Abstract
The World Health Organization (WHO) has declared that neurodegenerative diseases will be the biggest health issues of the twenty-first century. Among these, Alzheimer’s and Parkinson’s diseases can be considered as the most acute incurable neurological diseases. Researchers are studying and developing a new treatment approach that uses nanotechnology to diagnosis and treatment neurodegenerative diseases. This treatment strategy will be used to regress neurodegenerative diseases such as Alzheimer’s disease. Alzheimer’s disease (AD) is one of the most common forms of reduced brain function, which causes many devastating complications. Current neurodegenerative diseases treatment protocols only help to treat symptoms nevertheless with nanotechnology approaches, can regress nerve cells apoptosis, reduce inflammation, and improve brain drug delivery. In this paper, new nanotechnology methods such as nanobodies, nano-antibodies, and lipid nanoparticles have been investigated. Correspondingly blood-brain barrier drug delivery improvement methods have been suggested.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
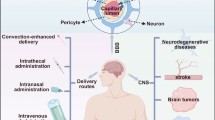
Given the growth rate of the elderly population, the World Health Organization predicts that the number of neurodegenerative disease subjects will triple over the next 30 years [1]. Striving for effective therapies is critical in neurodegenerative diseases treatment and prevention. The use of nanoparticles which are very small particles (1–1000 nm) in diagnosis and treatment malignancies and neurodegenerative diseases has led to new treatment methods [2, 3]. Nanoparticles have wide spread administration in food, electronics, and medical industries [4]. The preparation of nanoparticles smaller than 100 nm increases their surface area and increases their ability to react with organic and inorganic molecules [5]. Nanoparticles have expanded rapidly to tumor imaging, unravel of cancer biomolecules and biomarkers and nerve cells inflammation, as well as to target drug delivery [6]. Drug stabilization, especially enzymatic ones, in polymer nanoparticles increases their stability against heat, pH, proteases, and other destructive factors of their structure. It is now known that nanoparticles are capable to destroying inflammatory molecules without side effects in normal cells (Fig. 1) [7].
Biomimetic nanoparticles for inflammation targeting in neurodegenerative diseases
Inflammation is immune response to tissue damage in which inflammatory protein cytokines production and immune system activated cells are considered [8]. Inflammation is protective response to various pathological factors, including physical and chemical damage, immune reactions, microbial infections, toxins, hypoxia, and tissue damage [9], which leads to foreign factors elimination and tissue structure reconstruction and physiological function [10]. Although inflammation begins as a protective phenomenon, dysregulation of this process can lead to a variety of inflammatory disorders [11]. Inflammation has been considered as a key factor in neurological diseases pathophysiology. Nerve inflammation can be caused by nerve cells damage or induced by peripheral inflammation [12]. This process is mediated by microglia activation, stimulation of astrocytes, blood-brain barrier damage or increase of permeability, activated nerve immune cells, nerve overproduction of cytokines, nitric oxide, reactive oxygen species, as well as prostaglandins, and finally neuronal death is determined [13].
In Alzheimer’s disease, neuritis has been reported as inflammatory cytokines increase along with a decrease in neurotrophic factors and synaptic relapse. Microglia are attached to amyloid via cell surface receptors such as TLR, which promotes the activation of inflammatory cytokine [14].
The accumulation of Tau protein a microtubule structure is important phosphoprotein involved in vesicle transport, followed by oxidative stress caused by inflammation process that may lead to neurodegeneration, loss of synapses, and memory disorders [15]. Nerve cells inflammation is known as neuroinflammation and can be triggered by destructed neurons, invading microbes such as viruses, bacteria, and harmful chemical compounds. It is also stimulated by deformed proteins such as amyloid beta peptides [16].
Two major mechanisms lead to brain inflammation; systemic inflammatory can stimulate brain immune system which leads to cerebral inflammation. Neuritis occurs in a variety of pathological conditions such as stroke, infection, and neurodegenerative disorders (Fig. 2) [17]. This process, with the activation of microglia, increases blood-brain barrier permeability and the entrance of peripheral immune cells into cerebral tissue, causing inflammation [18].
Microglia cell changes in Alzheimer’s. As explained in the text, systemic inflammation in the body’s body is caused by changes in the cell proteins of brain cells, including the Tao protein. Taken from (C. Balducci et al.) With permission (https://www.sciencedirect.com/) Pharmacological Research
Of course, these processes are not only affected by microglia but also by astrocytes, neurons, endothelial cells of brain blood vessels, T cells, and foreign carnivorous organisms. Microglial cells undergo two major changes depending on the interaction of regulatory messages [19].
Neurotoxicity phenotype (called M1-like phenotype). Due to its resemblance to the M1 phenotype, it has peripheral macrophages that, by producing inflammatory cytokines and reactive oxygen species (ROS), create a harmful environment for neurons [20].
The neuroprotective phenotype, called the M2-like phenotype, produces a supportive environment for neurons by producing neurotrophic and anti-inflammatory mediators. Microglial cells, in response to factors such as bacterial lipopolysaccharide (LPS) and interferon-gamma (γ-INF), adopt M1-like phenotypes and produce inflammatory cytokines such as TNF α, NO, and IL-1,6, and in response to non-inflammatory cytokines such as IL-4 and IL-13, they adopt an M2-like phenotype and produce IL-10, anti-inflammatory cytokines and suppress the M1-like phenotype [21]. Naturally, when the central nervous system is infected, the microglia first take on the M1-like phenotype and have significant role in germicidal and xenophobic cleansing of the environment [22].
When microglia like M2 in reducing inflammation involved and the fate of harmful M1-like microglia with uncontrolled activity cause chronic inflammation, IL-6, IL-1, and IL-NO, proteolytic enzymes and glutamate cause damage or even neurons’ death.
Biomimetic nanoparticles which imitate immune cells can concerned as treatment agent to achieve molecular imaging and accurate drug delivery to inflammatory sites [23]. Biomimetic nanoparticles mimic immune system natural mechanisms for the sake of inflammation targeting [24].
Biomimetic nanoparticles based on synthetic nanoparticles modified with targeting ligands, screening targeting ligands with high affinity with CAMs and selectins, and optimizing the physiochemical characteristics of nanoparticles are imperative approaches for better inflammation targeting, as the interaction of nanoparticles with inflammatory such as silver nanoparticles [25, 26].
Among the various nanoparticles, silver nanoparticles have gained more importance due to their biological properties and potential applications [27]. Silver has been used since ancient times to treat wounds and inflammation. Silver nanoparticles have strong anti-inflammatory activity. Extensive applications of metal nanoparticles are due to their unique properties [28].
Current research demonstrated that nanoparticles have anti-inflammatory effect which reducing IL-1b expression as inflammatory factor and increasing expression of the IL-10 as anti-inflammatory factor [29].
Govindappa M et al. investigated the anti-inflammatory effect of synthesized np-Ag in skin dermatitis and lymphocytes [30].
Silver ion is an active antimicrobial agent. This ion can react with thiol group (-SH) to stimulate reactive oxygen species (ROS) production, which is one of the most vigorous antibacterial properties [31]. Due to their high surface-volume ratio, silver nanoparticles pay considerable attention to wound reduction, detoxification, and anti-inflammatory effect. Synthesized silver nanoparticles have a strong anti-inflammatory and restorative activity. Silver nanoparticles also suppress TNF IL-12 and IL-1b and induce inflammatory cells apoptosis and can be used as an inflammation therapeutic agent. In addition, proteomic studies have shown that silver nanoparticles have a positive effect on reducing inflammation by reducing cytokines [32].
In vivo studies have shown that allergic contact with rat dermatitis suppresses expression of α-TNF and IL-12 and silver nanoparticles induces apoptosis of inflammatory cells. This study suggests that silver nanocrystals may exert mechanisms of anti-inflammatory effects [33].
Nanoparticles approaches in neurodegenerative diseases
Advances in nanomedicine field have generated several platforms that improve drug transport across the blood-brain barrier, which described in details in this paper.
Nanotechnology has beneficial effects on improvement of sensory motor and cognitive functions in stroke, Parkinson’s disease, Huntington’s disease, Alzheimer’s diseases, amyotrophic lateral sclerosis (ALS), and spinal muscular atrophy [34].
Nanoparticles parallel with stem-cell therapy improve cell-based therapy efficiency regarding nanomaterial’s unique characteristics [35]. Nanoparticles can interact with stem-cell niche proneurogenic factors and consequently endorse self-renewal, proliferation, and differentiation of endogenous and exogenous neural stem cells (NSCs) (Fig. 3).
Graphene-nanofiber for differentiation of neural stem cells. Taken from (S. Shah et al.) With permission (https://www.mdpi.com/) Applied sciences
One of the major advantages of nanotechnology methods have significant effects in stem-cell studies which led to proliferation stem cells in large scales.
Neural cells amplification is key indicator, which has significant effect on neurodegenerative diseases therapies development.
Nanobodies
Antibodies in most mammalian species contain two identical heavy chains and two identical light chains [36]. The Camelidae family contains fully functional antibodies without light chain [37].
Nanobodies have high stability and can be used in compounds as immunoaffinity-based receptors or in biosensors [37]. These antibodies, by accurately targeting their target molecules, facilitate the diagnosis and treatment of diseases, including various cancers, and the elimination of cancer cells and even the neutralization of toxins in the body and the like [38, 39].
Because of their very small size, which is nano (2.5 nm in diameter and 4 nm in height), they are called nanobodies. Nanobodies have interesting properties such as very high specificity, small size, high solubility, low immunogenicity, high thermal stability, and good tissue permeability [40].
VHH (variable domain of heavy chain) is the smallest antibody fragment derived from camel heavy chain antibodies by recombinant DNA technology, also known as nanobody [41].
The crystal structure of a pure VHH indicates that the molecule is about 2.5 nm in diameter and less than 4-nm long. This is why it is called a nanobody molecule [42]. Thus, the second single VHH, or nanofiber, is the smallest antibody-binding antibody fragment with a total strength of about 12.5 kg in diameter. The antibody has a derivative binding power [43].
Camels have unique phenotypic and genetic characteristics that are not found in other mammals. Some of these features can be important in molecular and cellular aspects. Single-domain antibodies that are naturally only found in the camel’s immune system are called nanobodies [44]. In fact, nanobodies (ostrich monocotyledonous antibodies) are the smallest piece of intact, accessible, and antigen-binding antibody derived from heavy-chain, non-light-chain antibodies [43]. The creation and application of nanobodies in various studies shows that the unique physicochemical properties of these biomolecules have made them useful tools for biomedical applications and drug discovery. This is due to their small size, high binding affinity for secreted epitopes, low immunogenicity, and cost-effective production [45]. Due to these unique properties, nanobodies administration usage is very widespread, which targets tumors, cancer immunotherapy, cancer diagnosis, prevention of blood clotting, live imaging, neutralization of cytokines, and prevention of apoptosis, which are significant factors in Alzheimer’s progress [36, 38].
These single-domain antibodies can be applied to bacterial and other toxins, proteins, radioisotopes, fluorochromes, biotin, magnetic grains, and matrices of interest for a wide range of applications, including biosensor design, drug delivery, bacterial detection, endotoxin disposal, detection of infections by toxins, inhibition of virus secretion, and apoptosis prevention [46].
Biophysical and pharmacological properties of nanobodies, along with their suitable pharmaceutical flexibility therapeutic proteins, have led to being referred to as a new generation in antibody-based therapies [47].
Nanocrystals
Nanocrystals, crystals, are smaller than 1 μm. Nanocrystals can be an effective way to improve the pharmacokinetic and pharmacodynamic properties of low-density drugs. These nanoparticles can also increase bioavailability and solubility of other materials [48]. Compared to conventional fluorophores, nanocrystals are more photochemically stable because they have a narrow, balanced, and symmetrical emission spectrum [49]. Nanocrystals are very similar to onions, in that they contain a nucleus surrounded by a shell, which is a physical barrier between the external environment and the active optical core. Such a structure makes them less sensitive to oxidation and environmental changes [50].
Types of cerebral drug delivery nanoparticles
Conventional drugs spread after entering the body and affect many healthy organs as well, but this technology only targets a predetermined part [51]. Drug release from this nanotechnology-based pill is slow and, as a result, increases its effectiveness [52]. The result of such a mechanism is the reduction of drug side effects. For example, by targeting microglia cells and using patented FIU (electromagnetic nanoparticle system) systems for drug delivery, two anti-inflammatory drugs, CRID3 and Withaferin A, are delivered to the cell [53]. This process can inhibit inflammatory responses in microglia and help improve cognitive function in Alzheimer’s patients [54].
Nano-metal particles
The metal nanoparticles used for brain drug delivery, gold nanoparticles, are important issue due to being biocompatible, because lack of toxicity, no immune response and in some cases without the functionalization can penetrate to circulatory system [55]. Iron oxide nanoparticles and silver nanoparticles are other metal nanoparticle .
Lipid nanoparticles
This type of nanoparticles contain distinguished products: Liposomes first generation of nanoparticles for drug delivery and one or more two layers of vesicles made from lipid amphiphilic consist of an aqueous medium [56]. Using of nanoliposomes as nanocarriers not only reduces but also improves the antioxidant properties of drug during transport processes [57, 58].
It delivered drug slowly and in a controlled manner into the target tissue, causing a lasting effect of drug. Formulation of bicalin nanoliposomes bicalin was first performed by thin film method and then measure nanoparticles size and charge by DLS as well as their characterization and efficiency, were assessed DSC, spectrophotometry, fluorimetry, high pressure liquid chromatography, cell culture methods, confocal microscopy and erythrocyte hemolysis have been used.
Liposomal dual lipids are commonly biocompatible biodegradable lipids present in biomembranes. Liposomes have been used extensively for drug delivery to the brain, for the treatment of cerebral ischemia, for relaxation peptides, and for brain tumors.
Cationic liposomes contain positively charged lipids that are first carriers for genetic material transporting (such as DNA) into esophagus. Interaction between cationic lipids and nucleic acids leads to formation of lipoplex structure.
In contrast to liposomes, endogenous cationic liposomes are absorbed by the endosome and enter the endosome. In an environment with an acidity of 5 to 6, dioleole is mixed with the membrane of the endosome phosphatidylethanolamine (DOPE) to stabilize it and release the contents of the endosome. Therefore, drugs can invade endothelial cells, such as DNA, and increase their passage through the barrier to the neurons [59].
Solid lipid nanoparticles: Nanoparticles stable on a lipid base are a hydrophobic lipid nucleus in which drugs can be distributed which distinguished from biocompatible lipids such as triglycerides and small amounts of fatty acids and hair waxes (about 40 to 200). They are generally nanometers which allow them to cross tight endothelial cells and escape the reticuloendothelial system.
The ability to drug continuously release over several weeks is one of advantages of this type of nanoparticle, which increases drug delivery to the midbrain.
Nanoparticles are conjugated with antibodies
Semiconductor fluorescent nanocrystals (conductor semi-nanocrystals fluorescent) such as quantum particles (quantum dots) that conjugate with antibodies cause them to be labeled, and their exact amount is determined in a small piece of tissue [60].
Other nanoparticle particles such as nanocantilever, nanoprobes and nanoparticles coupled with specific ligands have also been investigated in cancer tumor imaging and Alzheimer diagnosis. Nanoparticles conjugated to antibodies can be used to simultaneously identify multiple molecular targets in small tissue fragments [61].
CD68 is a type of glycogen that increases the microglia cells inflammation. The development of nanoparticles bounds to CD68 antibody that reduces the level of this molecule in the cells. Previous studies have shown that this glycogen provokes immune cells involved in nerve cells inflammation.
Facilitate drug delivery over blood-brain barrier
There are various methods for drug delivery crossing blood-brain barrier, which several methods are invasive methods such as blood-brain barrier osmotic modification [62, 63]. Using hypertonic fluids causes contract of cerebral arteries endothelial cells as a result, which can lead to disruption of blood-brain barrier connections [64, 65]. Another way that can deliver drug to brain is by inhalation, but due to limited level of olfactory edges absorption, it is possible to deliver small extent of drug nanotechnology increase drug delivery to brain by crossing the blood-brain barrier [66]. PLGA nanoparticles are fabricated by a nanofoil mold that produces uniform nanoparticles and produces a specific size (loperamide drug) [67].
Nanoparticles toxicology
Due to the great diversity of nanoparticles and their properties, there is not enough nanoparticles toxicological and biological effects information, especially regarding body contact ways and its response [68].
Particle size, surface area, and chemical surface area are key factors in toxicology effects. Subjects who are directly or indirectly exposed to nanoparticle contamination may develop inflammatory, gastrointestinal, skin, cancer, and severe lung disease [69].
Another concern with nanoparticles is its reaction with other hazardous contaminants in water or air, thus facilitating their transport. Factors such as size and distribution, shape, properties, surface charge, mass, concentration, and distribution are effective in nanoparticles exposure risks assessing. Due to the rapid absorption of nanoparticles through skin, respiration and mucous cells, and distribution in target tissues, the nanoparticles disrupt the different parts function.
The relationship between particle size can affect biological properties and different ways of exposure. Decreased particle size causes a sudden increase in absorption or toxicity. These effects are small at first but become irreversible overtime. Contamination occurs through respiratory, gastrointestinal, blood, and skin, depending on the nanoparticles composition.
Studies by the Scientific Committee of the European Commission have shown that nanoparticles may have different toxic properties to bulk materials, but their risks and harms need to be considered on a case-by-case basis [70].
Numerous nanoparticles are made every year, many of which are released into environment and absorbed with organisms, so careful consideration of the toxicity of nanoparticles seems necessary.
Conclusion
Due to the therapeutic importance of nanoparticles, several studies are currently being conducted on them for use in diseases including cancers, infectious diseases, inflammation, and neurodegenerative diseases [71]. The unique properties of nanoparticles have led to advantages over conventional therapeutic such as antibodies in neurodegenerative inflammatory diseases including Alzheimer. Ability to detect missed and unusual epitopes, binding to active sites of proteins, suitable pharmaceutical flexibility, very weak immunogenicity and finally convenient production made nanoparticles wide speared functional in therapeutic protocols. Nanoparticles are very suitable for immunotherapy purposes due to their resistance to very high pHs and their target binding capacity.
Although inflammation plays a prominent role in the occurrence of neurological diseases, but unfortunately there is still no safe and effective treatment to control unregulated inflammatory processes in the brain. Drugs commonly used to control inflammation are less effective in nerve cells due to the lack of penetration of the blood-brain barrier. Since many inflammatory processes are active in nerve cells, controlling inflammation will be a more effective method of treatment strategies rather than suppressing inflammation. Recently, researchers have been able to make advances in the design of nanoparticles that block cell surface molecules that has role in inflammation and swelling. This treatment for Alzheimer’s is very safe and secure by using nanotechnology science [72]. Although there are not enough studies on nanoparticles toxicity, it is still difficult to draw a conclusion because studies have been conducted without characterization and complete description of nanoparticles and these nanoparticles have been used in vitro.
References
Noble W, Burns MP (2010) Challenges in neurodegeneration research. Front Psychiatr 1:7
Spuch C, Saida O, Navarro C (2012) Advances in the treatment of neurodegenerative disorders employing nanoparticles. Recent Patents Drug Deliv Formul 6(1):2–18
Abbasi-Oshaghi E, Mirzaei F, Mirzaei A (2018) Effects of ZnO nanoparticles on intestinal function and structure in normal/high fat diet-fed rats and Caco-2 cells. Nanomedicine. 13(21):2791–2816
Eftekhari A, Maleki Dizaj S, Sharifi S, Salatin S, Rahbar Saadat Y, Zununi Vahed S, Samiei M, Ardalan M, Rameshrad M, Ahmadian E, Cucchiarini M (2020) The use of nanomaterials in tissue engineering for cartilage regeneration; current approaches and future perspectives. Int J Mol Sci 21(2):536
Schmid G (2011) Nanoparticles: from theory to application. John Wiley & Sons
Caracciolo G, Vali H, Moore A, Mahmoudi M (2019) Challenges in molecular diagnostic research in cancer nanotechnology. Nano Today 27:6–10
Tierney T, Bodnár K, Rasmuson Å, Hudson S (2017) Carrier particle design for stabilization and isolation of drug nanoparticles. Int J Pharm 518(1-2):111–118
Amirrasouli H, Asefy Z, Taghikhani M (2011) Study of serum cystatin C as a reliable marker for metabolic syndrome. J Diab Metab Disord 10:6
Ley K (2003) The role of selectins in inflammation and disease. Trends Mol Med 9(6):263–268
Asefy Z, Mirinejad M, Amirrasooli H, Tagikhani M (2014) Assessing validity of serum cystatin C for predicting metabolic syndrome. Pak J Biol Sci 17(4):582–585
Ospelt C, Gay S (2010) TLRs and chronic inflammation. Int J Biochem Cell Biol 42(4):495–505
Villapol S (2018) Roles of peroxisome proliferator-activated receptor gamma on brain and peripheral inflammation. Cell Mol Neurobiol 38(1):121–132
Clark AK, Gentry C, Bradbury EJ, McMahon SB, Malcangio M (2007) Role of spinal microglia in rat models of peripheral nerve injury and inflammation. Eur J Pain 11(2):223–230
Parisi V. (ed) (2003) Correlation between morphological and functional retinal impairment in patients affected by ocular hypertension, glaucoma, demyelinating optic neuritis and Alzheimer’s disease. Seminars in ophthalmology. Taylor & Francis
Šimić G, Babić Leko M, Wray S, Harrington C, Delalle I, Jovanov-Milošević N, Bažadona D, Buée L, de Silva R, di Giovanni G, Wischik C, Hof P (2016) Tau protein hyperphosphorylation and aggregation in Alzheimer’s disease and other tauopathies, and possible neuroprotective strategies. Biomolecules. 6(1):6
Enache TA, Oliveira-Brett AM (2017) Alzheimer’s disease amyloid beta peptides in vitro electrochemical oxidation. Bioelectrochemistry. 114:13–23
Stern Y (2012) Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 11(11):1006–1012
Readnower RD, Chavko M, Adeeb S, Conroy MD, Pauly JR, McCarron RM et al (2010) Increase in blood–brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury. J Neurosci Res 88(16):3530–3539
Denieffe S, Kelly RJ, McDonald C, Lyons A, Lynch MA (2013) Classical activation of microglia in CD200-deficient mice is a consequence of blood brain barrier permeability and infiltration of peripheral cells. Brain Behav Immun 34:86–97
Tang Y, Le W (2016) Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol 53(2):1181–1194
Matias D, Dubois LG, Pontes B, Rosário L, Ferrer VP, Balça-Silva J, Fonseca ACC, Macharia LW, Romão L, e Spohr TCLS, Chimelli L, Filho PN, Lopes MC, Abreu JG, Lima FRS, Moura-Neto V (2019) GBM-derived Wnt3a induces M2-like phenotype in microglial cells through Wnt/β-catenin signaling. Mol Neurobiol 56(2):1517–1530
Song M, Liu T, Shi C, Zhang X, Chen X (2016) Bioconjugated manganese dioxide nanoparticles enhance chemotherapy response by priming tumor-associated macrophages toward M1-like phenotype and attenuating tumor hypoxia. ACS Nano 10(1):633–647
Sushnitha M, Evangelopoulos M, Tasciotti E, Taraballi F (2020) Cell membrane-based biomimetic nanoparticles and the immune system: immunomodulatory interactions to therapeutic applications. Front Bioeng Biotechnol 8
Alkhalifa H, Alshebber E, Taurin S (2021) Regenerative nanomedicine applications for neurodegenerative diseases of central nervous system. Theory and Applications of Nonparenteral Nanomedicines: Elsevier, pp 259-87
Parodi A, Molinaro R, Sushnitha M, Evangelopoulos M, Martinez JO, Arrighetti N, Corbo C, Tasciotti E (2017) Bio-inspired engineering of cell-and virus-like nanoparticles for drug delivery. Biomaterials. 147:155–168
Cui W, Fu W, Lin Y, Zhang T (2021) Application of nanomaterials in neurodegenerative diseases. Curr Stem Cell Res Ther 16(1):83–94
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27(1):76–83
Kim JS, Kuk E, Yu KN, Kim J-H, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, Kim YK, Lee YS, Jeong DH, Cho MH (2007) Antimicrobial effects of silver nanoparticles. Nanomedicine 3(1):95–101
Sheikpranbabu S, Kalishwaralal K, Venkataraman D, Eom SH, Park J, Gurunathan S (2009) Silver nanoparticles inhibit VEGF-and IL-1β-induced vascular permeability via Src dependent pathway in porcine retinal endothelial cells. J Nanobiotechnol 7(1):8
Govindappa M, Hemashekhar B, Arthikala M-K, Rai VR, Ramachandra Y (2018) Characterization, antibacterial, antioxidant, antidiabetic, anti-inflammatory and antityrosinase activity of green synthesized silver nanoparticles using Calophyllum tomentosum leaves extract. Results Phys 9:400–408
AshaRani P (2009) Low Kah Mun G, Hande MP, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 3(2):279–290
Patel CB, Jyoti A. Promises of nanomaterials as antimicrobial agents: a review
Wilkinson L, White R, Chipman J (2011) Silver and nanoparticles of silver in wound dressings: a review of efficacy and safety. J Wound Care 20(11):543–549
Mandoli C, Pagliari F, Pagliari S, Forte G, Di Nardo P, Licoccia S et al (2010) Stem cell aligned growth induced by CeO2 nanoparticles in PLGA scaffolds with improved bioactivity for regenerative medicine. Adv Funct Mater 20(10):1617–1624
Adams CF, Pickard MR, Chari DM (2013) Magnetic nanoparticle mediated transfection of neural stem cell suspension cultures is enhanced by applied oscillating magnetic fields. Nanomedicine 9(6):737–741
Revets H, De Baetselier P, Muyldermans S (2005) Nanobodies as novel agents for cancer therapy. Expert Opin Biol Ther 5(1):111–124
Muyldermans S (2013) Nanobodies: natural single-domain antibodies. Annu Rev Biochem 82:775–797
Hassanzadeh-Ghassabeh G, Devoogdt N, De Pauw P, Vincke C, Muyldermans S (2013) Nanobodies and their potential applications. Nanomedicine. 8(6):1013–1026
Jovčevska I, Muyldermans S (2020) The therapeutic potential of nanobodies. BioDrugs. 34(1):11–26
Helma J, Cardoso MC, Muyldermans S, Leonhardt H (2015) Nanobodies and recombinant binders in cell biology. J Cell Biol 209(5):633–644
Muyldermans S (2020) Applications of nanobodies. Ann Rev Anim Biosci 9
Vincke C, Muyldermans S (2012) Introduction to heavy chain antibodies and derived Nanobodies. Single Domain Antibodies: Springer, p. 15-26
Gibbs WW (2005) Nanobodies. Sci Am 293(2):78–83
Deffar K, Shi H, Li L, Wang X, Zhu X (2009) Nanobodies-the new concept in antibody engineering. Afr J Biotechnol 8(12)
De Meyer T, Muyldermans S, Depicker A (2014) Nanobody-based products as research and diagnostic tools. Trends Biotechnol 32(5):263–270
Vaneycken I, D’huyvetter M, Hernot S, De Vos J, Xavier C, Devoogdt N et al (2011) Immuno-imaging using nanobodies. Curr Opin Biotechnol 22(6):877–881
Steeland S, Vandenbroucke RE, Libert C (2016) Nanobodies as therapeutics: big opportunities for small antibodies. Drug Discov Today 21(7):1076–1113
Alivisatos P (2004) The use of nanocrystals in biological detection. Nat Biotechnol 22(1):47–52
Gao L, Liu G, Ma J, Wang X, Zhou L, Li X (2012) Drug nanocrystals: in vivo performances. J Control Release 160(3):418–430
Naasani I (2005) Nanocrystals. Google Patents
De Jong WH, Borm PJ (2008) Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine 3(2):133–149
Cho K, Wang X, Nie S, Shin DM (2008) Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res 14(5):1310–1316
Scott R Armstrong JH. Alzheimers Dis Res Grant Advis Board
Tehrani MD, Kim MO, Yoon J (2014) A novel electromagnetic actuation system for magnetic nanoparticle guidance in blood vessels. IEEE Trans Magn 50(7):1–12
Vio V, Jose Marchant M, Araya E, Kogan MJ (2017) Metal nanoparticles for the treatment and diagnosis of neurodegenerative brain diseases. Curr Pharm Des 23(13):1916–1926
Kassaee SM, Taghi Goodarzi M, Abbasi OE (2018) Antioxidant, antiglycation and anti-hyperlipidemic effects of Trigonella foenum and Cinnamon in type 2 diabetic rats. Jundishapur J Nat Pharm Prod 13(1)
Yadav N, Khatak S, Sara US (2013) Solid lipid nanoparticles-a review. Int J Appl Pharm 5(2):8–18
Shah R, Eldridge D, Palombo E, Harding I (2015) Lipid nanoparticles: production, characterization and stability. Springer
Weber S, Zimmer A, Pardeike J (2014) Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for pulmonary application: a review of the state of the art. Eur J Pharm Biopharm 86(1):7–22
Naja G, Bouvrette P, Hrapovic S, Luong JH (2007) Raman-based detection of bacteria using silver nanoparticles conjugated with antibodies. Analyst. 132(7):679–686
Jazayeri MH, Amani H, Pourfatollah AA, Pazoki-Toroudi H, Sedighimoghaddam B (2016) Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sens Bio-sens Res 9:17–22
Gao H (2016) Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm Sin B 6(4):268–286
Sarkar A, Fatima I, Mohammad Sajid Jamal Q, Sayeed U, Khan KA, Akhtar S et al (2017) Nanoparticles as a carrier system for drug delivery across blood brain barrier. Curr Drug Metab 18(2):129–137
Malhotra M, Prakash S (2011) Targeted drug delivery across blood-brain-barrier using cell penetrating peptides tagged nanoparticles. Curr Nanosci 7(1):81–93
Bhaskar S, Tian F, Stoeger T, Kreyling W, de la Fuente JM, Grazú V, Borm P, Estrada G, Ntziachristos V, Razansky D (2010) Multifunctional Nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: perspectives on tracking and neuroimaging. Part Fibr Toxicol 7(1):3
Fan Y, Chen M, Zhang J, Maincent P, Xia X, Wu W (2018) Updated progress of nanocarrier-based intranasal drug delivery systems for treatment of brain diseases. Crit Rev Ther Drug Carrier Syst 35(5)
Hersh DS, Wadajkar AS, Roberts NB, Perez JG, Connolly NP, Frenkel V et al (2016) Evolving drug delivery strategies to overcome the blood brain barrier. Curr Pharm Des 22(9):1177–1193
Elsaesser A, Howard CV (2012) Toxicology of nanoparticles. Adv Drug Deliv Rev 64(2):129–137
Murugadoss S, Lison D, Godderis L, Van Den Brule S, Mast J, Brassinne F et al (2017) Toxicology of silica nanoparticles: an update. Arch Toxicol 91(9):2967–3010
Park MV, Neigh AM, Vermeulen JP, de la Fonteyne LJ, Verharen HW, Briedé JJ et al (2011) The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials. 32(36):9810–9817
Petros RA, DeSimone JM (2010) Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov 9(8):615–627
Eftekhari A, Dizaj SM, Chodari L, Sunar S, Hasanzadeh A, Ahmadian E, Hasanzadeh M (2018) The promising future of nano-antioxidant therapy against environmental pollutants induced-toxicities. Biomed Pharmacother 103:1018–1027
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This research is involving no human participants and/or animals.
Conflict of interest
The authors declare no competing interests.
Ethical approval
Not applicable.
Informed consent
Authors declare their consent on this paper publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Asefy, Z., Hoseinnejhad, S. & Ceferov, Z. Nanoparticles approaches in neurodegenerative diseases diagnosis and treatment. Neurol Sci 42, 2653–2660 (2021). https://doi.org/10.1007/s10072-021-05234-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05234-x