Abstract
Background and purposes
The role of endovascular recanalization in the treatment of cancer patients with acute stroke remains elusive. Our study aimed to investigate the clinical and imaging outcomes of endovascular recanalization treatment in patients with acute large vessel occlusion stroke who had active cancer.
Methods
We retrospectively reviewed the data from our stroke registry from January 2011 to September 2016 which was collected prospectively. Acute stroke patients with large artery occlusion in the anterior circulation who had active cancer were identified. Baseline clinical characteristics and postprocedural and long-term clinicoradiological outcomes were evaluated. A good outcome was defined as a 90-day modified Rankin Scale score of 0 to 2. Outcomes were also compared with those of non-malignancy patients who had received endovascular therapy during the same period.
Results
A total of 378 ischemic stroke patients received endovascular treatment, of whom 27 (7.14 %) had current malignancy. In patients with current malignancy, a low baseline NIHSS score and male sex were associated with functional independency at 90 days. When comparing with non-malignancy patients, no significant differences in the proportions of patients with symptomatic intracranial hemorrhage (11.1% vs 16.2%, p = 0.60) and good functional outcome (37.0% vs 39.6%, p = 0.84) were found in the malignancy patients.
Conclusion
Endovascular treatment might be a feasible therapeutic option for acute ischemic stroke patients with current malignancy when candidates are selected carefully because the outcomes were not differed. Future large-scale prospective studies are necessary.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stroke is common in patients with cancer. Previous autopsy studies revealed that approximately 15% of patients with known cancer have cerebrovascular disease [1]. In addition, one study reported that 10% of hospitalized ischemic stroke patients have comorbid cancer [2]. Cancer-related ischemic strokes have two etiological mechanisms as follows: (1) tumor-related factors such as coagulation disorder, tumor embolism, and chemotherapy, and (2) conventional vascular risk factors such as hypertension, diabetes, and atrial fibrillation [2,3,4].
Timely revascularization using a stent-retriever-based endovascular procedure is the mainstay treatment for acute ischemic stroke (AIS). Endovascular treatment (EVT) for AIS has demonstrated clinical benefits in several recent clinical trials and is considered a new standard treatment for AIS with large vessel occlusion [5, 6]. However, AIS patients with active cancer have been excluded from randomized clinical trials of intravenous recombinant tissue-plasminogen activator (IV rt-PA) because of their increased risk of bleeding [7]. Therefore, evidence for the usefulness or feasibility of EVT for AIS with current malignancy (CM) is still lacking.
The aim of this study was to investigate the clinical and radiological outcomes of EVT in AIS patients with CM. The outcomes were compared with those in non-malignancy patients who received EVT for AIS during the same study period.
Methods
Study population
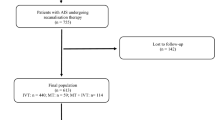
We retrospectively reviewed the medical records of patients with AIS who received endovascular therapy between January 2011 and September 2016. The analysis included patients with acute large vessel occlusion in the anterior circulation. The main occlusion sites are the internal carotid artery (ICA) and proximal middle cerebral artery (pMCA). The patients were divided into two groups according to the presence of CM (CM vs. non-CM group). Patients with CM were defined as subjects with any diagnosis of current or previous metastatic disease, those undergoing current treatment for malignancy, or those who refused treatment for current cancer. Patients were also included when the initial diagnosis of malignancy was made during hospitalization after the onset of stroke.
The inclusion criteria for endovascular therapy were as follows: (1) presentation within 8 h of stroke onset, (2) no evidence of intracranial hemorrhage on brain computed tomography (CT) or magnetic resonance imaging (MRI), (3) major arterial occlusion on magnetic resonance angiography or conventional angiography, (4) a target mismatch pattern on multimodal MRI according to visual estimation (time-to-peak map of perfusion imaging showing a lesion volume of ≥ 30% larger than that detected using diffusion-weighted imaging [DWI]), (5) infarct volume of less than one-third of the MCA territory on DWI or non-enhanced CT, and (6) premorbid modified Rankin Scale (mRS) score of ≤ 2.
Clinical and radiological findings were compared between the CM and non-CM groups. In addition, the characteristics of the patients in the CM group who had favorable long-term outcomes were investigated.
Ethics statement
This study was approved by the institutional review board (IRB) of Chonnam National University Hospital. All clinical investigations described in this study were conducted in accordance with the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from each patient or patient’s family member.
Clinical assessment
Information on the baseline clinical characteristics of the subjects was collected. We evaluated the location of the vessel occlusion, use of intravenous tPA prior to EVT, and the etiology of stroke categorized by TOAST classification (Trial of Org 10172 in Acute Stroke Treatment) [8]. We categorized the stroke mechanisms into conventional (large-artery atherosclerosis [LAA], small-vessel occlusion [SVO], and cardioembolism [CE]) and cryptogenic as previously described [9]. Systolic and diastolic blood pressures were initially measured in the emergency department. The characteristics of the patients’ cancer such as cancer type and systemic or brain metastasis were determined. The National Institutes of Health Stroke Scale (NIHSS) and modified Rankin Scale (mRS) scores at baseline, discharge, and 3 months were obtained by experienced stroke neurologists of the stroke team of our institution. Functional independency was defined as mRS scores of 0 to 2 after 90 days. Neurological and other medical complications were also evaluated. These clinical outcomes were compared between the two groups.
Radiological assessment
Recanalization status was assessed on the final digital subtraction angiography. The extent of recanalization after EVT was determined with the modified Treatment in Cerebral Infarction (mTICI) score, categorized as 0 (no reperfusion), 1, 2a, 2b, and 3 (complete reperfusion). intracerebral hemorrhages (ICHs) were assessed on post-treatment CT and gradient-echo magnetic resonance images, and classified as hemorrhagic infarction (HI) or parenchymal hemorrhage (PH) based on the European Cooperative Acute Stroke Study (ECASS) criteria [10]. We defined sICH according to the definitions of the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) trials (PH grade 2 and increase in NIHSS score by ≥ 4) [11]. These radiological outcomes were compared between the two groups.
Outcomes
We classified the outcomes as symptomatic intracerebral hemorrhage and in-hospital mortality as a short-term outcome of EVT and 3-month functional outcome and mortality at 90 days as a 3 months outcome.
Statistical analyses
Differences in baseline characteristics between the patients with and those without CM were evaluated. Values are presented as mean ± SD, median for continuous variables, or number (%) of subjects for categorical variables. The patients’ baseline characteristics were compared using the Pearson χ2 test, Mann-Whitney U test, or Student t test according to the type of variable. The Pearson χ2 test was used to evaluate the distribution of the modified Rankin Scale scores between the patients with CM and those without.
All statistical analyses were performed with the SPSS version 21.0 software for Windows (IBM SPSS, Chicago, IL, USA). A 2-sided p value of < 0.05 was generally considered a minimum level of statistical significance.
Results
A total 378 patients were enrolled in this study. Only 27 patients had CM (CM group). Vascular risk factors, baseline and discharge NIHSS scores, and functional independency at 90 days (mRS score of 0 to 2) were not significantly different between the non-CM and CM groups (Table 1).
The success rate of recanalization and incidence rate of ICH were comparable between the two groups (82.6% vs 85.2%, p = 0.80; 32.8% vs 44.4%, p = 0.29, respectively). Symptomatic ICH and in-hospital mortality were not significantly different between the two groups (16.2% vs 11.1%, p = 0.60; 2.3% vs 3.7%, p = 0.49, respectively). However, the CM group showed a higher 90-day mortality than the non-CM group (8.2% vs. 33.3%, p < 0.001). The distribution of 90-day mRS scores was not significantly different between the two groups (p = 0.07; Fig. 1). Short-term outcome was not differed between CM and non-CM groups.
Of the 27 patients with CM, 24 had solid-organ cancer and 3 had a hematologic malignancy (Table 2).
Thirteen patients had conventional stroke mechanisms and 14 had cryptogenic stroke mechanisms. Successful revascularization (mTICI ≥ 2b) was achieved in 23 patients and was not significantly different according to the stroke mechanism (11 in the conventional mechanism group and 12 in the cryptogenic group). Ten patients (37.0%) showed favorable outcomes at 3 months and all of them were male (Table 3). They had lower baseline NIHSS scores than the patients in the non-favorable outcome group (median [interquartile range]: 6.5 [5.0–13.25] vs. 11 [9–17], p = 0.035; Table 3). Long-term functional outcome was not affected by the occurrence of ICH, symptomatic ICH, or prior use of IV-tPA before EVT.
Discussion
In this study, 378 AIS patients with large artery occlusion in the anterior circulation received EVT. Functional outcome and radiological findings were not significantly different between the CM and non-CM groups. The incidence rate of symptomatic ICH and in-hospital mortality were comparable between the two groups. In the CM group, low baseline NIHSS score was associated with functional independency at 90 days.
In this study, EVT for AIS showed similar results between the CM and non-CM patients. The patients in the two groups showed no significant differences in the baseline clinical and radiological findings, including age, conventional vascular risk factors, initial DWI-ASPECT score, and time from symptom onset to reperfusion, which could affect prognosis [12]. Furthermore, the proportions of patients with favorable long-term outcome and hemorrhagic complication were comparable with those in the landmark randomized controlled mechanical thrombectomy trials [5]. Even in such past landmark large clinical trials, the patients with CM were excluded because the prognosis of cancer patients might be poor and hemorrhagic complications were predicted. Systemic malignancy and ischemic stroke are common medical conditions in CM and could increase mortality, especially in the elderly. Our study showed no significant increase in the incidence of hemorrhagic complications in the CM group after EVT. Although cancer patients may be more likely to have contraindications to thrombolysis than non-cancer patients, active cancer by itself is not exclusionary for recanalization therapies [2]. In this study, in spite of the small number of cancer patients, no significant difference in functional outcome was found between the CM and non-CM groups, which could emphasize the role of EVT in the management of acute ischemic stroke patients with active cancer. Despite the high mortality in the CM group, no significant differences were found in the success rate of recanalization, incidence of symptomatic ICH, and in-hospital mortality, which could allow the use of EVT to be considered when being appropriated. Large prospective studies might be needed to elucidate this result.
In the analysis of the CM group, the patients with functional independency at 90 days showed low baseline NIHSS score, more successful reperfusion status, shorter onset to reperfusion time, and low incidence rates of intracerebral hemorrhage and sICH. However, only low baseline NIHSS score was significantly associated with favorable outcome. EVT might be performed safely in AIS patients with CM, especially those presenting with relatively low initial NIHSS scores. It seemed that hematological malignancy caused worse clinical outcome, but there were no statistical differences. However, in interpreting the results, it should be noted that the CM group consisted of very few patients.
ICH was more frequently observed in our cohort (33.6%) than in those of previous clinical trials [13, 14]. However, the incidence rates of ICH and symptomatic ICH in the CM group were not higher than those in the non-CM group. The risk of bleeding tends to be increased in cancer patients owing to underlying coagulopathy [3]. However, our study showed that the incidence rate of hemorrhagic complication after EVT was not increased in the CM group. The use of IV-tPA before performing EVT can affect the development of ICH. In this study, the proportion of IV-tPA use was similar between the two groups. In the CM group, symptomatic ICH developed in 3 patients who did not receive IV-tPA before EVT. On the basis of this result, the association between prior use of IV-tPA and the severity of ICH is uncertain.
The 90-day mortality in the CM group was higher than that in the non-CM group. However, in-hospital mortality was similar between the two groups. In-hospital mortality was more strongly associated with management of acute stroke than 90-day mortality. The higher 90-day mortality rate in the CM group might be attributed to active cancer itself rather than to index stroke. Metastasis and thromboembolic events are leading causes of death in active cancer patients [15].
This study has several limitations. First, it is a retrospective study with a relatively small number of patients, especially in the CM group. Second, the study population is unlikely to be representative of the AIS patients with CM as a whole because the recruitment was performed in a single comprehensive stroke center. Third, selection bias was possible because in CM patients, EVT was performed at the discretion of the attending stroke physician. Also, selection bias on gender could be possible. Fourth, we had limited information on the CM patients. Information on cancer staging, cause of mortality, or treatment status of each CM patient was incomplete. CM itself is considered as a competing risk factor of 90-day mortality. However, the small number of CM patients might decrease the statistical power. We collected patient data prospectively through a registry-based design; however, this seems insufficient to overcome the aforementioned limitations. Fifth, multiple small infarction due to chronic disseminated intravascular coagulation or Trousseau syndrome might not be included, because endovascular treatment for AIS was performed only on the AIS due to large vessel occlusion
In conclusion, this study suggests that EVT might be feasible for AIS patients with CM. In addition, a low baseline NIHSS score was associated with favorable outcome in this setting. Prospectively designed large clinical studies are necessary.
References
Graus F, Rogers LR, Posner JB (1985) Cerebrovascular complications in patients with cancer. Medicine (Baltimore) 64(1):16–35
Navi BB, Iadecola C (2018) Ischemic stroke in cancer patients: a review of an underappreciated pathology. Ann Neurol 83(5):873–883. https://doi.org/10.1002/ana.25227
Grisold W, Oberndorfer S, Struhal W (2009) Stroke and cancer: a review. Acta Neurol Scand 119(1):1–16. https://doi.org/10.1111/j.1600-0404.2008.01059.x
Cestari DM, Weine DM, Panageas KS, Segal AZ, DeAngelis LM (2004) Stroke in patients with cancer: incidence and etiology. Neurology 62(11):2025–2030
Campbell BCV, Donnan GA, Lees KR, Hacke W, Khatri P, Hill MD, Goyal M, Mitchell PJ, Saver JL, Diener H-C, Davis SM (2015) Endovascular stent thrombectomy: the new standard of care for large vessel ischaemic stroke. Lancet Neurol 14(8):846–854. https://doi.org/10.1016/s1474-4422(15)00140-4
Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, Johnston KC, Johnston SC, Khalessi AA, Kidwell CS, Meschia JF, Ovbiagele B, Yavagal DR, American Heart Association Stroke C (2015) 2015 American Heart Association/American Stroke Association Focused Update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 46(10):3020–3035. https://doi.org/10.1161/STR.0000000000000074
Merkler AE, Marcus JR, Gupta A, Kishore SA, Leifer D, Patsalides A, DeAngelis LM, Navi BB (2014) Endovascular therapy for acute stroke in patients with cancer. Neurohospitalist 4(3):133–135. https://doi.org/10.1177/1941874413520509
Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24(1):35–41
Kim SG, Hong JM, Kim HY, Lee J, Chung PW, Park KY, Kim GM, Lee KH, Chung CS, Bang OY (2010) Ischemic stroke in cancer patients with and without conventional mechanisms: a multicenter study in Korea. Stroke 41(4):798–801. https://doi.org/10.1161/STROKEAHA.109.571356
Fiorelli M, Bastianello S, von Kummer R, del Zoppo GJ, Larrue V, Lesaffre E, Ringleb AP, Lorenzano S, Manelfe C, Bozzao L (1999) Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke 30(11):2280–2284
Wahlgren N, Ahmed N, Davalos A, Ford GA, Grond M, Hacke W, Hennerici MG, Kaste M, Kuelkens S, Larrue V, Lees KR, Roine RO, Soinne L, Toni D, Vanhooren G, investigators S-M (2007) Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 369 (9558):275-282. doi:https://doi.org/10.1016/S0140-6736(07)60149-4
Yoon W, Kim SK, Park MS, Baek BH, Lee YY (2017) Predictive factors for good outcome and mortality after stent-retriever thrombectomy in patients with acute anterior circulation stroke. J Stroke 19(1):97–103. https://doi.org/10.5853/jos.2016.00675
Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen MA, Staals J, Hofmeijer J, van Oostayen JA, Lycklama A, Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk LC, Kappelle LJ, Lo RH, van Dijk EJ, de Vries J, de Kort PL, van Rooij WJ, van den Berg JS, van Hasselt BA, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog HM, Gerrits DG, van den Berg-Vos RM, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam WH, Roos YB, van der Lugt A, van Oostenbrugge RJ, Majoie CB, Dippel DW, Investigators MC (2015) A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 372(1):11–20. https://doi.org/10.1056/NEJMoa1411587
Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo JH, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn SI, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD, Investigators ET (2015) Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 372(11):1019–1030. https://doi.org/10.1056/NEJMoa1414905
Wun T, White RH (2009) Venous thromboembolism (VTE) in patients with cancer: epidemiology and risk factors. Cancer Investig 27(Suppl 1):63–74. https://doi.org/10.1080/07357900802656681
Funding source
This study was supported by a grant (CRI 16040-22) from Chonnam National University Hospital Biomedical Research Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics statement
This study was approved by the institutional review board (IRB) of Chonnam National University Hospital. All clinical investigations described in this study were conducted in accordance with the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from each patient or patient’s family member.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Disclaimer
The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cho, BH., Yoon, W., Kim, JT. et al. Outcomes of endovascular treatment in acute ischemic stroke patients with current malignancy. Neurol Sci 41, 379–385 (2020). https://doi.org/10.1007/s10072-019-04103-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-019-04103-y