Abstract
Background and purpose
This study aimed to evaluate the efficacy of intra-arterial thrombectomy (IAT) and prognosis for acute ischaemic stroke patients with active cancer.
Methods
We retrospectively reviewed 253 patients who underwent IAT within 24 h after stroke onset between January 2012 and August 2017. We classified the patients into active cancer (n = 26) and control groups (n = 227) and compared clinical data. Primary outcome was a modified Rankin scale score at 3 months with ordinal logistic regression (shift analysis).
Results
Initial National Institutes of Health Stroke Scale (NIHSS) and rate of successful recanalisation did not differ between groups, but the active cancer group showed poor outcomes at 3 months on shift analysis (P = 0.001). The independent predictors of poor prognosis were age [adjusted common odds ratio (aOR) 1.03, 95% confidence interval (CI) 1.01–1.05], baseline NIHSS (aOR 1.14, 95% CI 1.09–1.19), baseline C-reactive protein level (aOR 1.14, 95% CI 1.03–1.25), any cerebral haemorrhage (aOR 1.92, 95% CI 1.21–3.06), and active cancer (aOR 2.35, 95% CI 1.05–5.25). Mortality at 90 days was 30.8% in the cancer group and 8.8% in the control group (P = 0.003).
Conclusions
Although baseline characteristics and recanalisation rate after IAT up to 24 h after stroke onset were similar between acute ischaemic stroke patients with active cancer and without any cancer, stroke-related death and short-term outcome were significantly poorer in patients with active cancer than the controls. Post-procedural haemorrhage and active cancer itself were independent predictors of a decrease in functional independence at 3 months.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The steep increase in incidence of cancer-related ischaemic stroke (CRIS) it attributed to improvements in survival rates and the longevity of cancer patients, who are now at an increased risk of ischaemic stroke due to various causes, including cancer-associated hypercoagulable states chemotherapy- or radiotherapy-related vasculopathies and ‘classical’ migratory thrombosis [1]. There is an increase in the number of CRIS patients requiring thrombolysis [2]. However, patients with active cancer are frequently excluded as candidates for intravenous thrombolysis for various reasons, including a history of recent surgery or bleeding diathesis [3,4,5,6]. Therefore, there is a need for treatments other than recombinant tissue plasminogen activator (rtPA) in the hyperacute stage of CRIS that can improve prognosis.
Intra-arterial thrombectomy (IAT) does not require the systemic administration of a thrombolytic agent, and it may be applicable to patients with haematological abnormalities. Thus, it may be hypothesised that IAT may improve the prognosis of acute ischaemic stroke (AIS) patients with active cancer who are ineligible for intravenous thrombolysis. Some case reports have reported less favourable outcomes than expected with IAT treatment in patients with active cancer, recommending against its use for AIS in patients with active cancer [7,8,9]. Furthermore, five pivotal trials have excluded patients with active cancer as candidates for IAT [10,11,12,13,14].
This study aimed to investigate the clinical characteristics associated with poor outcomes of IAT for patients with acute CRIS up to 24 h and who had a clinical diffusion–perfusion mismatch, to establish predictive prognostic factors.
Materials and methods
Study sample
This study was conducted with the approval of the institutional review board of Asan Medical Center (IRB number: 2018–0879), which waived the need for written informed consent because of the retrospective nature of the study.
We reviewed the records of all consecutive patients who presented with AIS treated with IAT in Asan Medical Center between January 2012 and August 2017. The following exclusion criteria were applied: AIS without occlusion of the relevant artery; arterial reperfusion performed > 24 h after symptom onset; intracranial neoplasm or metastasis; other causes of AIS (e.g., arterial dissection, collagen vascular disease, in stent thrombosis and Moyamoya disease); failure of IAT due to technical reasons and if clinical follow-up with a modified Rankin scale (mRS) value at 90 days was unavailable.
The patients who underwent IAT were classified according to their cancer history into those with active cancer, inactive cancer, and no history of cancer (controls). Active cancer patients were those with any metastatic disease, were undergoing current treatment for a malignancy, and were offered treatment for a malignancy, but declined [4]. Inactive cancer patients were those whose records indicated an inactive past history of malignancy with no history or evidence of metastatic disease, and who had completed any planned treatments. We defined the period of 5 years as the absence of evidence of active cancer after the end of treatment. Nevertheless, patients with inactive cancer were excluded from the analysis because of uncertainty over their current cancer activity and the possibility of different features and prognosis compared to those with active cancer.
AIS treatment protocol
On admission, neurologists performed neurological examinations, including assessments using the National Institutes of Health Stroke Scale (NIHSS) and mRS. All patients underwent a non-enhanced cranial computed tomography (CT) scan and multimodal magnetic resonance (MR) imaging before IAT and within 48 h after the procedure. CT angiography was performed on patients unable to undergo MRI. In most cases, diffusion-weighted imaging–perfusion-weighted imaging mismatch was evaluated (by visual inspection of the perfusion images). Clot sign was defined as a hypointense signal that exceeded the contralateral vessel diameter [15]. Stroke severity was assessed using the NIHSS at both baseline and discharge. For patients who met the relevant criteria, intravenous rtPA was used within 3–4.5 h after symptom onset. Aspirin and clopidogrel were administered orally or via a nasogastric tube to the patients who underwent balloon angioplasty or intracranial stenting. After the IAT procedure, the patients continued medical treatment in the neurologic intensive care unit. Follow-up brain MR imaging and MR angiography (or brain CT and CT angiography) were performed 24–48 h after IAT.
Intra-arterial thrombectomy
The criterion for IAT at our hospital is evidence on CT angiography or MR angiography of the occlusion or severe stenosis of a large cerebral artery, including the anterior cerebral artery, middle cerebral artery (MCA) M1 or M2, internal carotid artery (ICA), common carotid artery and posterior circulation (the posterior cerebral artery, basilar artery and vertebral artery). Within 24 h after symptom onset, AIS was targeted by IAT and when the exact onset time could not be verified, the last time the patient felt normal was considered.
All procedures were performed via a transfemoral approach under local anaesthesia by two highly experienced neurointerventionists. Intravenous sedation under consciousness was used when necessary. The endovascular techniques used were chosen according to the circumstances; these included stent-retriever thrombectomy, suction thrombectomy, direct balloon angioplasty and/or stenting, direct stenting, intra-arterial urokinase and mechanical disruption.
Clinical and angiographic outcome measures
Patients were classified according to the Trial of ORG 10,172 in Acute Stroke Treatment (TOAST) criteria [16]. If there were more than two arterial occlusion sites, each was counted. The data related to cancer included the type of cancer, pathology, TNM staging, metastasis status, and modality of treatment. The clinical outcome was assessed by a stroke neurologist using the mRS at an outpatient visit 3 months after discharge; the assessment was made by telephone if the patient was unable to attend. The severity of 90-day disability was assessed according to the distribution of scores across the mRS (shift analysis) as primary outcome [17]. The patient’s reperfusion status was evaluated according to the modified thrombolysis in cerebral infarction (mTICI) scale [18]. Successful reperfusion was defined as mTICI 2b or 3. Two neuroradiologists, blinded to the clinical information, independently analysed the angiographic data, with any conflict resolved through consensus. Partial and complete reperfusion in the follow-up image was also considered as reperfusion. Time variables including symptom onset-to-groin puncture time, symptom onset-to-reperfusion time, groin puncture to reperfusion time, and total intervention time were investigated. The reperfusion time was defined as the first reperfusion with mTICI ≥ 2a. Cerebral haemorrhage included any subarachnoid or intracerebral haemorrhage (ICH) found on follow-up imaging. ICH was classified into four types: haemorrhagic infarction types 1 and 2 and parenchymal hematoma types 1 and 2 [19].
Statistical analysis
Student’s t test and the Mann–Whitney U test were used to compare continuous demographic and clinical characteristics variables. Categorical variables were compared using Pearson’s Chi-square test and Fisher exact test. Adjusted common odds ratios (aORs) were calculated comparing mRS outcomes after IAT in the cancer and control groups (shift analysis). The variables tested in the logistic regression models were those with P < 0.1 in the univariate analysis. Ordinal logistic regression analyses were performed to determine the independent factors that predicted the outcome after IAT. An interaction between risk factors and outcomes at 3 months was evaluated after adjustment of the same confounding variables. A two-tailed P value < 0.05 was considered significant. SPSS version 21.0 for Windows (IBM Corp., Armonk, NY, USA) and SAS version 9.4 (SAS Institute, Cary, NC, USA) were used for statistical analyses.
Results
Patients
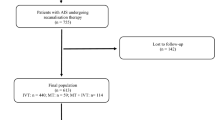
Between January 2012 and August 2017, 295 AIS patients with large vessel occlusion were treated by IAT within 24 h after symptom onset. Of these, 42 were excluded (Fig. 1); 13 patients in the control group due to technical failure and one patient in the cancer group and 12 in the control group due to failure to obtain mRS at 3 months. The remaining 253 patients were classified into the cancer (n = 26) or control group (n = 227). Baseline characteristics of the two groups are presented in Table 1. Patients in the cancer group were younger (63.2 vs. 68.8 years, P = 0.029) and fewer experienced atrial fibrillation (23.1% vs. 52.0%, P = 0.006). The initial NIHSS and premorbid mRS scores did not differ between groups (Table 1 and Supplementary Table 1). The mean C-reactive protein (CRP) was higher in the cancer group (3.4 vs. 0.7 mg/dL, P < 0.001), but there were no significant differences between groups in white blood cell and platelet counts. Detailed profiles of the patients’ cancer types are presented in Supplementary Table 2. The most common cancer pathology was adenocarcinoma and 11 patients (42%) in the cancer group experienced distant metastasis.
Clinical, radiographic, and angiographic characteristics
Although initial stroke severity was similar in both groups, the presumed stroke mechanisms and locations of the occluded arteries differed. In the control group, most patients had significant atherosclerotic lesions or embolic heart diseases that caused the ischaemic stroke. However, in the cancer group, undetermined aetiology was most common (Supplementary Table 1). The proportion with occlusion of the MCA was similar in both groups but occlusion of the ICA was more prevalent in the cancer group (57.7% vs. 29.5%; P = 0.007).
Clinical and angiographic efficacy outcomes
No differences between groups were noted in the time variables (Table 2). In addition, there was no difference in neurointerventional procedures performed, except suction thrombectomy. Successful recanalisation (mTICI 2b/3) after IAT was achieved in 88.5% (23/26) patients in the cancer group and 90.7% (205/227) patients in the control group, with no significant difference (P = 0.723). ICH was significantly higher in the cancer group (57.7% vs. 38.7%; P = 0.034), but there was no significant difference with regard to the type of ICH. There was a significant difference between groups in the proportion of patients with any cerebral haemorrhage (61.5% vs. 40.9%; P = 0.021). NIHSS at discharge was significantly higher in the cancer group [11 (interquartile range, 4.5–19.0) vs. 5 (1.5–3.0); P = 0.015] despite the lack of difference in reperfusion rates in the follow-up imaging.
In the cancer group, IAT was associated with a significant shift in the distribution of scores toward greater disability (P < 0.001 by the Cochran–Mantel–Haenszel test) (Fig. 2). The treatment effect was maintained even after adjusting for age, baseline NIHSS score, atrial fibrillation and ICA occlusion (aOR for 1 point improvement on the mRS, 3.5; 95% confidence interval (CI), 1.63–7.51; P < 0.001). Ordinal logistic regression showed that age (aOR 1.03; 95% CI 1.01–1.05), CRP (aOR 1.14; 95% CI 1.03–1.25), the presence of any cerebral haemorrhage (aOR 1.92; 95% CI 1.21–3.06), and active cancer (aOR 2.34 95% CI 1.05–5.25) were significant independent predictors of a shift toward poor outcomes; however, the presence of atrial fibrillation (aOR 0.56; 95% CI 0.35–0.89) was associated with a shift toward better outcomes in mRS at 90 days (Table 3). Mortality at 90 days was 30.8% (8/26) in the cancer group and 8.8% (20/227) in the control group (P = 0.003). In addition, stroke-related deaths were more common than cancer-related deaths in the cancer group: of the eight patients who died in this group, five died of cerebral infarction, two from progression of cancer, and one from sepsis. There was no difference in the prognosis regardless of distant metastasis (P = 0.491) (Table 4).
Modified Rankin Scale scores at 3 months after endovascular treatment. The bars show the distributions of modified Rankin Scale scores at 3 months for the patients in the cancer and control groups. Possible scores ranged from 0 to 6, with 0 indicating no symptoms, 1 no clinically significant disability, 2 slight disability, 3 moderate disability, 4 moderately severe disability, 5 severe disability, and 6 death. Endovascular thrombectomy in the cancer group was associated with a significant shift in the distribution of scores toward greater disability (P < 0.001 by the Cochran–Mantel–Haenszel test)
Discussion
This study showed significantly worse prognosis and increased risk of ICH after IAT for AIS patients with active cancer than for those without cancer, even with the application of various exclusion criteria regarding brain imaging or laboratory tests. In addition, post-procedural haemorrhage and active cancer itself were independent predictors of a decrease in functional independence at 3 months after the procedure. Active cancer was associated with shifts toward poor outcomes across the entire spectrum of disability, as were being female, being aged < 70 years, or having an NIHSS score < 10 or > 17 points in the subgroup analysis.
CRIS has a poor prognosis, which may be due to the cancer itself or to cancer-associated coagulopathy [20, 21]. The presence of cancer has a hazard ratio of 1.8 for a 6-month cumulative incidence of ischaemic stroke [22]. However, herein, the prognosis for acute CRIS patients after IAT was worse, although IAT might be expected to improve their prognosis. Furthermore, although the use of intravenous rtPA in the two study groups was not statistically different, the prognosis after IAT was poor for CRIS patients.
It is controversial whether active cancer causes haemorrhagic transformation or post-procedural haemorrhage more frequently in AIS, even when intravenous rtPA is administered [3, 4, 23, 24]. Herein, in the cancer group, ICH and any cerebral haemorrhage were more common, and any cerebral haemorrhage was an independent predictor of an unfavourable shift in the mRS score at 3 months. There was no difference in laboratory findings reflecting bleeding tendency between groups of patients with any cerebral haemorrhage (Supplementary Table 3). Given that asymptomatic cases can show deterioration in long-term clinical outcomes, it is necessary to develop post-procedural management that improves the prognosis after IAT [25, 26].
C-reactive protein has a high predictive value for stroke severity and early neurobehavioral outcomes and is an independent factor that predicts long-term outcomes and early mortality [27,28,29]. Herein, patients in the cancer group had significantly higher CRP levels, and this was an independent predictor of a poor outcome, suggesting that inflammation contributed to the haemorrhagic transformation. In addition, the hypercoagulable state represented by the elevation of D-dimer levels has been reported to be associated with poor prognosis in CRIS, [30, 31] which in the CRIS group in our study showed a mean D-dimer level of 9.2 μg/dL. In a prospective study, adequate correction of hypercoagulable state may be helpful in improving the survival of patients with CRIS [31].
Chemotherapy or radiotherapy may have accelerated the deterioration of the stroke; however, many patients discontinued treatment for several months before the stroke stabilised. We concluded that these treatments were not related to the unfavourable 3-month outcome.
This study had some limitations. First, it was a retrospective study with data from a single hospital, and the number in the cancer group was too small to draw valid conclusions for the outcome of IAT in active cancer patients. Our findings are corroborated by the findings of a recently published other single-centre study, which also revealed poorer short-term outcomes with active cancer compared to other stroke subtype after IAT, with implications future studies [32]. Second, the heterogeneity in the cancer group indicate that there may have been a bias in selecting IAT candidates, because patients who refused to receive IAT because of the shortened life expectancy and patients, whose neurologists decided against IAT were excluded, resulting in unfavourable results following IAT of CRIS patients. In addition, unlike other IAT trials, we used broad inclusion criteria for IAT and patients in the cancer group had relatively good functional status before AIS (all patients were within the range of premorbid mRS 0–2). Third, several procedures were used in IAT; however, this diversity is likely to reflect real-world data as the most advanced treatments were used, and importantly, the same treatments also applied to the control group.
Summary
In AIS patients, IAT with an onset-to-recanalisation time < 24 h resulted in worse outcomes for patients with active cancer than for the controls. Active cancer was an independent predictor of an unfavourable shift in mRS score distribution. Candidates for IAT among cancer patients should, therefore, be selected with caution. Given the poor prognosis of cancer patients despite the same recanalisation rate, further studies are needed on post-IAT management to improve their prognosis.
References
Grisold W, Oberndorfer S, Struhal W (2009) Stroke and cancer: a review. Acta Neurol Scand 119(1):1–16. https://doi.org/10.1111/j.1600-0404.2008.01059.x
Cronin KA, Lake AJ, Scott S, Sherman RL, Noone AM, Howlader N, Henley SJ, Anderson RN, Firth AU, Ma J, Kohler BA, Jemal A (2018) Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 124(13):2785–2800. https://doi.org/10.1002/cncr.31551
Cappellari M, Carletti M, Micheletti N, Tomelleri G, Ajena D, Moretto G, Bovi P (2013) Intravenous alteplase for acute ischemic stroke in patients with current malignant neoplasm. J Neurol Sci 325(1–2):100–102. https://doi.org/10.1016/j.jns.2012.12.008
Masrur S, Abdullah AR, Smith EE, Hidalgo R, El-Ghandour A, Rordorf G, Schwamm LH (2011) Risk of thrombolytic therapy for acute ischemic stroke in patients with current malignancy. J Stroke Cerebrovasc Dis 20(2):124–130. https://doi.org/10.1016/j.jstrokecerebrovasdis.2009.10.010
Casado-Naranjo I, Calle ML, Falcon A, Serrano A, Portilla JC, Ramirez-Moreno JM (2011) Intravenous thrombolysis for acute stroke in patients with cancer. J Neurol Neurosurg Psychiatry 82(12):1404–1405. https://doi.org/10.1136/jnnp.2010.207472
Graber JJ, Nayak L, Deangelis LM (2012) Use of recombinant tissue plasminogen activator in cancer patients with acute stroke. J Neurooncol 107(3):571–573. https://doi.org/10.1007/s11060-011-0780-5
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL (2018) 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 49(3):e46–e110. https://doi.org/10.1161/str.0000000000000158
Matsumoto N, Fukuda H, Handa A, Kawasaki T, Kurosaki Y, Chin M, Yamagata S (2016) Histological examination of trousseau syndrome-related thrombus retrieved through acute endovascular thrombectomy: report of 2 cases. J Stroke Cerebrovasc Dis 25(12):e227–e230. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.08.041
Merkler AE, Marcus JR, Gupta A, Kishore SA, Leifer D, Patsalides A, DeAngelis LM, Navi BB (2014) Endovascular therapy for acute stroke in patients with cancer. Neurohospitalist 4(3):133–135. https://doi.org/10.1177/1941874413520509
Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, Jansen O, Jovin TG, Mattle HP, Nogueira RG, Siddiqui AH, Yavagal DR, Baxter BW, Devlin TG, Lopes DK, Reddy VK, du Mesnil de Rochemont R, Singer OC, Jahan R, Investigators SP, (2015) Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 372(24):2285–2295. https://doi.org/10.1056/NEJMoa1415061
Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Roman L, Serena J, Abilleira S, Ribo M, Millan M, Urra X, Cardona P, Lopez-Cancio E, Tomasello A, Castano C, Blasco J, Aja L, Dorado L, Quesada H, Rubiera M, Hernandez-Perez M, Goyal M, Demchuk AM, von Kummer R, Gallofre M, Davalos A, Investigators RT (2015) Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 372(24):2296–2306. https://doi.org/10.1056/NEJMoa1503780
Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo JH, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn SI, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD, Investigators ET (2015) Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 372(11):1019–1030. https://doi.org/10.1056/NEJMoa1414905
Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, Ang T, Scroop R, Barber PA, McGuinness B, Wijeratne T, Phan TG, Chong W, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Donnan GA, Davis SM, Investigators E-I (2015) Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 372(11):1009–1018. https://doi.org/10.1056/NEJMoa1414792
Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen MA, Staals J, Hofmeijer J, van Oostayen JA, Lycklama a Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk LC, Kappelle LJ, Lo RH, van Dijk EJ, de Vries J, de Kort PL, van Rooij WJ, van den Berg JS, van Hasselt BA, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog HM, Gerrits DG, van den Berg-Vos RM, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam WH, Roos YB, van der Lugt A, van Oostenbrugge RJ, Majoie CB, Dippel DW, Investigators MC. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20. https://doi.org/10.1056/NEJMoa1411587.
Cho KH, Kim JS, Kwon SU, Cho AH, Kang DW (2005) Significance of susceptibility vessel sign on T2*-weighted gradient echo imaging for identification of stroke subtypes. Stroke 36(11):2379–2383. https://doi.org/10.1161/01.STR.0000185932.73486.7a
Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24(1):35–41
Saver JL, Gornbein J (2009) Treatment effects for which shift or binary analyses are advantageous in acute stroke trials. Neurology 72(15):1310–1315. https://doi.org/10.1212/01.wnl.0000341308.73506.b7
Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, Marks MP, Prabhakaran S, Kallmes DF, Fitzsimmons BF, Mocco J, Wardlaw JM, Barnwell SL, Jovin TG, Linfante I, Siddiqui AH, Alexander MJ, Hirsch JA, Wintermark M, Albers G, Woo HH, Heck DV, Lev M, Aviv R, Hacke W, Warach S, Broderick J, Derdeyn CP, Furlan A, Nogueira RG, Yavagal DR, Goyal M, Demchuk AM, Bendszus M, Liebeskind DS (2013) Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 44(9):2650–2663. https://doi.org/10.1161/strokeaha.113.001972
Fiorelli M, Bastianello S, von Kummer R, del Zoppo GJ, Larrue V, Lesaffre E, Ringleb AP, Lorenzano S, Manelfe C, Bozzao L (1999) Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke 30(11):2280–2284
Zhang YY, Chan DK, Cordato D, Shen Q, Sheng AZ (2006) Stroke risk factor, pattern and outcome in patients with cancer. Acta Neurol Scand 114(6):378–383. https://doi.org/10.1111/j.1600-0404.2006.00709.x
Cutting S, Wettengel M, Conners JJ, Ouyang B, Busl K (2017) Three-month outcomes are poor in stroke patients with cancer despite acute stroke treatment. J Stroke Cerebrovasc Dis 26(4):809–815. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.10.021
Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, Panageas KS, DeAngelis LM (2017) Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol 70(8):926–938. https://doi.org/10.1016/j.jacc.2017.06.047
Murthy SB, Karanth S, Shah S, Shastri A, Rao CP, Bershad EM, Suarez JI (2013) Thrombolysis for acute ischemic stroke in patients with cancer: a population study. Stroke 44(12):3573–3576. https://doi.org/10.1161/STROKEAHA.113.003058
Sobolewski P, Brola W, Szczuchniak W, Fudala M, Sobota A (2015) Safety of intravenous thrombolysis for acute ischaemic stroke including concomitant neoplastic disease sufferers—experience from Poland. Int J Clin Pract 69(6):666–673. https://doi.org/10.1111/ijcp.12586
Desilles JP, Rouchaud A, Labreuche J, Meseguer E, Laissy JP, Serfaty JM, Lapergue B, Klein IF, Guidoux C, Cabrejo L, Sirimarco G, Lavallee PC, Schouman-Claeys E, Amarenco P, Mazighi M (2013) Blood-brain barrier disruption is associated with increased mortality after endovascular therapy. Neurology 80(9):844–851. https://doi.org/10.1212/WNL.0b013e31828406de
Lei C, Wu B, Liu M, Chen Y (2014) Asymptomatic hemorrhagic transformation after acute ischemic stroke: is it clinically innocuous? J Stroke Cerebrovasc Dis 23(10):2767–2772. https://doi.org/10.1016/j.jstrokecerebrovasdis.2014.06.024
Ghabaee M, Zandieh A, Mohebbi S, Fakhri M, Sadeghian H, Divani F, Amirifard H, Mousavi-Mirkala M, Ghaffarpour M (2014) Predictive ability of C-reactive protein for early mortality after ischemic stroke: comparison with NIHSS score. Acta Neurol Belg 114(1):41–45. https://doi.org/10.1007/s13760-013-0238-y
Pandey A, Shrivastava AK, Saxena K (2014) Neuron specific enolase and c-reactive protein levels in stroke and its subtypes: correlation with degree of disability. Neurochem Res 39(8):1426–1432. https://doi.org/10.1007/s11064-014-1328-9
VanGilder RL, Davidov DM, Stinehart KR, Huber JD, Turner RC, Wilson KS, Haney E, Davis SM, Chantler PD, Theeke L, Rosen CL, Crocco TJ, Gutmann L, Barr TL (2014) C-reactive protein and long-term ischemic stroke prognosis. J Clin Neurosci 21(4):547–553. https://doi.org/10.1016/j.jocn.2013.06.015
Nam KW, Kim CK, Kim TJ, An SJ, Oh K, Mo H, Kang MK, Han MK, Demchuk AM, Ko SB, Yoon BW (2017) Predictors of 30-day mortality and the risk of recurrent systemic thromboembolism in cancer patients suffering acute ischemic stroke. PLoS ONE 12(3):e0172793. https://doi.org/10.1371/journal.pone.0172793
Lee MJ, Chung JW, Ahn MJ, Kim S, Seok JM, Jang HM, Kim GM, Chung CS, Lee KH, Bang OY (2017) Hypercoagulability and mortality of patients with stroke and active cancer: the OASIS-CANCER study. J Stroke 19(1):77–87. https://doi.org/10.5853/jos.2016.00570
Jung S, Jung C, Hyoung Kim J, Se Choi B, Jung Bae Y, Sunwoo L, Geol Woo H, Young Chang J, Joon Kim B, Han MK, Bae HJ (2018) Procedural and clinical outcomes of endovascular recanalization therapy in patients with cancer-related stroke. Interv Neuroradiol 24(5):520–528. https://doi.org/10.1177/1591019918776207
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to report.
Ethical standards
This study was approved by Asan Medical Center institutional review board.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, D., Lee, D.H., Suh, D.C. et al. Intra-arterial thrombectomy for acute ischaemic stroke patients with active cancer. J Neurol 266, 2286–2293 (2019). https://doi.org/10.1007/s00415-019-09416-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09416-8