Abstract
Background
Posterior communicating artery (PcomA) aneurysm can be classified into sidewall or bifurcation types based on the anatomical variation of fetal-type posterior cerebral artery (fPCA). The aims of this study were to investigate the significance of fPCA as an independent risk factor for the rupture of PcomA aneurysm and to evaluate other associated morphological and clinical risk factors.
Methods
We retrospectively reviewed clinical and radiological findings of 255 patients with PcomA aneurysms, which were treated in a single tertiary institute between January 2009 and December 2016. Univariate and multivariate analyses were performed to evaluate the associations between morphological and clinical variables and rupture status. Subgroup analysis was also performed based on the aneurysms with and without fPCA.
Results
Fifty-five out of 255 PcomA aneurysms (21.6%) were associated with fPCA. Multivariate logistic regression analysis showed that the superior direction of aneurysm dome (OR 9.106, p = 0.007), the presence of a bleb (OR 4.780, p < 0.001), a high aspect ratio (OR 1.878, p = 0.045), and fPCA (2.101, p = 0.040) were significantly associated with PcomA aneurysm rupture. In the fPCA group, only the presence of a bleb varied significantly between ruptured and unruptured PcomA aneurysms. However, in the non-fPCA group, larger aneurysms, the superior direction of dome, the presence of a bleb, and a high aspect and dome-to-neck ratio were significantly higher in the ruptured aneurysm group than in the unruptured aneurysm group.
Conclusions
The results demonstrate that fPCA may be an independent risk factor for rupture, especially together with the presence of a bleb.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Posterior communicating artery (PcomA) aneurysms are one of the most frequently detected forms of cerebral aneurysms, accounting for about 15–25% of all intracranial aneurysms [1, 2]. While the natural history of unruptured intracranial aneurysms (UIAs) remains unclear, recent studies suggest that PcomA aneurysms are prone to rupture compared with aneurysms located at other sites [2,3,4]. Because subarachnoid hemorrhage (SAH) due to aneurysmal rupture carries approximately 40–50% mortality rate, the ability to predict the risk of rupture for an incidental aneurysm is of great clinical value [5,6,7]. Therefore, investigators have proposed various clinical, morphological, and hemodynamic risk factors for the rupture of UIAs [2, 3, 8,9,10,11]. However, many studies have assessed the risk of rupture for saccular aneurysm in a monolithic fashion without distinction between sidewall and bifurcation types, despite varying features of aneurysm between the two subsets [7, 12]. Aneurysms involving PcomA can be classified into sidewall and bifurcation types because they represent unique anatomical variants of fetal-type posterior cerebral artery (fPCA) [7].
The fPCA is a common anatomical variation around the circle of Willis and defined as PCA that completely originates from the internal carotid artery (ICA) or has a small connection with the basilar artery (BA) [13, 14]. In this situation, the PCA is mainly supplied by ICA instead of BA [15]. A few studies have shown that fPCA is associated with occipital lobe infarction, life-threatening headache, and white matter degeneration. Other studies have reported that fPCA is associated with the occurrence of PcomA aneurysms [14, 16]. However, to the best of our knowledge, few studies evaluated the morphological risk factors for the rupture of PcomA aneurysms, with a focus on fPCA [16,17,18]. The aim of this study was to investigate the significance of fPCA as an independent risk factor for the rupture of PcomA aneurysm and to evaluate other associated morphological and clinical risk factors.
Materials and methods
Study population and clinical characteristics
A total of 2396 consecutive cases of intracranial ruptured and unruptured aneurysms were treated with open surgery or endovascular treatment (EVT) in a single tertiary institute between January 2009 and December 2016. Of these patients, we included only those whose aneurysms arose from the ICA-PcomA junction. Aneurysms involving other locations including the paraclinoid, anterior choroidal artery (AchoA), ICA bifurcation, middle cerebral artery (MCA), A1, anterior communicating artery (AcomA), distal anterior cerebral artery (DACA), and posterior circulation were excluded, leaving a total of 284 patients with PcomA aneurysm. Among these patients, we excluded 20 patients with PcomA aneurysm because there was no preoperative diagnostic digital subtraction angiography (DSA). Nine patients who underwent repeated treatment following initial treatment at another hospital were also excluded because we could not evaluate the characteristics of the initial aneurysm. After exclusion, a total of 255 aneurysms were finally included in this study.
We retrospectively reviewed the patients’ medical records to obtain their baseline information (age, sex), medical history (hypertension, hyperlipidemia, prior stroke history), and smoking history (current smoker or not). Hypertension was defined as taking antihypertensive drugs, a systolic blood pressure ≥ 140 mmHg, or a diastolic blood pressure ≥ 90 mmHg. Hyperlipidemia was defined as taking antihyperlipidemic agents or a total cholesterol level ≥ 240 mg/dL.
Morphological assessment of PcomA aneurysms
Morphological characteristics of all PcomA aneurysms included in this study were evaluated by DSA including three-dimensional rotational angiography (3DRA). Radiologic features, including aneurysm size, the direction of aneurysm dome, the presence of a bleb, aspect ratio, dome-to-neck ratio, and fPCA, were retrospectively reviewed by two observers who were blinded to information pertaining to subarachnoid hemorrhage (SAH), under the supervision of an experienced neuroradiologist.
The direction of aneurysm dome was classified as anterior, posterior, superior, inferior, medial, or lateral directions. The direction was defined based on the positional relation between the aneurysm dome and the skull base on routine DSA and 3DRA. Aneurysm size was measured based on the largest diameter. The fPCA was defined as a PCA arising from only ICA without visualization of the P1 segment on vertebral artery (VA) angiography.
Statistical analysis
We performed statistical analysis using SPSS version 17 (SPSS, Chicago, IL, USA). All categorical variables were analyzed by Fisher’s exact test or chi-square test. Univariate analysis to evaluate the relevant factors for PcomA aneurysm rupture was performed using Fisher’s exact test or chi-square test. Multivariate logistic regression analysis to determine risk factors for rupture was performed including variables with p value < 0.05 in univariate analysis. Less than 0.05 of p value was regarded as statistically significant.
Results
Univariate and multivariate analyses of potential clinical and morphological risk factors associated with aneurysm rupture
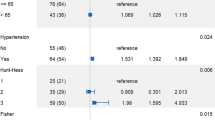
The results of univariate and multivariate analyses of clinical and morphological risk factors are summarized in Table 1. The proportion of old age (more than 70 years) and current smokers was significantly higher in the ruptured group than in the unruptured group by univariate analysis. The mean aneurysm size was significantly greater in the ruptured group than in the unruptured group. Aneurysm with size greater than 7 mm, superior direction, a bleb, high aspect ratio, and fPCA was shown to occur at a significantly higher proportion in the ruptured group by univariate analysis. Multivariate logistic regression analysis was performed with clinical and morphological variables that were significantly associated with aneurysm rupture (p < 0.05) in univariate analysis. The results of multivariate analysis showed that superior direction of aneurysm dome (odds ratio (OR) 9.106, 95% confidence interval (CI) 1.849–44.841), presence of a bleb (OR 4.780, 95% CI 2.414–9.467), high aspect ratio (OR 1.878, 95% CI 1.015–3.473), and aneurysms arising in fPCA (OR 2.101, 95% CI 1.033–4.276) were significantly associated with rupture (p < 0.05) (Fig. 1).
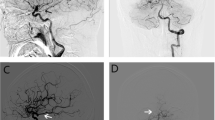
Illustrative cases of ruptured aneurysm with fPCA (a–c) and unruptured aneurysm with non-fPCA (d, e). a Non-enhanced brain CT of a 55-year-old female patient demonstrates SAH in basal cistern and both Sylvian cisterns. b Lateral view of DSA shows PcomA aneurysm with superior direction and fPCA (black arrow). c 3DRA shows an elongated and irregularly shaped aneurysm with blebs. d, e On the other hand, in case of unruptured PcomA aneurysm, the DSA and 3DRA show a round and smooth aneurysm with small diameter PcomA (arrowhead)
Clinical and morphological differences between unruptured and ruptured aneurysms with or without fPCA
Of the 255 aneurysms included in this study, 55 aneurysms (21.5%) were arising from fPCA. In the fPCA group, only the presence of a bleb was significantly different between the ruptured and unruptured groups. Other clinical characteristics or morphological features showed no significant differences between the ruptured and unruptured groups for aneurysms with fPCA. On the other hand, for aneurysms without fPCA, the proportion of larger aneurysm size, superior direction of dome, presence of a bleb, and high aspect and dome-to-neck ratio was significantly higher in the ruptured aneurysm group than in the unruptured aneurysm group (Table 2).
Discussion
Intracranial aneurysm involving PcomA is a unique aneurysm that can be divided into bifurcation (aneurysm arising from fPCA) and sidewall (aneurysm arising from non-fPCA) types [7]. In cases with non-fPCA, the PCA is mainly supplied by vertebrobasilar-P1 artery rather than PcomA [14, 19]. On the other hand, the blood supply into the PCA is predominantly or exclusively from the ipsilateral ICA-PcomA in cases with fPCA [14, 19]. Therefore, blood flow and blood pressure of PcomA from ipsilateral ICA are increased, leading to more impact against the wall of the ICA-PcomA junction in cases with fPCA [20]. In addition, the vessel wall of the arterial bifurcation lacks the media layer, which can result in thinning of the arterial wall [21]. For these reasons, some authors have reported that fPCA can be a risk factor for the formation of ICA-PcomA aneurysm [14, 16]. Baharoglu et al. clearly distinguished the morphological and hemodynamic characteristics between bifurcation and sidewall aneurysms [7]. They also emphasized that ruptured bifurcation aneurysms represent 66.3% of all ruptured aneurysms in their report [7]. Therefore, we assumed that PcomA aneurysms arising from fPCA (bifurcation type) might be associated with an increased rupture risk compared with those arising from non-fPCA (sidewall type). In this study, a significantly higher proportion of fPCA was observed in the ruptured PcomA aneurysm group (OR 2.101, 95% CI 1.033–4.276, p = 0.040) by multivariate analysis. Thus, our results supported this hypothesis.
Matsukawa et al. reported that fPCA is not an independent risk factor for the rupture of PcomA aneurysm in 2014 [17]. However, their study evaluated fPCA mainly based on computed tomogram angiography (CTA) rather than DSA, and the sample size was relatively small. Lv et al. also reported that fPCA showed no significant difference between ruptured and unruptured PcomA aneurysms [16]. However, they investigated morphological risk factors, with a focus on small PcomA aneurysms (< 7 mm), and thus, this result might not represent all PcomA aneurysms. In another study by Zhang et al., investigators showed that PCA type was significantly associated with PcomA aneurysm rupture, but they divided PCA into lateral (if PcomA diameter/ICA diameter < 1/5) and bifurcation types (if PcomA diameter/ICA diameter ≥ 1/5) [18]. In our study, we included all available PcomA aneurysms treated in our hospital and fPCA was evaluated by DSA.
In our study, subgroup analyses of morphological and clinical differences between unruptured and ruptured aneurysms were performed according to a presence of fPCA. Baharoglu et al. have reported the need for analyses based on aneurysm type because sidewall and bifurcation aneurysms showed a clear morphological and hemodynamic dichotomy and they behave differently under identical 3D shape-based analysis [7]. The most commonly used risk factors, such as large maximal diameter, high aspect ratio, and high size ratio, were shown to be independently associated with rupture in sidewall aneurysms. However, these size-related features failed to discriminate rupture status in aneurysms arising at bifurcations. Moreover, they suggested that aneurysm dome shape and related bleb formation could be more important for rupture in bifurcation aneurysms [7]. Similar with a previous study, our results also showed different characteristics for rupture status between fPCA and non-fPCA groups. In PcomA aneurysms without fPCA, size-related features (maximal diameter, aspect ratio, and dome-to-neck ratio), direction of dome, and bleb formation showed significant difference between the rupture and unruptured groups. Accordingly, unruptured PcomA aneurysms without fPCA may be considered to treat if currently used size-related risk factors are observed. In contrast, only bleb formation was significantly different between the ruptured and unruptured groups in PcomA aneurysms with fPCA. Therefore, unruptured PcomA aneurysms with fPCA and a bleb can be considered as indication for treatment regardless of other clinical and morphological features.
There were some limitations to this study. First, the study was conducted retrospectively at a single institution and the number of patients with fPCA may not be large enough to draw definite conclusions about the relationship between fPCA and aneurysm rupture, possibly leading to selection bias and wide confidence interval. Second, the intraaneurysmal hemodynamics of PcomA aneurysm were not investigated. Third, alcohol consumption and smoking status were not quantitatively analyzed in our study. Further prospective large population data should be investigated to exclude any limitations.
Conclusions
The present result demonstrated that fPCA may be an independent risk factor for rupture, especially along with the presence of a bleb.
References
Golshani K, Ferrell A, Zomorodi A, Smith TP, Britz GW (2010) A review of the management of posterior communicating artery aneurysms in the modern era. Surg Neurol Int 1:88. https://doi.org/10.4103/2152-7806.74147
Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, Hashimoto N, Nakayama T, Sakai M, Teramoto A, Tominari S, Yoshimoto T (2012) The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med 366:2474–2482. https://doi.org/10.1056/NEJMoa1113260
Wiebers DO, Whisnant JP, Huston J 3rd, Meissner I, Brown RD Jr, Piepgras DG, Forbes GS, Thielen K, Nichols D, O’Fallon WM, Peacock J, Jaeger L, Kassell NF, Kongable-Beckman GL, Torner JC (2003) Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 362:103–110
Brown RD Jr, Broderick JP (2014) Unruptured intracranial aneurysms: epidemiology, natural history, management options, and familial screening. Lancet Neurol 13:393–404. https://doi.org/10.1016/s1474-4422(14)70015-8
Fogelholm R, Hernesniemi J, Vapalahti M (1993) Impact of early surgery on outcome after aneurysmal subarachnoid hemorrhage. A population-based study. Stroke 24:1649–1654
van Gijn J, Kerr RS, Rinkel GJ (2007) Subarachnoid haemorrhage. Lancet 369:306–318. https://doi.org/10.1016/s0140-6736(07)60153-6
Baharoglu MI, Lauric A, Gao BL, Malek AM (2012) Identification of a dichotomy in morphological predictors of rupture status between sidewall- and bifurcation-type intracranial aneurysms. J Neurosurg 116:871–881. https://doi.org/10.3171/2011.11.Jns11311
Hoh BL, Sistrom CL, Firment CS, Fautheree GL, Velat GJ, Whiting JH, Reavey-Cantwell JF, Lewis SB (2007) Bottleneck factor and height-width ratio: association with ruptured aneurysms in patients with multiple cerebral aneurysms. Neurosurgery 61:716–722; discussion 722-713. https://doi.org/10.1227/01.Neu.0000298899.77097.Bf
Prestigiacomo CJ, He W, Catrambone J, Chung S, Kasper L, Pasupuleti L, Mittal N (2009) Predicting aneurysm rupture probabilities through the application of a computed tomography angiography-derived binary logistic regression model. J Neurosurg 110:1–6. https://doi.org/10.3171/2008.5.17558
Ujiie H, Tachibana H, Hiramatsu O, Hazel AL, Matsumoto T, Ogasawara Y, Nakajima H, Hori T, Takakura K, Kajiya F (1999) Effects of size and shape (aspect ratio) on the hemodynamics of saccular aneurysms: a possible index for surgical treatment of intracranial aneurysms. Neurosurgery 45:119–129 discussion 129-130
Frosen J (2016) Flow dynamics of aneurysm growth and rupture: challenges for the development of computational flow dynamics as a diagnostic tool to detect rupture-prone aneurysms. Acta Neurochir Suppl 123:89–95. https://doi.org/10.1007/978-3-319-29887-0_13
Hassan T, Timofeev EV, Saito T, Shimizu H, Ezura M, Matsumoto Y, Takayama K, Tominaga T, Takahashi A (2005) A proposed parent vessel geometry-based categorization of saccular intracranial aneurysms: computational flow dynamics analysis of the risk factors for lesion rupture. J Neurosurg 103:662–680. https://doi.org/10.3171/jns.2005.103.4.0662
Arjal RK, Zhu T, Zhou Y (2014) The study of fetal-type posterior cerebral circulation on multislice CT angiography and its influence on cerebral ischemic strokes. Clin Imaging 38:221–225. https://doi.org/10.1016/j.clinimag.2014.01.007
He Z, Wan Y (2018) Is fetal-type posterior cerebral artery a risk factor for intracranial aneurysm as analyzed by multislice CT angiography? Exp Ther Med 15:838–846. https://doi.org/10.3892/etm.2017.5504
Lv X, Li Y, Yang X, Jiang C, Wu Z (2012) Potential proneness of fetal-type posterior cerebral artery to vascular insufficiency in parent vessel occlusion of distal posterior cerebral artery aneurysms. J Neurosurg 117:284–287. https://doi.org/10.3171/2012.4.Jns111788
Lv N, Feng Z, Wang C, Cao W, Fang Y, Karmonik C, Liu J, Huang Q (2016) Morphological risk factors for rupture of small (<7 mm) posterior communicating artery aneurysms. World Neurosurg 87:311–315. https://doi.org/10.1016/j.wneu.2015.12.055
Matsukawa H, Fujii M, Akaike G, Uemura A, Takahashi O, Niimi Y, Shinoda M (2014) Morphological and clinical risk factors for posterior communicating artery aneurysm rupture. J Neurosurg 120:104–110. https://doi.org/10.3171/2013.9.Jns13921
Zhang Y, Jing L, Liu J, Li C, Fan J, Wang S, Li H, Yang X (2016) Clinical, morphological, and hemodynamic independent characteristic factors for rupture of posterior communicating artery aneurysms. J Neurointerv Surg 8:808–812. https://doi.org/10.1136/neurintsurg-2015-011865
Shaban A, Albright KC, Boehme AK, Martin-Schild S (2013) Circle of Willis variants: fetal PCA. Stroke Res Treat 2013:105937–105936. https://doi.org/10.1155/2013/105937
Xu J, Yu Y, Wu X, Wu Y, Jiang C, Wang S, Huang Q, Liu J (2013) Morphological and hemodynamic analysis of mirror posterior communicating artery aneurysms. PLoS One 8:e55413. https://doi.org/10.1371/journal.pone.0055413
Lin W, Ma X, Deng D, Li Y (2015) Hemodynamics in the circle of Willis with internal carotid artery stenosis under cervical rotatory manipulation: a finite element analysis. Med Sci Monit 21:1820–1826. https://doi.org/10.12659/msm.892822
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, Z., Kim, B.S., Lee, K.S. et al. Morphological and clinical risk factors for the rupture of posterior communicating artery aneurysms: significance of fetal-type posterior cerebral artery. Neurol Sci 40, 2377–2382 (2019). https://doi.org/10.1007/s10072-019-03991-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-019-03991-4