Abstract
Mucormycosis is a perilous fungal infection that primarily affects individuals with compromised immune systems. The escalating incidence of conditions such as diabetes, cancer and the use of immunosuppressive drugs renders more individuals susceptible to contracting this disease. This report delves into the case of a 45-year-old woman from Tanzania with diabetes who succumbed to rhino-cerebral mucormycosis. Despite aggressive treatment, which often involves disfiguring surgical debridement and administration of antifungal drugs, the mortality rate remains high. Additionally, we present a comprehensive literature review of the various clinical aspects of Mucormycosis, an uncommon yet fatal condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mucormycosis is an uncommon severe fungal infection caused by fungi of the Mucorales order which are part of a group of fungi known as Mucormycetes. Mucorales are ubiquitous and can be found in diversified environments such as soil, decaying organic matter, compost, and contaminated food sources [27, 36]. While the most common causative agents in human infections are Rhizopus (Rhizopus oryzae), Mucor circinelloides, Mucor velutinosus, and Rhizomucor pusillus, other organisms of clinical relevance in this order include, Cunninghamella bertholletiae, Apophysomyces elegans, Lichtheimia (previously referred to as Absidia), Saksenaea vasiformis, Actinomucor elegans, and Syncephalastrum racemosum [6, 18, 27].
The actual incidence of mucormycosis remains uncertain; however, its mortality rate ranges from 31% and approaches 96% in the settings of disseminated disease [18, 28, 34]. The disease almost always occurs in immunocompromised patients, diabetes mellitus (DM) being one of the most common underlying medical comorbidities [5, 18, 22]. Establishing the diagnosis of this condition is often challenging and requires a high index of suspicion, many a time necessitating invasive diagnostic investigations [32]. Here, we present a case of fatal mucormycosis involving the orbital and sinonasal regions in a patient with diabetes.
Case presentation
A 45-year-old female, known diabetic on metformin for 3 years, presented at our facility with left eyelid and facial swelling for 1 week associated with bloody nasal discharge mixed with pus, congestion and stuffiness, blurred vision, fever, and black lesions on the nasal bridges. This was also associated with headache and pain above the eyes. She had no history of smoking tobacco or alcohol use. On physical examination, she was febrile (38.1 °C axillary temperature), with the rest of the vital signs within the normal limits. Local examination of the face and left eyelid revealed a tender, swollen, hyperpigmented left eyelid with crusted yellowish discharge, diminished vision, and restricted eye movement. A rhinoscopy was done which revealed a gangrenous anterior septum and a necrotic left inferior turbinate with no septal perforation. Laboratory investigation results indicated a high random blood glucose level, elevated blood urea nitrogen, serum creatinine, and leukocytosis with a predominance of neutrophils (Table 1). A Computer Tomography (CT) scan of the head showed bilateral air pockets in the orbits (Fig. 1A, B), bilateral cortical bone discontinuity of the medial–lateral walls of the maxillary sinuses with multiple air pockets seen in the maxillary sinuses as well as subcutaneous multiple air locules surrounding left maxillary and zygomatic regions in keeping with emphysematous changes (Fig. 1C, D), the brain parenchyma was normal (Fig. 1E, F).
A, B Non-contrasted CT scan of the brain at the level of the orbits, axial plane, soft tissue window (A) and bone window (B) show bilateral air pockets in the orbits (arrow) and left extraconal region (white curved arrow) and greater wing of sphenoid bone (yellow curved arrow). The lens, optic nerve and extraocular muscles are intact. C, D Both are CT scan images of the head, axial views at the level of the maxillary sinuses, bone window shows bilateral cortical bone discontinuity of the medial lateral walls of the maxillary sinuses (white arrows), bilateral soft tissue densities (24–37 HU) with multiple air pockets seen in the maxillary sinuses LT > > RT (star), subcutaneous multiple air locules are also seen surrounding left maxillary and zygomatic regions (curved arrows) in keeping with emphysematous changes. E is non-contrasted brain CT axial plane and F is contrasted brain CT axial plane. Both images show normal brain parenchyma and attenuation
The patient was started on broad-spectrum antibiotics including Metronidazole, Ceftriaxone and Meropenem, hematinic, and other drugs as per diabetic treatment protocols. Renal function monitoring as well as dietary control were done accordingly. She then underwent a left hemi-maxillectomy, orbital exenteration, and surgical debridement a week after admission. Tissue submitted for histopathological examination revealed multiple broad pauci-septate/nearly aseptate fungal hyphae which were thin-walled and occasionally branched at right angles as well as surrounding necrosis and invasion of the fibrous and adipose tissues (Fig. 2). She was then started on IV Liposomal Amphotericin B at 250 mg OD for a total of 21 days. The response to treatment in the first three weeks of admission was satisfactory and was later kept on itraconazole 200 mg OD for 7; however, the patient’s condition deteriorated thereafter, necessitating the placement of a tracheostomy and mechanical ventilation. On the fourth week of admission, she succumbed to the illness.
Discussion
Epidemiology
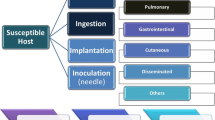
Mucormycosis infection results from inhalation of sporangiospores. However, in rare instances, the infection results from wound inoculation by contaminated dressing materials and instruments [27, 36]. Individuals at an increased risk of contracting this infection include diabetic patients with recurrent episodes of ketoacidosis, burn and trauma patients, patients receiving iron chelation therapy, and immunocompromised individuals suffering from cancer and those receiving chemotherapy [18]. Additionally, chronic sinusitis with impaired mucociliary function has also been associated with an increased likelihood of developing the rhino-cerebral form of mucormycosis [21]. Nonetheless, the major underlying risk factors in the order of frequency in Africa include DM, malignancies, and neutropenia [22].
The incidence of mucormycosis is unclear and underreported due to the challenges in diagnosing the condition as most cases require histological or microbiological confirmation [27]. However, a study conducted in France revealed an increasing annual incidence from 0.7 to 1.2 per million persons between 1997 and 2006, where, a higher incidence rate was observed among patients with hematological malignancies, with an annual increase of 24% from 1997 to 2006 [15]. In France, mucormycosis is now the fifth leading cause of invasive fungal infections, accounting for 1.5% of all cases [2]. In India, where the disease is more prevalent, the incidence rose from 13 cases per year between 1990 and 1999 to 50 cases per year between 2006 and 2007 [28].
The condition has a male predilection and the most common clinical forms include rhino-orbital-cerebral (ROC) and sinus (39%) due to the mode of infection which begins with inhalation of the spores and that allows the fungus to spread on the paranasal sinuses and adjacent sinuses [30], pulmonary (24%), cutaneous (19%), gastrointestinal (2 to 9%) as well as disseminated mucormycosis [28]. However, the underlying medical conditions that increase the risk of infection can influence the clinical presentation and outcome [4]. In diabetic patients, as of the presented case, the most common presentation is ROC mucormycosis [12, 21, 37]. The mortality rate for mucormycosis varies depending on the clinical presentation and underlying medical condition, with an overall mortality rate as high as 50% [4]. In patients with diabetes, mortality rates range from 4 to 75% [4, 22, 28]. Disseminated mucormycosis presents with the highest mortality rates ranging up to 96%, while the cutaneous form presents with the lowest reported overall mortality of 25% [4, 22, 26, 27].
Pathophysiology
Several virulent factors are implicated in mucormycotic infections, one of which includes the organism’s capacity to sequester iron, which is not readily available under normal physiological conditions in humans [27]. Mucormycetes exhibit a high affinity for iron permease (FTR1), enabling their survival in iron-deprived environments [36]. While iron chelators used in iron toxicity treatment are inhibitory, deforaxime, acting as a xenosiderophore, has been found to increase the risk of mucormycosis by enhancing Mucorales growth and pathogenicity, akin to elevated plasma concentrations of free iron [7, 27]. This poses a particular risk for diabetic patients since ketoacidosis in diabetics is often associated with elevated serum plasma free iron levels compared to non-diabetic individuals [14]
Mucorales display angiotropic behavior and possess a propensity to invade endothelial cells, leading to both local and disseminated disease. This invasion is facilitated by the spore surface protein, coat protein homology 3 (CotH3), which binds to the endothelial cell receptor and glucose-related protein 78 (GRP78), thereby promoting invasion and subsequent thrombosis and gangrene [5, 27]. Additionally, vascular endothelial growth factor (VEGF), a well-known pro-angiogenic factor that fosters neovascularization has been associated with mucormycosis; facilitating disease progression and spread into adjacent tissues [41].
Notably, the condition is more prevalent in patients with neutropenia as opposed to those with HIV-related immunodeficiency and those with neutrophil dysfunction such as those with diabetes and ketocidosis underpinning the crucial role of neutrophils rather than T cells in phagocytosis and limitation of spore proliferation [4, 16, 41]. Mucorales also produce enzymes such as protease and ketone reductase, which aid survival in high glucose and acidic conditions, thereby increasing the risk of infection and severe diseases in diabetic ketoacidosis cases, as observed in this scenario [8, 14, 27].
The primary host defense against Mucorales involves phagocytes, which are essential in controlling spore proliferation and killing through the production of oxidative metabolites, cationic peptides, and defensins [8]. These pathogens bind to toll-like receptor-2 (TLR-2) and stimulate the release of nuclear factor kappa β (NK-kβ), (interleukin-6) IL-6, interleukin-8 (IL-8), and tumor necrosis factor-α (TNF-α) [14]. However, conditions like hyperglycemia, acidosis, and steroid usage can impair phagocytic function, leading to a weak host defense mechanism [27]. Moreover, other risk factors, such as chronic persistent sinusitis, result in the downregulation of epidermal differentiation complex factors like S100 proteins and the SPINK5 gene, which play a role in maintaining barrier functions in the upper respiratory tract mucosa [29].
Certain Rhizopus species like Rhizopus microsporus and Rhizopus chinensis harbor an endosymbiotic bacterium called Bulkorlderia that produces a mycotoxin known as rhizotoxin. This mycotoxin acts as an antimitotic microcyclic polyketide metabolite, inhibiting the host cell cycle [23].
Symptomatology
Symptoms of mucormycosis are non-specific and may overlap with symptoms of other fungal infections such as aspergillosis, candidiasis, fusariosis, scedosporiosis, and histoplasmosis [4, 27]. The hallmark of this disease is tissue necrosis as a result of angioinvasion resulting in a black eschar [27]. The spread of the disease is usually fast, but there are rare descriptions of infections with an indolent course [7].
The commonest presentation is the ROC form, as of the presented case. Most patients with the ROC disease present with fever, diplopia, headache, nasal congestion, purulent nasal discharge, and sinus pain [7, 27, 36]. Infection involving the sinuses can spread to adjacent tissues such as the palate, orbit, and brain,this spread generally occurs over a short course of days. Few cases of ROC infection have been reported to progress slowly over the course of weeks [12]
Another form of presentation is related to pulmonary infection which manifests as fever, chest pain, and hemoptysis due to angioinvasion. Extension of the infection to the heart and the mediastinum can also occur [27]. Pulmonary infections occur mainly in patients with neutropenia and hematological malignancies [4, 28].
Cutaneous presentation can occur in both immunocompetent and immunocompromised patients with an equal distribution. These lesions start as painful erythema and induration and progressively become necrotic, forming a blackish eschar [4, 5, 36]. Other rare forms of mucormycosis include gastrointestinal mucormycosis, renal and disseminated mucormycosis.
Diagnosis
Diagnosing mucormycosis calls for a high index of suspicion. Imaging, histology, microbiology, and advanced molecular techniques are some of the modalities used in diagnosing this condition [32]. However, the gold standard test is the identification of the organism by direct microscopic examination, culture, and histopathology. Nonetheless, cultures are only positive in 50% of cases [4, 32].
Fungal elements are visible on hematoxylin and eosin sections. Periodic acid-Schiff or Grocott-Gomori’s methenamine silver staining is also used to highlight fungal hyphae, hence allowing a detailed evaluation of the morphology. Mucorales hyphae range in width from 6 to 25 µm, are non-septate or pauci-septate, and have an uneven, ribbon-like appearance. The branching angle varies and includes wide-angle (90°) bifurcations [32]. Moreover, Inflammation, whether neutrophilic or granulomatous, dominates tissue histology and may be absent in cases of severe immunosuppression [4, 32, 36]. There are histopathological features that predict prognosis and treatment outcome. Higher fungal load, inflammatory infiltrates in particular neutrophils, degree of necrosis, and extent of angioinvasion are said to be associated with poor outcomes. Shanmugasubdaram et al. and Jain et al. recommended grading the stage of mucormycosis based on those histopathological features [17, 31].
Molecular testing and immunohistochemistry with monoclonal antibodies, anti-Rhizomucor-antibodies, are used to further classify the species [4, 32]. Molecular testing such as polymerase chain reaction (PCR), restriction fragment length polymorphism (RFLP), and DNA sequencing in paraffin-embedded tissue blocks have high sensitivity and therefore useful in culture-negative samples when there is a high index of clinical suspicion and can be used to confirm the presence of Mucorales in histological specimens [10, 32].
While various studies have demonstrated the efficacy of serum or plasma PCR assays, their clinical relevance remains unknown [19]. However, invasive sampling can be avoided if PCR-based testing on serum or plasma samples is used. Furthermore, the detection of Mucorales-specific T–cell lymphocytes by serological tests such as enzyme-linked immunospot (ELISpot) assay has been tested. The utility of such techniques as surrogate diagnostic markers for Mucorales remains under investigation [25].
Imaging also plays a crucial role in diagnosing mucormycosis; however, radiological features are usually not specific. CT scan for pulmonary mucormycosis has been well studied, presenting classically as a reversed halo sign showing enhanced consolidation with central ground glass appearance. Other features include multifocal pneumonia which is usually indistinguishable from bacterial or aspiration pneumonia [9]. In ROC mucormycosis, both CT scan and contrasted MRI are useful, nonetheless, the former is cost-effective and readily available [13, 35]. However, MRI is useful in detecting the disease at an early stage before bone destruction is evident on CT scans. Furthermore, MRI has proven to be useful in the detection of sinus, extra sinus, intracranial, vascular, orbital, optic nerve, and skull base involvement, all of which have benefits to prognostication, staging, treatment planning, and follow-up [35].
Treatment
The mainstay treatment for mucormycosis is the combination of surgical debridement, anti-fungal therapy, and elimination of the underlying risk factors mentioned earlier. Surgical debridement should be done as soon as the diagnosis of mucormycosis is suspected. Debulking of the infection by surgical debridement is associated with improved outcomes for ROC and pulmonary mucormycosis [6, 38]. Amphotericin B is the most active antifungal drug against the Mucolares, started as an initial dose of 5–10 mg/kg for several months to years. Early treatment with Amphotericin B is crucial in achieving disease control, however, renal impairment following the drug is not uncommon particularly for non-lipid-based Amphotericin B. The lysosomal formulation as compared to deoxycholate is well tolerated and has less nephrotoxicity [3, 11, 40]. Step-down therapy following Amphotericin B is with broad-spectrum azoles, i.e., Posaconazole and Isavuconazole for patients who have responded to Amphotericin B. The use of Posaconazole delayed-release tablets (300 mg every 12 h on the first day, then 300 mg once daily) [33] taken with food is favorable when switching to an oral regimen. One study done in patients with COVID-19-associated Mucormycosis showed improvement with triazoles, i.e., Posaconazole or Isavuconazole as predominant antifungal therapy [33]. In this case, Amphotericin B dosage was low due to impaired renal function, and step-down treatment was done with itraconazole due to scarcity of the triazole, which may have contributed to the mortality.
Combined antifungal therapy is still under clinical trials but studies have shown better outcomes when Amphotericin B is combined with triazoles such as Posaconazole and Isavuconazole as well Echinocandins particularly Caspofungin [20, 34] and especially in diabetic patient as in this case [24]. Although the use of these antifungal therapies is well known, susceptibility varies across the species [20, 32]. In general, Mucor spp. are more susceptible to Posaconazole, Isavuconazole, and Itraconazole as opposed to Rhizopus and Lichtheimiaceae [1, 39]. Other antifungal medications such as Voriconazole, Fluconazole, and Flucytosine are ineffective in mucormycosis [39].
Conclusions
Mucormycosis remains a fatal disease with poor outcomes and necessitates high-index clinical suspicion. The clinical presentation and outcome are influenced by underlying risk factors, promptness of aggressive treatment, and comorbidities altering treatment. Mucormycosis should be staged early on to assess response to treatment and follow-up should be done by imaging and monitoring the drug minimum inhibitory concentration (MIC) to predict response. In Africa, where limited information exists about mucormycosis, it is imperative to conduct additional research to identify underlying risk factors and understand the true burden of the disease, given the increasing prevalence of diabetes mellitus and other risk factors in the region.
Availability of data and materials
Not applicable.
References
Arendrup MC, Jensen RH, Meletiadis J. In vitro activity of isavuconazole and comparators against clinical isolates of the Mucorales Order. Antimicrob Agents Chemother. 2015;59(12):7735–42. https://doi.org/10.1128/AAC.01919-15.
Bitar D, et al. Population-based analysis of invasive fungal infections, France, 2001–2010. Emerg Infect Dis. 2014;20(7):1163–9. https://doi.org/10.3201/eid2007.140087.
Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B–based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47(4):503–9. https://doi.org/10.1086/590004.
Danion F, et al. Mucormycosis: new developments into a persistently devastating infection. Semin Respir Crit Care Med. 2015;36(05):692–705. https://doi.org/10.1055/s-0035-1562896.
Darwish RM, AlMasri M, Al-Masri MM. Mucormycosis: the hidden and forgotten disease. J Appl Microbiol. 2022;132(6):4042–57. https://doi.org/10.1111/jam.15487.
Farmakiotis D, Kontoyiannis DP. Mucormycoses. Infect Dis Clin North Am. 2016;30(1):143–63. https://doi.org/10.1016/j.idc.2015.10.011.
Ferguson BJ. Mucormycosis of the nose and paranasal sinuses. Otolaryngol Clin North Am. 2000;33(2):349–65. https://doi.org/10.1016/S0030-6665(00)80010-9.
Gale GR, Welch AM. Studies of opportunistic fungi. I. Inhibition of Rhizopus oryzae by human serum. Am J Med Sci. 1961;241:604–12.
Hammer MM, Madan R, Hatabu H. Pulmonary mucormycosis: radiologic features at presentation and over time. Am J Roentgenol. 2018;210(4):742–7. https://doi.org/10.2214/AJR.17.18792.
Hammond SP, et al. Molecular methods to improve diagnosis and identification of mucormycosis. J Clin Microbiol. 2011;49(6):2151–3. https://doi.org/10.1128/JCM.00256-11.
Harbarth S, et al. The epidemiology of nephrotoxicity associated with conventional amphotericin B therapy. Am J Med. 2001;111(7):528–34. https://doi.org/10.1016/S0002-9343(01)00928-7.
Harrill WC, et al. Chronic rhinocerebral mucormycosis. Laryngoscope. 1996;106(10):1292–7. https://doi.org/10.1097/00005537-199610000-00024.
Herrera D, et al. Imaging findings of rhinocerebral mucormycosis. Skull Base. 2009;19(02):117–25. https://doi.org/10.1055/s-0028-1096209.
Ibrahim AS, et al. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(suppl_1):S16–22. https://doi.org/10.1093/cid/cir865.
Increasing Incidence of Zygomycosis (Mucormycosis), France, 1997–2006. n.d. https://doi.org/10.3201/eid1509.090334.
Iqbal N, et al. Chronic pulmonary mucormycosis: an emerging fungal infection in diabetes mellitus. J Thorac Dis. 2017;9(2):E121–5. https://doi.org/10.21037/jtd.2017.02.31.
Jain K, et al. Clinical and histology features as predictor of severity of mucormycosis in post-COVID-19 patients: an experience from a rural tertiary setting in Central India. SAGE Open Med. 2022;10:205031212210747. https://doi.org/10.1177/20503121221074785.
Jeong W, et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25(1):26–34. https://doi.org/10.1016/j.cmi.2018.07.011.
Legrand M, et al. Detection of circulating mucorales DNA in critically ill burn patients: preliminary report of a screening strategy for early diagnosis and treatment. Clin Infect Dis. 2016;63(10):1312–7. https://doi.org/10.1093/cid/ciw563.
Meena DS, Kumar D, Bohra GK. Combination therapy in Mucormycosis: current evidence from the world literature, a mini review. J Med Mycol. 2023;33(1):101332. https://doi.org/10.1016/j.mycmed.2022.101332.
Mignogna MD, et al. Mucormycosis in immunocompetent patients: a case-series of patients with maxillary sinus involvement and a critical review of the literature. Int J Infect Dis. 2011;15(8):e533–40. https://doi.org/10.1016/j.ijid.2011.02.005.
Osaigbovo II, et al. Mucormycosis in Africa: epidemiology, diagnosis and treatment outcomes. Mycoses. 2023;66:555–62. https://doi.org/10.1111/myc.13581.
Partida-Martinez LP, Hertweck C. Pathogenic fungus harbours endosymbiotic bacteria for toxin production. Nature. 2005;437(7060):884–8. https://doi.org/10.1038/nature03997.
Patel A, et al. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin Microbiol Infect. 2020;26(7):944.e9-944.e15. https://doi.org/10.1016/j.cmi.2019.11.021.
Potenza L, et al. Mucorales-specific T cells emerge in the course of invasive mucormycosis and may be used as a surrogate diagnostic marker in high-risk patients. Blood. 2011;118(20):5416–9. https://doi.org/10.1182/blood-2011-07-366526.
Prakash S, Kumar A. Mucormycosis threats: a systemic review. J Basic Microbiol. 2023;63(2):119–27. https://doi.org/10.1002/jobm.202200334.
Riley TT, et al. Breaking the mold. Ann Pharmacother. 2016;50(9):747–57. https://doi.org/10.1177/1060028016655425.
Roden MM, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–53. https://doi.org/10.1086/432579.
Schleimer RP, et al. Epithelium, inflammation, and immunity in the upper airways of humans: studies in chronic rhinosinusitis. Proc Am Thorac Soc. 2009;6(3):288–94. https://doi.org/10.1513/pats.200808-088RM.
Serris A, Danion F, Lanternier F. Disease entities in mucormycosis. J Fungi. 2019;5(1):23. https://doi.org/10.3390/jof5010023.
Shanmugasundaram S, Ramasamy V, Shiguru S. Role of histopathology in severity assessments of post-COVID-19 rhino-orbital cerebral mucormycosis - a case-control study. Ann Diagn Pathol. 2023;67:152183. https://doi.org/10.1016/j.anndiagpath.2023.152183.
Skiada A, et al. Challenges in the diagnosis and treatment of mucormycosis. Med Mycol. 2018;56(suppl_1):S93–101. https://doi.org/10.1093/mmy/myx101.
Soman R, Chakraborty S, Joe G. Posaconazole or isavuconazole as sole or predominant antifungal therapy for COVID-19-associated mucormycosis. A retrospective observational case series. Int J Infect Dis. 2022;120:177–8. https://doi.org/10.1016/j.ijid.2022.04.009.
Spellberg B, Edwards J, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556–69. https://doi.org/10.1128/CMR.18.3.556-569.2005.
Sreshta K, et al. Magnetic resonance imaging in rhino-orbital-cerebral mucormycosis. Indian J Ophthalmol. 2021;69(7):1915. https://doi.org/10.4103/ijo.IJO_1439_21.
Steinbrink JM, Miceli MH. Mucormycosis. Infect Dis Clin North Am. 2021;35(2):435–52. https://doi.org/10.1016/j.idc.2021.03.009.
Sun H-Y, et al. Rhino-orbital-cerebral zygomycosis in solid organ transplant recipients. Transplantation. 2010;90(1):85–92. https://doi.org/10.1097/TP.0b013e3181dde8fc.
Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Wagner L, et al. A revised species concept for opportunistic Mucor species reveals species-specific antifungal susceptibility profiles. Antimicrob Agents Chemother. 2019;63(8):e00653-19. https://doi.org/10.1128/AAC.00653-19.
Wingard JR, et al. A randomized, double-blind comparative trial evaluating the safety of liposomal amphotericin B versus amphotericin B lipid complex in the empirical treatment of febrile neutropenia. Clin Infect Dis. 2000;31(5):1155–63. https://doi.org/10.1086/317451.
Zumla A. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. Lancet Infect Dis. 2010;10(5):303–4. https://doi.org/10.1016/S1473-3099(10)70089-X.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conceptualization: SBM and ERM; writing—original draft preparation: SBM and IS; investigations especially radiology: AEK and AMM; writing—literature review: SBM; writing—review and editing: GM and DWK; supervision AK, ARM, and ERM. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This case report study was exempted from ethical approval at our institution as this paper reports a single case that emerged during normal surgical practice.
Consent for publication
Written informed consent to publish this article was provided by the next of kin.
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Matiku, S.B., Murenzi, G., Shaban, I. et al. Mucormycosis: a rare forgotten but fatal disease—a case report and literature review. J Rare Dis 3, 9 (2024). https://doi.org/10.1007/s44162-024-00033-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44162-024-00033-2