Abstract
The CD64 receptor has been described as an interesting bacterial infection biomarker. Its expression has not been studied in previously healthy children admitted to pediatric critical care unit (PICU). Our objective was firstly to describe the CD64 expression and secondly study its diagnostic accuracy to discriminate bacterial versus viral infection in this children. We made a prospective double-blind observational study (March 2016–February 2018). A flow cytometry (FC) was done from peripheral blood at PICU admission. We studied the percentage of CD64+ neutrophils and the CD64 mean fluorescence intensity (MFI) on neutrophils (nCD64) and monocytes (mCD64). Statistical analyses were performed with non-parametric tests (p < 0.05). Twenty children in the bacterial infection group (BIG) and 25 in the viral infection group (VIG). Children in BIG showed higher values of CD64+ neutrophils (p = 0.000), nCD64 (p = 0.001), and mCD64 (p = 0.003). In addition, CD64+ neutrophils and nCD64 expression have positive correlation with procalcitonin and C reactive protein. The nCD64 area under the curve (AUC) was 0.83 (p = 0.000). The %CD64+ neutrophils showed an AUC of 0.828 (p = 0.000). The mCD64 AUC was 0.83 (p = 0.003). The nCD64 and %CD64+ neutrophils also showed higher combined values of sensitivity (74%) and specificity (90%) than all classical biomarkers.In our series CD64 expression allows to discriminate between bacterial and viral infection at PICU admission. Future studies should confirm this and be focused in the study of CD64 correlation with clinical data and its utility as an evolution biomarker in critical care children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infectious diseases are a main cause of admission in pediatric intensive care unit (PICU). Bacterial and viral infections are the principal causes in healthy children. Added to the supportive therapies used in PICU, to start a correct and prompt antimicrobial treatment is critical in order to anticipate complications and minimize morbidity [1]. The inexistence of accurate and precocious biomarkers of viral or bacterial infection forces the physician to initiate broad spectrum therapies that maybe are not indicated [8]. This, plus a not adequate de-escalation antibiotherapy politic, increases the probability of bacterial antibiotic resistance. Also, the PICU and hospital stance are prolonged with an impact in cost each admission [8, 13, 23].
Nowadays, in acute or critical context, early etiological recognition of infection remains a matter of concern. There is no doubt about how the etiological diagnosis of infection has been improved in recent years. The introduction of molecular diagnosis tools, such reactive polymerase chain reaction or rapid immunological test, has collaborated in this new status. But, gold standard, as the blood culture or other body fluid cultures, requires at least 24–48 h to offer its results. Also, negative cultures do not completely exclude the presence of suspected bacterial infection [8, 13]. Biomarkers that may facilitate early diagnosis and the assessment of therapeutic responses are in focus and widely explored. The evidence that support the use of C-reactive protein (CRP) or procalcitonin (PCT) has been complemented with recent investigations that show the utility and interest of new biomarkers [19, 26]. This new molecules have high sensibility and specificity and also inform about the inflammatory status, the presence of bacteraemia or the respond to the therapy initiated. In this paper we describe the utility of flow cytometry (FC) to study the expression of the high-affinity immunoglobulin-Fc fragment receptor I (FcγRI) CD64 on neutrophils and monocytes [5, 21, 23].
The flow cytometry is a laboratory technique that allows evaluating immune status of leukocyte populations. In spite of its advantages, it remains not routinely employed in clinical practice. Its use is generally restricted to circumstances in which determination of leukocyte populations is essential (e.g., diagnosis of leukemia or HIV). Flow cytometer is able to measure leukocyte populations instantaneously, facing a stream of cells to a laser and capturing the emergent light. Thus, both basic cellular features (size, complexity, etc.) and immune characteristics (immunophenotyping) are determined [5, 10]. Since it allows evaluating the immune response in a dynamic way, its use might provide unique clinical information. That would permit detecting immunological changes in real time, substantiating diagnostic suspicion, anticipating the evolution, and modifying therapeutic attitudes. Its use and interpretation, mainly in the context of inflammation (whether from an infectious etiology or not), it is a novel and interesting approach to these patients [25].
The CD64 is a type I high-affinity receptor for the Fc fraction of the immunoglobulin G, located on the surface of monocytes, macrophages, dendritic cells, and neutrophils. It induces phagocytosis, superoxide anion generation, and cytokine production in monocytes and macrophages. Its expression is upregulated by the presence in peripheral blood of gamma interferon (IFN-γ), released in infection conditions. Increasing of its density on surface is directly related to the intensity of stimulation received by inflammatory cytokines, quantified in cytometry as mean fluorescence intensity (MFI). Thus, different studies have shown an increase in the expression of CD64 as a response to bacterial infection (sepsis in newborns, preterm very low weight, pediatric population, and adults) [27]. In case of viral infections CD64 expression has been described as lower that in bacterial diseases [5, 13, 18, 21].
The aim of this study was to describe the CD64 expression in healthy children requiring PICU admission because of an infectious disease. For this purpose, the percentage of CD64-positive cells and the mean fluorescence intensity (IFM) of CD-64 on monocytes (mCD64) and neutrophils (nCD64) were determined. Afterward, the possible differences in CD64 expression between viral or bacterial infection were analyzed in order to compare them and describe the diagnostic accuracy to discriminate viral versus bacterial infection at PICU admission.
Material and methods
Prospective double-blind observational study was conducted in a single pediatric institution (Hospital Infantil Universitario Niño Jesús) (HIUNJ) after Ethics Committee for clinical research approval. Children admitted to the PICU because of infectious disease from March 2016 to February 2018 were consecutively included. One peripheral blood sample was extracted after parents or legal guardians consent at admission in PICU. Always a previously established intravenous line was used when present. The volume obtained was 0.5 ml per sample and collected in sterile EDTA tube. The sample handling was based on item 59 of the Spanish law on Biomedical Research. Study was designed to not influence the treatment of the participating patients.
Sample processing and analysis by flow cytometry
Samples were collected in sterile EDTA at room temperature or refrigerated at 4 °C, used for CD45+ cells marking, and analyzed by flow cytometry in a time period shorter than 24 h. CD64 surface expression was measured by BD FACS Canto II flow cytometer (Becton Dickinson, New York, USA). It was measured in monocytes (mCD64) and neutrophils (nCD64) staining a blood sample with a CD64 antibody from Biolegend®, San Diego (clone 10.1). Cell viability was confirmed by 7-AAD staining. At least 10,000 events were recorded for each sample. The flow cytometer settings and samples were prepared according to the manufacturer’s instructions. Neutrophils, monocytes, and lymphocytes were identified on dot-plot profile and gated (Fig. 1 and Fig. S1). The intensity of CD64 surface expression was measured as mean fluorescence intensity (MFI) in arbitrary units. The positive CD64 cells were expressed as percentage. Results were blinded for clinicians. The experimental team did not know any clinical data from the children included. The cost for each experiment per patient was 7€.
Inclusion criteria and collected data
All children with infectious disease as main cause of PICU admission were recruited. Demographic, neutrophil percentage, monocyte percentage, lymphocyte percentage, CRP at admission, PCT at admission, CD64 expression at admission, and final etiological diagnosis were collected. No previously healthy children were excluded. Later also were excluded: (1) no etiological diagnosis or children with a confirmed coinfection (viral and bacterial) or the presence of positive cultures considered as contaminations (Staphylococcus epidermidis positivity from a culture not obtained at admission and/or from a venous access later instituted), (2) primary or secondary immunodeficiency, (3) refusal to participate, (4) children with suspected bacterial or viral infection but no microbiological confirmation, and (5) one or more antibiotherapy doses to PICU admission. Neutrophil count, PCT, and CRP were obtained from the same blood sample used to determine CD64 by FC. In addition, neutrophil count, PCT, and CRP were considered as references to compare the diagnosis accuracy of CD64.
Definitions for a microbiologically confirmed case of bacterial infection included (1) isolation of an organism by culture from blood, urine in a patient with clinical symptoms and signs of urinary tract infection or pyelonephritis, needle aspiration of abscess or empyema, stool sample of a patient with symptoms of gastroenteritis, pus sample from deep wound infection, or detection of Streptococcus pneumoniae antigen from the pleural effusion sample of a patient with pneumonia.
The diagnosis of a microbiologically confirmed viral infection required (1) detection of IgM antibodies or a fourfold increase in IgG antibodies in serum samples, (2) viral antigen from nasopharyngeal aspirate, or (3) viral nucleic acids by polymerase chain reaction of a nasopharyngeal aspirate, biological fluid, or blood.
The data obtained from CD64 expression on leukocytes were (1) % of CD64+ neutrophils: percentage of neutrophils with CD64 expression on surface, (2) nCD64: mean fluorescence intensity of CD64 on neutrophils, and (3) mCD64: mean fluorescence intensity of CD64 on monocytes.
Statistical study
Statistical analysis was performed with the statistical program SPSS version 19.0 (IBM®). The quantitative values are expressed as mean and standard deviation. The Kolmogorov-Smirnov test was applied to establish the goodness of fit to normality for the variables studied. To compare quantitative variables between the bacterial and viral groups, a Mann-Whitney U test was used. Spearman’s rank correlation coefficient was bi-marginally calculated to measure the relationship between two continuous variables. A receiver operating characteristic (ROC) analysis with area under curve (AUC), sensitivity, and specificity and cutoff values was performed for each biomarker, and their diagnostic accuracy for PBI or antibiotherapy was calculated. The cutoff values were calculated by Youden index. Findings of two-tailed p < 0.05 were considered statistically significant.
Results
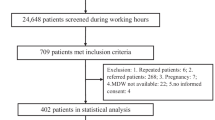
One-hundred and forty-five children were recruited. After discard by exclusion criteria, 54 children were classified as viral or bacterial infection. Later, 9/54 were excluded because of the existence of an immunodeficiency, mainly secondary due to oncological therapies (Fig. 1). Finally, there was 20 children in the bacterial infection group (BIG; 2 urinary tract infection, 12 non-respiratory infection, 2 central nervous system infection, and 6 respiratory tract infection) and 25 in the viral infection group (VIG; 22 respiratory tract infection and 3 central nervous system infection). There were 20 females and 25 males. The children in the VIG were younger than the IBG (mean 590 days ± 431 versus 1592 days ± 225, p = 0.002). It was the only difference observed in epidemiological data between groups. About the classical biomarkers, all of them were higher in the IBG (Table 1 and see in Fig. S1 the differences observed in the FC dot-plot charts).
CD64 expression was higher in bacterial infections and correlates with classical infection biomarkers
Children with BIG showed higher values of % CD64+ neutrophils (p = 0.000), nCD64 (p = 0.001), and mCD64 (p = 0.003) (see Table 1). The nCD64, % of neutrophils CD64+ and mCD64 showed positive correlation with PCT (p = 0.001, p = 0.002, p = 0.04). Also, nCD64 and mCD64 showed positive correlation with CRP (p = 0.02 and p = 0.04; see Table 2). No differences were observed between gender. The CD64 expression was not correlated with age or days of prior to admission in PICU.
ROC analyses: CD64 expression is useful as a bacterial infection biomarker
To study the usefulness of CD64 surface expression as a tool to predict bacterial infection, we evaluated the ROC curve (Fig. 2) for these data compared to the classical analytical data. As seen in Table 3, CRP showed the best AUC, been of 0.851 (p = 0.000; with a cut point of 3.5 mg/dl and 89% specificity and 71% sensitivity). All three AUC from CD64 were higher than the rest of biomarkers. The nCD64 AUC was 0.83 (p = 0.000 with a cut point of 4353.5 MFI and 90% specificity and 74% sensitivity). The % CD64+ neutrophils showed an AUC of 0.828 (p = 0.000 with a cut point of 99.5% MFI and 90% specificity and 74% sensitivity). Finally, the mCD64 AUC was 0.83 (p = 0.003 with a cut point of 1033 0MFI and 57% specificity and 95% sensitivity).
Receiver operating characteristic curve comparison between CD64 expression and classical biomarkers. PCT procalcitonin, CRP C reactive protein, % of CD64+ neutrophils percentage of neutrophils with CD64 expression on surface, nCD64 mean fluorescence intensity of CD64 on neutrophils, and mCD64 mean fluorescence intensity of CD64 on monocytes
Discussion
In the present paper, we describe for the first time the CD64 expression on monocytes and neutrophils in previously healthy children admitted to PICU because of an infectious disease. We observed that in bacterial infection cases the CD64 expression was higher than in case of viral infection. In addition, CD64 correlates with classical infection biomarkers. Finally, the CD64 expression on neutrophils and the percentage of CD64+ neutrophils have cutoff values with high specificity to diagnosis bacterial infection.
As it is known, several methods are used and studied to identify or anticipate bacterial infections. Improve the rational use of antibiotics is a worldwide priority and the interest of new methods of precocious bacterial diagnosis is rising. At PICU admission, the initiation of antibiotic is mainly influenced by clinical sings and analytical biomarkers [8, 13, 23]. Therefore, the study and introduction of new diagnostic techniques to improve antibiotherapy choices is of great interest. Neutrophil count, CRP, or PCT are the classical biomarkers [8, 23]. Individually, they do not possess high specificity or sensitivity and are generally more helpful when considered together [26].
The interest in CD64 as a bacterial infection biomarker has rose in recent years [3, 4, 8, 11, 19, 21, 22]. The nCD64 expression is considered a very early phase of a host’s immune response to bacterial infection. It is strongly upregulated within 4–6 h in case of infection, and it starts to increase 1 h after invasion. Currently, CD64 expression could be quickly and precisely measured by flow cytometric technology using minimal blood volumes. The main problem is the absence of optimal cutoff values and observational studies that confirm its utility [8]. About the flow cytometry, it is a laboratory technique based on marking leukocyte populations with monoclonal antibodies. This technique allows to evaluate in a dynamic way immune status and response of a patient both under basal and disease conditions. Its use results in a novel approach with great interest for these patients [4, 9, 24, 25]. Mainly in the context of an infection, its use and interpretation may provide unique information letting know immunological changes in real time [11]. These properties would help to substantiate diagnostic suspicion, anticipate the evolution, and modify therapeutic attitudes dynamically.
In our study, we demonstrated that CD64 expression on a blood sample at PICU admission was higher in case of bacterial infection (Table 1). In addition, we observed that this molecule on monocytes and neutrophils have positive correlation with classical biomarkers (Table 2). Both observations have not been previously described in pediatric PICU population [12, 15, 26]. As our group has seen in other infectious scenarios (severe acute bronchiolitis), the CD64 rise in parallel to the CRP and the PCT [10]. The CD64 expression adds data about the patient immunophenotypic status. It informs about a pro-phagocytosis status in case of bacterial infection [17]. Besides, we describe and know the immune response to the infection and to define if the patient has a normal response to it [6, 16, 27]. The CD64 has been also described as a highly sensitive marker of culture positive sepsis or bacteremia [7, 19, 20, 23]. All children in the BIG were microbiologically confirmed, so we were able to see that. The main issue about this is that we exclude all suspicion of bacterial infection, with no positive culture or other method, and also we did not consider those children with at least one antibiotic dose. To really confirm this data, we should analyze this population in future studies in order to know if CD64 really anticipate bacteremia.
About cutoff points, in the last years, two meta-analyses of observational studies have been published on the accuracy of nCD64 as an early diagnostic biomarker in bacterial infections. Pooled sensitivity of nCD64 for the diagnosis of early onset of bacterial infections ranged from 76% (95% CI 74–78) to 79% (95% CI 70–86), whereas pooled specificity was between 85% (95% CI 83–86) and 91% (95% CI 85–95). Importantly, both meta-analyses have results from adult, pediatric, and neonatal studies, which may explain their high level of heterogeneity [2, 14]. Our study has similar results than these papers. The AUC of nCD64 showed similar sensitivity and specificity that in previous publications. In addition, we add to this previous data the analysis of the percentage of positive neutrophils to CD64 and the increase in MFI of mCD64. Both parameters showed good performance in order to obtain bacterial infection diagnosis with an AUC superior to all the classical biomarkers analyzed except CRP (Table 3). This has not been previously described nor in children or adult population.
This study presents some limitations. It is done in two groups of infection: viral or bacterial. It will be of great interest to analyze CD64 performance in case of fungal infections. We choose to study these two groups because they are the main cause of infections in healthy children. Its realization in a single center, with a small number of patients and without strictly defined admission criteria, despite minimizing clinical management variability, may not display/show the different clinical approach of these patients in a reliable way. At the same time, it cannot be assumed that CD64 is the only bacterial infection biomarker. Also, the positive children may well represent the most severe or acute cases in the spectrum, and may not be representative of the performance of the test on children in general. Their values should be compared and added to traditional biomarkers in larger samples. This aspect could also be attenuated with the inclusion of other cell surface markers in further analysis. Finally, a single blood sample was obtained and serial samples could offer new interest data about its utility to modify or de-escalate antibiotic treatment.
In conclusion, the present study has demonstrated that the neutrophil and monocyte CD64 expression is higher in bacterial infection and could be useful as precocious biomarker. The simplification and extension of availability of this tool could improve the rational use of antibiotics. Based on these findings, it seems of interest to continue the study with other biomarkers and periodic determinations, providing dynamic and reliable information about the actual utility of the flow cytometry in these patients.
References
Boeddha NP, Schlapbach LJ, Driessen GJ, Herberg JA, Rivero-Calle I, Cebey-Lopez M, Klobassa DS et al (2018) Mortality and morbidity in community-acquired sepsis in European pediatric intensive care units: a prospective cohort study from the European childhood life-threatening infectious disease study (EUCLIDS). Crit Care 22:143
Cid J, Aguinaco R, Sanchez R, Garcia-Pardo G, Llorente A (2010) Neutrophil CD64 expression as marker of bacterial infection: a systematic review and meta-analysis. J Infect 60:313–319
Dai J, Jiang W, Min Z, Yang J, Tan Y, Ma T, Ge Z (2017) Neutrophil CD64 as a diagnostic marker for neonatal sepsis: meta-analysis. Adv Clin Exp Med 26:327–332
Daix T, Guerin E, Tavernier E, Mercier E, Gissot V, Herault O, Mira JP et al (2018) Multicentric standardized flow cytometry routine assessment of patients with Sepsis to predict clinical worsening. In: Chest
Davis BH, Olsen SH, Ahmad E, Bigelow NC (2006) Neutrophil CD64 is an improved indicator of infection or sepsis in emergency department patients. Arch Pathol Lab Med 130:654–661
de Jong E, de Lange DW, Beishuizen A, van de Ven PM, Girbes AR, Huisman A (2016) Neutrophil CD64 expression as a longitudinal biomarker for severe disease and acute infection in critically ill patients. Int J Lab Hematol 38:576–584
Efe Iris N, Yildirmak T, Gedik H, Simsek F, Aydin D, Demirel N, Yokus O (2017) Could neutrophil CD64 expression be used as a diagnostic parameter of bacteremia in patients with febrile neutropenia? Turk J Haematol 34:167–173
Fontela PS, O'Donnell S, Papenburg J (2018) Can biomarkers improve the rational use of antibiotics? Curr Opin Infect Dis 31:347–352
Garcia-Salido A, Melen G, Gomez-Pina V, Onoro-Otero G, Serrano-Gonzalez A, Casado-Flores J, Ramirez M (2018) Circulating soluble RAGE and cell surface RAGE on peripheral blood mononuclear cells in healthy children. J Pediatr Endocrinol Metab 31:649–654
Garcia-Salido A, Serrano-Gonzalez A, Casado-Flores J, Sierra-Colomina M, de Azagra-Garde AM, Garcia-Teresa MA, Melen GJ, Ramirez-Orellana M (2018) CD64 on monocytes and granulocytes in severe acute bronchiolitis: pilot study on its usefulness as a bacterial infection biomarker. J Leukoc Biol 103:965–971
Garcia-Salido A, Serrano-Gonzalez A, de Azagra-Garde AM, Nieto-Moro M, Melen GJ, Ramirez-Orellana M (2017) Flow cytometry analysis of CD64, CD18, CD11a and CD11b in four children with Bordetella pertussis infection and admitted to critical care: New biomarkers? Medicina intensiva
Jamsa J, Ala-Kokko T, Huotari V, Ohtonen P, Savolainen ER, Syrjala H (2018) Neutrophil CD64, C-reactive protein, and procalcitonin in the identification of sepsis in the ICU - post-test probabilities. J Crit Care 43:139–142
Larsen FF, Petersen JA (2017) Novel biomarkers for sepsis: a narrative review. Eur J Intern Med 45:46–50
Li S, Huang X, Chen Z, Zhong H, Peng Q, Deng Y, Qin X, Zhao J (2013) Neutrophil CD64 expression as a biomarker in the early diagnosis of bacterial infection: a meta-analysis. Int J Infect Dis 17:e12–e23
Liu Y, Hou JH, Li Q, Chen KJ, Wang SN, Wang JM (2016) Biomarkers for diagnosis of sepsis in patients with systemic inflammatory response syndrome: a systematic review and meta-analysis. SpringerPlus 5:2091
Muzlovic I, Ihan A, Stubljar D (2016) CD64 index on neutrophils can diagnose sepsis and predict 30-day survival in subjects after ventilator-associated pneumonia. J Infect Dev Countries 10:260–268
Nancy Hilda J, Das S (2018) Neutrophil CD64, TLR2 and TLR4 expression increases but phagocytic potential decreases during tuberculosis. Tuberculosis 111:135–142
Ng PC, Li K, Wong RP, Chui KM, Wong E, Fok TF (2002) Neutrophil CD64 expression: a sensitive diagnostic marker for late-onset nosocomial infection in very low birthweight infants. Pediatr Res 51:296–303
Rogina P, Stubljar D, Lejko Zupanc T, Ihan A, Skvarc M (2017) Neutrophil CD64 molecule expression can predict bloodstream infection in septic shock patients. Clin Chem Lab Med 55:e130–e132
Rudensky B, Sirota G, Erlichman M, Yinnon AM, Schlesinger Y (2008) Neutrophil CD64 expression as a diagnostic marker of bacterial infection in febrile children presenting to a hospital emergency department. Pediatr Emerg Care 24:745–748
Sack U (2017) CD64 expression by neutrophil granulocytes. Cytometry B Clin Cytom 92:189–191
Sheneef A, Mohamed T, Boraey NF, Mohammed MA (2017) Neutrophil CD11b, CD64 and Lipocalin-2: early diagnostic markers of neonatal Sepsis. Egypt J Immunol 24:29–36
Shores DR, Everett AD (2018) Children as biomarker orphans: Progress in the field of pediatric biomarkers. J Pediatr 193(14–20):e31
Umlauf VN, Dreschers S, Orlikowsky TW (2013) Flow cytometry in the detection of neonatal sepsis. Int J Pediatr 2013:763191
Venet F, Lepape A, Monneret G (2011) Clinical review: flow cytometry perspectives in the ICU - from diagnosis of infection to monitoring of injury-induced immune dysfunctions. Crit Care 15:231
Yang AP, Liu J, Yue LH, Wang HQ, Yang WJ, Yang GH (2016) Neutrophil CD64 combined with PCT, CRP and WBC improves the sensitivity for the early diagnosis of neonatal sepsis. Clin Chem Lab Med 54:345–351
Zinsly Sampaio Camargo T, Marra AR, Bacal NS, Casaroto E, Pinto LM, Pasternak J, Victor ED, Dos Santos OF, Edmond MB (2016) Evaluation of two methods for determination of CD64 as a diagnostic marker of infection in critically ill adults. Biomed Res Int 2016:6593232
Acknowledgments
We are grateful to all the medical doctors, nurses, and nurse assistants working in our PICU. We extend our appreciation to all the children who participated in this study, as well as their caregivers for their patience and understanding.
Funding
Funding was provided by “Fundación de Investigación Biomédico”, Hospital Infantil Universitario Niño Jesús.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Ethics Committee for clinical research, Hospital Infantil Universitario Niño Jesús.
Informed consent
The blood samples were extracted after parents or legal guardians consent at admission in pediatric critical care unit. Also, the children were recruited by this procedure.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Fig. S1
Three flow cytometry dot-plots representing CD64 expression on leukocytes surface. The leukocytes are gated by side scatter characteristics (SSC) and CD64 expression. The positive CD64 region is indicated by a striped arrow. A: healthy control, no expression on surface in neutrophils and lymphocytes. The lymphocytes do not express CD64 so they act as an internal negative control in each dot-plot chart. Monocytes are always positive to CD64 and its mean fluorescence intense (MFI) is 5556 and change in case of infection. B: children with viral infection. The lymphocytes are negative to CD64. The neutrophils has move to the right and 83% of them have CD64 expression on surface. The MFI of CD64 in neutrophils is 1800 and the MFI on monocytes is 8638. C: children with bacterial infection. The lymphocytes are negative to CD64. The CD64 expression on neutrophils is increased. Now 100% of neutrophils are CD64 positive and MFI is 9551. The CD64 MFI in monocytes is 15,556. (PNG 1396 kb)
Rights and permissions
About this article
Cite this article
García-Salido, A., de Azagra-Garde, A.M., García-Teresa, M.A. et al. Accuracy of CD64 expression on neutrophils and monocytes in bacterial infection diagnosis at pediatric intensive care admission. Eur J Clin Microbiol Infect Dis 38, 1079–1085 (2019). https://doi.org/10.1007/s10096-019-03497-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-019-03497-z