrophils the day after admission at the emergency department. We followed the patients until full recovery or death. There were 115 (87%) patients with bacterial infection and 108 (94%) of them survived. There were 17 (13%) patients without bacterial infection and 12 (71%) of them survived. Patients with bacterial infection and patients who survived showed a CD64 index higher when compared with patients without bacterial infection and patients who died, respectively (3.7 ± 3.2 vs. 2.5 ± 2.3; p = 0.03; and 3.7 ± 3.1 vs. 1.7 ± 0.6; p = 0.002; Mann-Whitney U test). The receiver operating characteristic (ROC) curve analysis for detecting bacterial infection and predicting survival with the CD64 index showed an area under curve (AUC) of 0.66 (95% CI, 0.52–0.8; p = 0.03) and 0.71 (95% CI, 0.57–0.85; p = 0.01), respectively. Diagnostic accuracy and prognostic value of CD64 expression was good in adult patients with fever.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacterial infections are a major cause of morbidity and mortality in the world [1]. Diagnosis of bacterial infections is sometimes challenging, because clinical presentation of infections from different causative agents can be similar [2]. Moreover, inflammatory states might also have a clinical presentation similar to that for an infection [3]. The treatment of bacterial infections has evolved in the past with improved antibiotics but little has changed to improve diagnosis [4]. Although untreated bacterial infections may cause serious complications, treating viral illnesses or no infective causes of inflammation with antibiotics is not only ineffective, but also contributes to the development of resistance, increases costs, and add the risks of toxicity and allergic reactions [5]. Thus, an improved diagnostic test for bacterial infection would have both economic and therapeutic healthcare benefits [6].
Laboratory diagnosis of infection still relies on diagnostic tests that have been available since the 1970s and earlier, such as neutrophil counts, the presence of myeloid-immature forms in the peripheral blood, and C-reactive protein (CRP) levels [7, 8]. More recently, the new-generation and improved diagnostics for infection have been directed towards soluble biomarkers in the serum or plasma, such as TNFα, IL-6, IL-1α, IL-10, and procalcitonin [6, 9, 10]. One step forward is the measurement of CD64 expression on the membrane of the neutrophils, because it offers the potential to be the first clinically-useful diagnostic cell-based parameter of a systemic acute inflammatory response [6, 11]. Neutrophils play an important role as primary phagocytes. The surface receptors of neutrophils recognize bacterial antigens and this interaction activates the neutrophils to phagocyte. Phagocytosis is facilitated by various receptors for immunoglobulin-G (IgG) and neutrophils can express three classes of IgG receptors. Fcγ receptor I (FcγRI), which is the high affinity receptor for monomeric IgG1 and IgG3, is recognized by the monoclonal antibody CD64 [5].
We were interested in this new test and we performed a systematic review and meta-analysis of neutrophil CD64 expression of bacterial infection [12]. The pooled sensitivity and specificity for CD64 expression on neutrophils were 79% (95% CI, 70–86%) and 91% (95% CI, 85–95%), respectively. To gain more knowledge with the use of this test, we designed a prospective study aimed to evaluate the diagnostic accuracy and the prognostic value of neutrophil CD64 expression for bacterial infection in febrile adult patients presenting to our hospital emergency department.
Materials and methods
We conducted this prospective study at the emergency department of the University Hospital Joan XXIII, located in Tarragona, Spain. We started the study in October 2009 and finished in February 2010. We wrote a protocol before we undertook this study; the ethical committee of our hospital approved the protocol study and all enrolled patients gave a written informed consent. We included patients if they were 17 years old or older, if they self-reported to have fever ≥ 38ºC (≥100.4ºF) at least during the last 24 hours and if the attending physician considered it necessary to order a complete blood count and a blood culture. We excluded patients if they had taken antibiotics during the last 48 hours and if they were admitted at the emergency room during the weekend. We collected data including sex, age, co-morbidity with the Charlson index [13], and the suspected origin of the fever. We also collected clinical and laboratory data: body temperature, heart and breath frequencies, blood pressure, oxygen saturation, complete blood count, liver enzymes, creatinine, prothrombin time, C-reactive protein, and arterial blood gasometry.
We performed blood cultures for each included patient at the time of admission and before starting antibiotic therapy. We placed 5 mL of blood in aerobic and anaerobic blood culture bottles and performed the cultures with the VersaTREK system (TREK Diagnostic Systems, Cleveland, OH). We identified positive results with standard methods.
We measured neutrophil CD64 expression with a diagnostic kit (Leuko64™; Trillium Diagnostic, Brewer, ME, USA), following manufacturer’s instructions. Briefly, we mixed 50 μL of EDTA-anticoagulated whole blood with 50 μL of Reagent A and 20 μL of Reagent C-II of the Leuko64™ kit, and we incubated this mixture for ten minutes. We measured the CD64 expression on neutrophils with the CELL-DYN Sapphire™ haematology analyzer (Abbott Diagnostics, Santa Clara, CA, USA) because we used a variant version of the Leuko64™ kit that was specifically designed for use on this haematology analyzer [5]. Additionally, we used the lot-specific Leuko64™ QuantiCALC automated software (Trillium Diagnostic) that reports neutrophil expression of CD64 as an index using fluorescein-labeled calibration beads. We used the blood sample collected to perform the complete blood count and we maintained this sample at 4°C until we did the measurement of CD64 on neutrophils the day after the admission of the patient at the emergency department.
One author (GGP) collected demographic, clinical and laboratory data of the patients and determined the presence or absence of infection as well as the suspected origin of the fever. This author followed the patients and assigned a final clinical bacterial diagnosis based on clinical, laboratory and radiologic data. When the patient had a positive culture, the author assigned a final proven bacterial diagnosis. He also followed the patients until full recovery or death. However, he didn’t know the results of the CD64 measurements. Two authors (RA and RS) performed the CD64 measurements and the blood cultures, respectively. They didn’t know the final diagnosis of the patients. Two authors (JC and AL) designed the study, analyzed data and reported the results to the rest of authors at the end of the study period.
We compared quantitative variables between four groups of patients using the non-parametric Kruskal-Wallis test. We compared qualitative and quantitative variables between two groups of patients using the non-parametric chi-squared and the Mann-Whitney U tests, respectively. We considered a p value less than 0.05 as statistically significant. We performed the statistical analysis using the Statistical Package for the Social Sciences (SPSS) version 15.0 (SPSS, Chicago, IL, USA). We also constructed a receiver operating characteristic (ROC) curve and we calculated the point of maximum test efficiency with GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA). This software calculates the sensitivity and specificity for the assay. It also calculates the area under curve (AUC) and its 95% CI and reports a p value that tests the null hypothesis that the AUC really equals 0.50. When the p value is small, one can conclude that the test actually does discriminate between abnormal patients and normal controls.
Results
Our series comprised 132 patients. Figure 1 shows the flow diagram of our study and Table 1 shows the demographic and clinical characteristics of the study population. We performed a blood culture in all patients and 13 (9.8%) of them were positive. We also performed cultures from other locations in 36 patients (27.3%) and 35 (97.2%) of them were positive. The source of these positive bacterial cultures was urine (19 cases), skin (4 cases), sputum (3 cases), stool (3 cases), abscess (2 cases), and cerebrospinal fluid, ascites, pleural effusion and bone marrow aspirate (1 case each). One patient had a positive blood culture and a positive stool culture. We did a diagnosis of clinical and proven bacterial infection in 67 (50.8%) cases and 48 (36.3%) cases, respectively. There were 17 patients (13%) without bacterial infection who suffered from small bowel obstruction (5 cases), systemic lupus erythematosus (2 cases), heart failure (2 cases), pleural effusion (2 cases), lymphoma (2 cases), renal cell carcinoma (2 cases), prostate cancer (1 case), and pleural mesothelioma (1 case). Table 2 shows the demographic and clinical characteristics of the patients according to the final diagnosis. Of note, patients with a final diagnosis of bacterial infection showed a lower systolic blood pressure, and higher leukocyte and neutrophil counts when compared with patients without bacterial infection. Table 3 shows the final diagnosis of the patients with bacterial infection. After the follow-up, 120 patients (91%) recovered and 12 patients (9%) died. Table 4 shows the demographic and clinical characteristics of the patients according to survival. Of note, patients who died showed a higher Charlson index, heart frequency, urea levels, and lower haemoglobin value when compared with patients who survived.
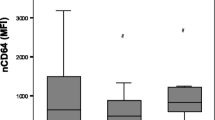
The values of the CD64 index in the different groups of patients are shown in Table 5. Overall, patients with bacterial infection showed a mean CD64 index higher when compared with patients who did not suffer a bacterial infection (3.7 ± 3.2 vs. 2.5 ± 2.3; p = 0.03; Mann-Whitney U test). The ROC curve analysis for detecting bacterial infection with the CD64 index showed an AUC of 0.66 (95% CI, 0.52–0.8; p = 0.03; Fig. 2a). Patients who survived showed a CD64 index higher when compared with patients who died (3.7 ± 3.1 vs. 1.7 ± 0.6; p = 0.002; Mann-Whitney U test). The ROC curve for predicting survival with the CD64 index showed an AUC of 0.71 (95% CI, 0.57–0.85; p = 0.01; Fig. 2b). The ROC curve analysis for detecting bacterial infection and survival with CRP showed an AUC of 0.63 (95% CI, 0.51–0.75; p = 0.09; Fig. 2c) and 0.51 (95% CI, 0.35–0.67; p = 0.9; Fig. 2d), respectively. According to the ROC curve analysis, the cut-off points with the best sensitivity and specificity for CD64 index and CRP were 1.5 and 4.2 mg/dL, respectively, and Table 6 shows their operative characteristics.
Discussion
We investigated the diagnostic accuracy of neutrophil CD64 expression for bacterial infection in febrile adult patients presenting to our hospital emergency department. According to our results, a CD64 index ≥ 1.5 had a good sensitivity (87%) but a low specificity (41%) to detect bacterial infection in adult patients who presented to the emergency department with fever. We also found that the expression of neutrophil CD64 was elevated in the group of patients who survived when compared with the group of patients who died.
The low specificity of the CD64 index form detecting bacterial infection in the present study could be explained by different reasons. First, 115 (87%) patients of our series had a bacterial infection but only 48 (42%) of them had bacterial growth in any cultures performed. In the meta-analysis performed by our group, the diagnostic accuracy of the CD64 assay increased when bacterial infection was diagnosed by a positive culture [12]. Second, we couldn’t exclude viral infection in the group of patients with clinical infection and negative cultures. Third, 35 patients with positive cultures but negative blood cultures had a CD64 index similar to patients with positive blood cultures. Interestingly, to increase the specificity of the CD64 expression on neutrophils, some authors have proposed to combine it with other infectious markers, such as CD64 expression on monocytes [14] and lipopolysaccharide-binding protein [15].
There is strong evidence that neutrophil CD64 expression represents a promising screening test for infection because of its kinetics and laboratory characteristics [5]. Regarding the kinetics of this marker, in resting neutrophils, CD64 is expressed at very low levels; upon neutrophil activation it is strongly upregulated by the proinflammatory cytokines interferon (IFN)-γ and granulocyte colony stimulating factor (G-CSF), which are produced during infections or exposure to endotoxin [16]. CD64 expression on PMN significantly increased from baseline by 24 h after initiating in vivo IFN treatment, substantially decreased within 48 h of cessation of IFN and were back to normal baseline levels by day seven [16]. Regarding the laboratory characteristics, PMN CD64 has negligible expression on the healthy individual and shows no evidence of sensitivity to blood sample manipulation [17]. Some authors have reported that PMN CD64 expression is stable at room temperature for more than 30 h, in contrast to the labile expression of CD11b and other PMN antigens [18, 19]. Moreover, other authors have measured CD64 expression at baseline, and after 24 h, 48 h, and 72 h and they did not observe significant differences in CD64 expression in stored blood samples at 4°C as compared to baseline values [20].
However, our group performed a systematic review and meta-analysis about the use of neutrophil CD64 as a marker for bacterial infection and we found that the included studies showed a low methodological quality [12]. We measured the methodological quality with the 25-item criteria developed by the STARD committee [21, 22]. Accordingly, one study can score from 0 to 25 points, and in our previous meta-analysis, we showed that the 13 included studies had a total score for the STARD checklist that ranged from 9 to 16 points. Moreover, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group developed a two-step process of how the accuracy of a test indirectly changes patient-important outcomes [23, 24]. In the first step, investigators should perform a diagnostic test accuracy study. In the second step, judgements about patient-importance of test accuracy are based on the consequences of being correctly or incorrectly classified as having or not having the disease.
For all these reasons, we decided to perform the current study in order to analyze the diagnostic accuracy of neutrophil CD64 expression for bacterial infection in “real life”, i.e. in febrile adult patients presenting to our hospital emergency department. To do that, first, we decided to measure the neutrophil CD64 expression in adult patients with fever because this is the group of patients to whom clinicians would apply the test in the course of regular clinical practice [23]. Moreover, we decided to exclude children because we wanted to focus on adult patients to have a homogeneous group of patients and to avoid a possible source of variation. Second, we did not use healthy people as controls because it is well known that the apparent accuracy of a test is likely to be misleadingly high [25, 26].
Following the two-step process of the (GRADE) Working Group of how the accuracy of a test indirectly changes patient-important outcomes, we also detected that a high expression of CD64 was related to survival of the patients. To our knowledge, there are only two previous studies that analyzed the prognostic value of CD64. Livaditi et al. showed that neutrophil CD64 expression and serum IL-8 were sensitivity early markers of severity and 28-day mortality in patients with sepsis [27]. In that study, the median (range) CD64 values for patients who died compared with those who survived were respectively 7,396 (3,863–9,947) and 4,123 (2,206–6,571) molecules/cell (p = 0.004). In contrast, Danikas et al. found that patients with severe sepsis and higher levels of neutrophil CD64 expression had better outcome [28]. Interestingly, other authors found that neutrophil CD64 expression was associated with severity and prognosis of disseminated intravascular coagulation [29]. In the present study, we found that patients who survived showed a higher CD64 index when compared with patients who died. We recommend that future studies analyze this important outcome because the CD64 expression could be used as a prognostic factor.
The main limitation of our study is the number of patients included. However, the majority of previous studies with adult patients enrolled around 100 subjects, and we only know two studies that enrolled more adult patients than this study [30, 31]. However, we recommend performing more studies with a high number of patients to obtain more powerful data and making strong recommendations with the use of CD64 in clinical practice.
Finally, we used the Leuko64™ kit (Trillium Diagnostics) to measure the CD64 expression on neutrophils. This kit allows rapid and precise, quantitative measurement of CD64 expression on neutrophils. The kit contains a mixture of FITC-labeled CD64 (clone 22 and 32.2) and PE-labeled monocyte specific CD163 antibodies, fluorescent beads for calibration and standardization and dedicated software for automated calculation of the neutrophil CD64 index. This assay requires only 50 μL of EDTA-anticoagulated whole blood and can be completed within 30 minutes. Moreover, we used the version of the kit that was specifically designed for use on the CELL-DYN Sapphire™ haematology analyzer (Abbott Diagnostics). The test is almost fully automated, the results are available rapidly and the assay can be performed on a 24/7 basis without specific expertise in flow cytometry [5].
In conclusion, the present study showed that the diagnostic accuracy of neutrophil CD64 expression in febrile adult patients presenting to our hospital emergency department had a good sensitivity but a low specificity to detect bacterial infection and we also showed that higher expression of CD64 was related to survival of the patients.
References
Lever A, Mackenzie I (2007) Sepsis: definition, epidemiology, and diagnosis. BMJ 335:879–883
Martin GS, Mannino DM, Eaton S, Moss M (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546–1554
Ng PC, Lam HS (2006) Diagnostic markers for neonatal sepsis. Curr Opin Pediatr 18:125–131
Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J (2004) Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis 39:206–217
Hoffmann JJ (2009) Neutrophil CD64: a diagnostic marker for infection and sepsis. Clin Chem Lab Med 47:903–916
Davis BH (2005) Improved diagnostic approaches to infection/sepsis detection. Expert Rev Mol Diagn 5:193–207
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 31:1250–1256
Calandra T, Cohen J (2005) The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med 33:1538–1548
Sarmati L, Beltrame A, Dori L, Maffongelli G, Cudillo L, De AG, Picardi A, Ottaviani L, Cefalo MG, Venditti A, Amadori S, Arcese W, Andreoni M (2010) Procalcitonin is a reliable marker of severe systemic infection in neutropenic haematological patients with mucositis. Am J Hematol 85:380–383
Zeitoun AA, Gad SS, Attia FM, bu Maziad AS, Bell EF (2010) Evaluation of neutrophilic CD64, interleukin 10 and procalcitonin as diagnostic markers of early- and late-onset neonatal sepsis. Scand J Infect Dis 42:299–305
Icardi M, Erickson Y, Kilborn S, Stewart B, Grief B, Scharnweber G (2009) CD64 index provides simple and predictive testing for detection and monitoring of sepsis and bacterial infection in hospital patients. J Clin Microbiol 47:3914–3919
Cid J, Aguinaco R, Sanchez R, Garcia-Pardo G, Llorente A (2010) Neutrophil CD64 expression as marker of bacterial infection: a systematic review and meta-analysis. J Infect 60:313–319
Charlson M, Szatrowski TP, Peterson J, Gold J (1994) Validation of a combined comorbidity index. J Clin Epidemiol 47:1245–1251
Nuutila J, Hohenthal U, Laitinen I, Kotilainen P, Rajamaki A, Nikoskelainen J, Lilius EM (2007) Simultaneous quantitative analysis of FcgammaRI (CD64) expression on neutrophils and monocytes: a new, improved way to detect infections. J Immunol Methods 328:189–200
Groselj-Grenc M, Ihan A, Pavcnik-Arnol M, Kopitar AN, Gmeiner-Stopar T, Derganc M (2009) Neutrophil and monocyte CD64 indexes, lipopolysaccharide-binding protein, procalcitonin and C-reactive protein in sepsis of critically ill neonates and children. Intensive Care Med 35:1950–1958
Schiff DE, Rae J, Martin TR, Davis BH, Curnutte JT (1997) Increased phagocyte Fc gammaRI expression and improved Fc gamma-receptor-mediated phagocytosis after in vivo recombinant human interferon-gamma treatment of normal human subjects. Blood 90:3187–3194
Davis BH, Bigelow NC, Curnutte JT, Ornvold K (1995) Neutrophil CD64 expression: potential diagnostic indicator of acute inflammation and therapeutic monitor of interferon-gamma therapy. Lab Hematol 1:3–12
Briggs C, Kunka S, Fujimoto H, Hamaguchi Y, Davis BH, Machin SJ (2003) Evaluation of immature granulocyte counts by the XE-IG master: upgraded software for the XE-2100 automated hematology analyzer. Lab Hematol 9:117–124
Sauer M, Tiede K, Fuchs D, Gruhn B, Berger D, Zintl F (2003) Procalcitonin, C-reactive protein, and endotoxin after bone marrow transplantation: identification of children at high risk of morbidity and mortality from sepsis. Bone Marrow Transplant 31:1137–1142
Tillinger W, Jilch R, Jilma B, Brunner H, Koeller U, Lichtenberger C, Waldhor T, Reinisch W (2009) Expression of the high-affinity IgG receptor FcRI (CD64) in patients with inflammatory bowel disease: a new biomarker for gastroenterologic diagnostics. Am J Gastroenterol 104:102–109
Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Moher D, Rennie D, de Vet HC, Lijmer JG (2003) The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Clin Chem 49:7–18
Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC (2003) Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Standards for Reporting of Diagnostic Accuracy. Clin Chem 49:1–6
Schunemann HJ, Oxman AD, Brozek J, Glasziou P, Bossuyt P, Chang S, Muti P, Jaeschke R, Guyatt GH (2008) GRADE: assessing the quality of evidence for diagnostic recommendations. Evid Based Med 13:162–163
Schunemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, Williams JW Jr, Kunz R, Craig J, Montori VM, Bossuyt P, Guyatt GH (2008) Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 336:1106–1110
Rutjes AW, Reitsma JB, Di Nisio M, Smidt N, van Rijn JC, Bossuyt PM (2006) Evidence of bias and variation in diagnostic accuracy studies. CMAJ 174:469–476
Lijmer JG, Mol BW, Heisterkamp S, Bonsel GJ, Prins MH, van der Meulen JH, Bossuyt PM (1999) Empirical evidence of design-related bias in studies of diagnostic tests. JAMA 282:1061–1066
Livaditi O, Kotanidou A, Psarra A, Dimopoulou I, Sotiropoulou C, Augustatou K, Papasteriades C, Armaganidis A, Roussos C, Orfanos SE, Douzinas EE (2006) Neutrophil CD64 expression and serum IL-8: sensitive early markers of severity and outcome in sepsis. Cytokine 36:283–290
Danikas DD, Karakantza M, Theodorou GL, Sakellaropoulos GC, Gogos CA (2008) Prognostic value of phagocytic activity of neutrophils and monocytes in sepsis. Correlation to CD64 and CD14 antigen expression. Clin Exp Immunol 154:87–97
Song SH, Kim HK, Park MH, Cho HI (2008) Neutrophil CD64 expression is associated with severity and prognosis of disseminated intravascular coagulation. Thromb Res 121:499–507
Matsui T, Ohsumi K, Ozawa N, Shimada K, Sumitomo S, Shimane K, Kawakami M, Nakayama H, Sugii S, Ozawa Y, Tohma S (2006) CD64 on neutrophils is a sensitive and specific marker for detection of infection in patients with rheumatoid arthritis. J Rheumatol 33:2416–2424
Tanaka S, Nishino J, Matsui T, Komiya A, Nishimura K, Tohma S (2009) Neutrophil CD64 expression in the diagnosis of local musculoskeletal infection and the impact of antibiotics. J Bone Joint Surg Br 91:1237–1242
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cid, J., García-Pardo, G., Aguinaco, R. et al. Neutrophil CD64: diagnostic accuracy and prognostic value in patients presenting to the emergency department. Eur J Clin Microbiol Infect Dis 30, 845–852 (2011). https://doi.org/10.1007/s10096-011-1164-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-011-1164-7