Abstract
Presented here are the results of a retrospective analysis of all mucormycoses infections recorded at a tertiary hospital in Greece during the last 10 years. A total of 24 patients were identified, 15 male and 9 female, with ages ranging from 37 to 80 years. Twelve of the patients had soft tissue infections (2 with concomitant pulmonary infections), and 12 had rhinocerebral infections. Transmission could be traced in two cases; to nitroglycerin patches in one patient and to a lemon-tree-thorn scratch in the other. Among the 17 patients who underwent surgery, 11 survived. All seven patients on whom surgery was not performed died. Rapid diagnosis and treatment of mucormycosis are essential for patient survival. The severity of the patient’s underlying condition, the degree of immunosuppression, and prompt surgical treatment are the most important factors contributing to the outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mucormycosis is a rare fungal infection caused by saprophytic, fungal hyphae of the order Mucorales, to which several genera belong: Rhizopus, Mucor, Rhizomucor, Absidia and Apophysomyces [1]. Even with early diagnosis and aggressive surgical and medical therapy, the mortality rate is high. For this reason, a high degree of suspicion is needed in order to initiate treatment promptly. In recent years, mucormycosis has been increasingly recognized in Greece. Thus, we analyzed all cases of this disease recorded at the infectious diseases department of a tertiary hospital in Athens during the last 10 years, and we reviewed the literature for all other cases reported from Greece.
Materials and Methods
From January 1993 to December 2002, a total of 24 cases of mucormycoses were recorded at the Infectious Diseases Department of “Laiko” General Hospital in Athens, Greece. The patients were either hospitalized in this hospital or consultation was sought at our department for patients hospitalized elsewhere, and a specimen was brought to our laboratory for microscopy and culture. The diagnosis of mucormycosis was confirmed by histopathology, revealing non-septate, thick-walled hyphae with right-angle branching. For this study, the medical records, laboratory data, radiographic images, and operative reports of every patient were examined retrospectively.
A literature review was then conducted to identify other cases of mucormycosis in Greece. We searched both Medline and the Greek MedNet using the key words “mucormycosis”, “zygomycosis”, “Mucor”, and “Rhizopus”. A total of 11 publications were identified; one in 1988, one in 1995 and nine from 1999 onwards. The characteristics of these cases were analyzed.
Results and Discussion
In our hospital, 24 cases of mucormycosis were recorded during the 10-year period studied. Fifteen of the patients were male and nine were female. Their ages ranged from 37 to 80 years, with a mean age of 59 years. Eight patients were infected by Rhizopus spp. and 16 by Mucor spp. Nine of the patients had an hematologic malignancy (6 acute myelogenous leukemia, 1 chronic lymphocytic leukemia, and 2 myelodysplastic syndrome). The patient with chronic leukemia had received corticosteroids for 6 months, while the other eight patients had experienced prolonged neutropenia (15–45 days). Of these nine patients, four had rhinocerebral mucormycosis (1 extending to the orbit), three had mucormycosis of the paranasal sinuses (1 with extension to the soft palate), and two had mucormycosis of the soft tissues. One patient had recently undergone kidney transplantation and was receiving cyclosporine and steroids; he developed mucormycosis of the soft tissues of the leg.
Seven patients had underlying diabetes. In two of these patients the condition was steroid-induced (1 had dermatomyositis, 1 had nephrotic syndrome, and both had metabolic acidosis). These two patients, as well as two other patients with diabetes, had rhinocerebral mucormycosis with extension to the orbit. A fifth patient with diabetes developed mucormycosis due to contaminated nitroglycerin patches. This patient had kept patches at home for a long period of time and, after he was admitted for treatment of a soft tissue infection, it was found that the patches had been contaminated by a Mucor sp. (confirmed by culture on Sabouraud agar). The infection started at the site where the patch was adhered and progressively extended to the pleura. A sixth patient with diabetes developed Mucor infection of decubitus ulcers. The seventh patient with diabetes had infection of the soft tissues of the left leg.
Three patients with no predisposing factors developed mucormycosis of the skin and soft tissues as a result of car accidents. One of these patients had extensive injury of the scalp; she presented with necrotic eschars covering wide areas of her head, which were initially attributed to bacterial infection. One other patient was a burn victim who developed soft tissue mucormycosis after reconstructive surgery. Another patient developed invasive mucormycosis in a pre-existing lung cavity, due to previous tuberculosis.
The final two patients also had no identified predisposing factors. The first one had mucormycosis of the abdominal soft tissues and, interestingly, had a brother who had been treated for mucormycosis a few months earlier; he was not included in this study, since he was hospitalized at another institution and complete records were not available. She spent a lot of time with her brother, and her infection developed on an abdominal scar resulting from a previous cholecystectomy. On questioning, she said she often had pruritus and scratched the scar. Presumably, this was the mode of transmission. The siblings had no underlying immune defects, such as chronic granulomatous disease. The second patient with no recognized predisposing factor reported that a lemon-tree thorn had pricked his arm at the site where mucormycosis subsequently developed.
In summary, 11 patients had rhinocerebral mucormycosis, 12 had soft tissue infections, and 1 had a lung infection. Necrotic eschars were the characteristic clinical findings in all patients, whether on the nasal mucosa, the palate or the skin. In one case of limb mucormycosis, the initial diagnosis was gangrene and in another one the eschar was thought to be a cancerous growth. The patients with orbital involvement presented with intense headache, periorbital edema on the affected side, retro-orbital pain, exophthalmus, and vision disturbances. Three of the cases progressed to ophthalmoplegia and blindness. In the case of lung mucormycosis beginning in a pre-existing cavity, the infection extended to the mediastinum and soft tissues of the neck. Initially, the patient presented with cough and later he developed fever and hemoptysis.
All patients received medical treatment and 13 of them also underwent surgical intervention. The primary medication used was liposomal amphotericin B (AmBisome; Gilead Sciences, USA) or conventional amphotericin B. The total dose of liposomal amphotericin B given ranged between 1.6 g and 30 g (from 3 to 5 mg/kg/day). The duration of administration ranged from 8 days to 3 months. The short periods of treatment reflect cases that were already at an advanced stage when the diagnosis was made; despite initiation of treatment, these patients deteriorated rapidly and died soon thereafter. A total of 13 patients died as a result of their infections. The characteristics of the 11 patients who recovered are presented in Table 1. It should be noted that all of the patients who survived underwent extensive surgical debridement of the infected tissues in addition to medical treatment. A total of 17 patients underwent surgery, and 11 of them survived. All seven patients who did not undergo surgery died.
In our review of the literature, we found eleven publications pertaining to cases of mucormycosis in Greece. The characteristics of these cases are summarized in Table 2. As can be seen, one was in 1988, one in 1995, and nine in the years following 1999. This increase in reported incidence can be attributed to the heightened awareness of mucormycosis as a disease entity in Greece in recent years. This may be due both to a heightened index of suspicion and, perhaps, to an actual increase in incidence. It should be noted, however, that while there is a trend to publish more in recent years, for academic reasons, the recent reports of mucormycosis in Greece might not reflect the true incidence of the disease, but rather a sample of it.
The major mode of disease transmission for the Zygomycetes is presumed to be via inhalation of spores from environmental sources. There have been outbreaks of rhinocerebral or pulmonary zygomycosis linked to excavation and construction [2]. The spores may enter through the nose, deposit on the nasal turbinates and extend into the paranasal sinuses and orbit, or they may be inhaled directly into the lungs. Once the organism becomes invasive, tissue necrosis develops by arterial occlusion [3]. The organism continues to spread by direct extension along the injured blood vessels. The histopathological picture is consequently a mixture of necrosis and varying amounts of neutrophilic infiltration. The hyphae may not be prominent and the pathologist should be apprised of the clinical diagnosis in order to use stains that will demonstrate the fungal elements.
Percutaneous modes of exposure are also very important. The usual mode of transmission is traumatic implantation of spores contained in dirt [4]. Such was the case in the three patients presented here who had mucormycosis after being involved in car accidents. There have also been several reports of skin infection by Rhizopus spp. occurring in surgical patients following exposure to contaminated surgical adhesives [5], but no other case of transmission by nitroglycerin patches was found in the literature. Needle-sticks have been implicated in zygomycotic infections occurring at the site of injection [6]. Although we found no cases of mucormycosis due to a lemon-tree-thorn scratch in our literature search, since the fungus is ubiquitous in nature, it may be that in the case of our patient the thorn produced the wound that was later infected by a Mucor sp.
Several medical conditions have been associated with mucormycosis, though only two major factors clearly predispose patients to this disease. The first factor is metabolic acidosis and the second a defect in either neutrophil or monocytic function. Metabolic acidosis interferes with the ability of transferrin to bind iron, thereby leading to high iron levels in tissue that enhance the growth of fungal organisms. Reduced neutrophil chemotaxis and adhesion to hyphae have been demonstrated in patients with diabetes mellitus [7]. In our series only two patients had metabolic acidosis. Hematological malignancies, especially leukemias, present a high risk for mucormycosis [3]. Aggressive chemotherapy, resulting in sustained neutropenia, in conjunction with prolonged use of broad-spectrum antibiotics and immunosuppressive therapy, have been shown to increase the risk of fungal infections [1]. The usual site of infection in leukemia patients is the lung, but no such case was found in our series. Transplantation is another factor leading to immunosuppression and increased fungal infections [8]. Exactly what role, if any, cyclosporine plays in the development of fungal infections in transplant recipients is unknown, but some studies have shown that it primarily inhibits lymphokine production and not macrophage function [9].
Invasive burn wound infections have historically been the primary cause of death in patients with burn injuries. Burn injury causes generalized, multifactorial immunosuppression, but the main factor predisposing individuals to mucormycosis is skin trauma. Fungus ball in a pre-existing cavity is a known entity and is more often due to Aspergillus spp. In immunocompetent patients it may have an indolent course for a long time. However, there have been reports of Mucor infections that started as fungus balls and, producing necrotizing tissue, extended to the adjacent pleura or to the mediastinum [10]. Tojima et al. [11] published a case of chronic pulmonary mucormycosis that developed in pre-existing cavities caused by tuberculosis in a patient with diabetes mellitus and liver cirrhosis.
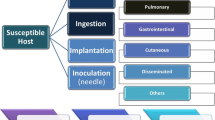
Although the vast majority of patients with mucormycosis have either an underlying disease or major trauma, there have been several reports of patients with no predisposing factors whatsoever who developed mucormycosis [12]. Mucormycosis can manifest as different clinical forms, namely rhinocerebral, cutaneous, pulmonary, disseminated, gastrointestinal, and other rare forms [3], but the black necrotic eschar is the hallmark of the disease. A similar clinical presentation can be the result of infection by Aspergillus spp., especially in patients with hematological malignancies.
Cutaneous and soft tissue disease may occur from primary inoculation or as a result of disseminated disease. Growth of the fungus in a pre-existing lesion may produce an acute inflammatory response with pus, abscess formation, tissue swelling, and necrosis. The lesions may appear red and indurated, but they often progress to form black eschars. Infections may be polymicrobial and are generally rapidly aggressive, even in the face of appropriate debridement and medical treatment [1]. Primary cutaneous mucormycosis may progress to necrotizing fasciitis. It extends to adjacent tissues, invading fat, muscle, and bone. Because it starts from the skin, it can be diagnosed at an early stage; however, the curative surgical intervention may be disfiguring or may involve amputation of the involved limb.
As a general rule, treatment of mucormycosis is multimodal. It includes the use of antifungal medications (either amphotericin B or its liposomal forms), surgical debridement, and medical management or correction of the underlying condition that is predisposing the patient to the disease. There have been reports of cure by medical treatment alone [13] in cases where surgical intervention is not possible or not preferable due to the site of the infection. There have also been reports of cure of mucormycosis by isolated surgical resection [14]. These cases, however, are the exception and survival rates are higher when all modalities are used [15]. In a review by Kontoyannis et al. [15], the survival rate was reported as 33% and favorable outcome in patients with hematologic malignancies seemed to correlate with lack of pulmonary involvement, surgical debridement, neutrophil recovery, and a cumulative total amphotericin B dose of 2000 mg. In our series, the survival rate in patients who underwent surgery was 65%, while the seven patients on whom surgery was not performed all died. It seems that the severity of the underlying condition, the degree of immunosuppression and surgical treatment were the most important factors contributing to the outcome.
References
Ribes JA, Vanover-Sams CL, Baker DJ (2000) Zygomycetes in human disease. Clin Microbiol Rev 13:236–301
Lueg EA, Ballagh RH, Forte V (1996) Analysis of the recent cluster of invasive fungal sinusitis at the Toronto Hospital for Sick Children. J Otolaryngol 25:366–370
Sugar AM (1992) Mucormycosis. Clin Infect Dis 14 (Suppl 1):126–129
Adam RD, Hunter G, DiTomasso J, Comerci G (1994) Mucormycosis: emerging prominence of cutaneous infections. Clin Infect Dis 19:67–76
Mead JH, Lupton GP, Dillavou CL, Odom RB (1979) Cutaneous Rhizopus infection: occurrence as a postoperative complication associated with an elasticized adhesive dressing. JAMA 242:272–274
Jain JK, Markowitz A, Khilanani PV (1978) Case report: localized mucormycosis following intramuscular corticosteroid, case report and review of the literature. Am J Med Sci 275:209–216
Chinn RY, Diamond RD (1982) Generation of chemotacticfactors by Rhizopus oryzae in the presence and absence of serum: relationship to hyphal damage mediated by human neutrophils and effects of hyperglycemia and ketoacidosis. Infect Immun 38:1123–1129
Fisher J, Tuazon CU, Geelhoed GW (1980) Mucormycosis in transplant patients. Am Surg 46:315–322
Thompson AW, Moon DK, Geczy CL, et al. (1983) Cyclosporin A inhibits lymphokine production but not the responses of macrophages to lymphokines. Immunology 48:291–299
Bigby TD, Serola ML, Tierny LM, Matthay MA (1986) Clinical spectrum of pulmonary mucormycosis. Chest 89:435–439
Tojima H, Tokudome T, Otsuka T (1997) Chronic pulmonary mucormycosis that developed in preexisting cavities caused by tuberculosis in a patient with diabetes mellitus and liver cirrhosis. Nihon Kyobu Shikkan Gakkai Zasshi 35:100–105
Radner AB,Witt MD, Edwards Jr JE (1995) Acute rhinocerebral zygomycosis in an otherwise healthy patient: case report and review. Clin Infect Dis 20:163–166
Ng TT, Campbell CK, Rothera M, Houghton JB, Hughes D, Denning DW (1994) Successful treatment of sinusitis caused by Cunninghamella bertholletiae. Clin Infect Dis 19:313–316
Tomford JW, Whittlesey D, Ellner JJ, Tomashefski Jr JF (1980) Invasive primary cutaneous phycomycosis in diabetic leg ulcers. Arch Surg 115:770–771
Kontoyannis DP, Wessel VC, Bodey GP, Rolston KV (2000) Zygomycosis in the 1990s in a tertiary-care cancer center. Clin Infect Dis 30:851–856
Kotzamanoglou K, Tzanakakis G, Michalopoulos E, Stathopoulou M (1988) Orbital cellulites due to mucormycosis. A case report. Graefes Arch Clin Exp Opthalmol 226:539–541
Economopoulou P, Laskaris G, Ferekidis E, Kanelis N (1995) Rhinocerebral mucormycosis with severe oral lesions: a case report. J Oral Maxillofac Surg 53:215–217
Athanasiou E, Barbanis S, Kostopoulos L, Kaloutsi V, Zois E, Ageloudi M, Malaka A, Papadimitriou C (1998) Opportunistic infections in immunosuppressed/immunodeficient patients: a histological and immunohistochemical study in three cases. Arch Hellenic Pathol 12:116–121
Kanellopoulou M, Velegraki A, Mylona E, Papaefstathiou K, Legakis N, Papafrangas E (1999) Cutaneous Rhizopus oryzae mucormycosis. Archi Hellenic Med 16:383–385
Papadogeorgakis N, Logothetis I, Marti-Zografou K, Rondogianni D, Foundas A, Chalevelakis G, Raptis SA (1999) Late surgical intervention in a diabetic patient with mucormycosis of the head and neck, after prolonged treatment with liposomal amphotericin B. Arch Hellenic Med 16:496–500
Tsaousis G, Koutsouri A, Gatsiou C, Paniara O, Peppas C, Chalevelakis G (2000) Liver and brain mucormycosis in a diabetic patient type II successfully treated with liposomal amphotericin B. Scand J Infect Dis 32:335–337
Tsoutsos D, Tsati E, Metaxotos N, Keramidas E, Rodopoulou S, Ioannovich J (2001) Extensive burn injury complicated by mucormycosis: a case report. Ann Burn Fire Disasters XIV(3)
Papadaki TH, Mastrodimos V, Tsilimbaris M, Pallikaris J (2001) Orbital mucormycosis Iatriki 79:74–78
Tryfon S, Stanopoulos I, Kakavelas E, Nikolaidou A, Kioumis I (2002) Rhinocerebral mucormycosis in a patient with latent diabetes mellitus: a case report. J Oral Maxillofac Surg 60:328–330
Kyrmizakis DE, Doxas PG, Hajiioannou JK, Papadakis CE (2002) Palate ulcer due to mucormycosis. J Laryngol Otol 116:146–147
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petrikkos, G., Skiada, A., Sambatakou, H. et al. Mucormycosis: Ten-Year Experience at a Tertiary-Care Center in Greece. Eur J Clin Microbiol Infect Dis 22, 753–756 (2003). https://doi.org/10.1007/s10096-003-1035-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-003-1035-y