Abstract
Background
Endovascular treatment (EVT) of posterior communicating artery aneurysms (PcomA) is challenging because of posterior communicating artery (Pcom) architecture. Additionally, these aneurysms have a high risk of recanalization compared with those located elsewhere.
Methods
The radiographic findings of 171 patients treated with EVT at two institutions were retrospectively reviewed. Univariate and multivariate analyses were performed, and subgroup analyses were performed based on Pcom characteristics.
Results
Recanalization of PcomAs occurred in 53 patients (30.9%). Seven patients (4.0%) were retreated (six endovascularly and one with microsurgical clipping). The mean follow-up duration was 27.7 months (range: 3.5–78.6). The maximum diameter (odds ratio [OR] 1.23, P = .006, 95% CI 1.07–1.44), a Raymond–Roy classification of grade II or III (OR 2.26, P = .03, 95% CI 1.08–4.82), and the presence of reinforcement (balloon or/and stent, OR 0.44, P = .03, 95% CI 0.20–0.91) were associated with recanalization using multivariate logistic regression. Significant differences were found in maximum aneurysm diameter (P = .03) between normal- and fetal-type Pcoms on analysis of variance.
Conclusions
The recanalization rate of PcomAs after EVT was 30.9%; the retreatment rate was 4.0%. Maximum diameter, Raymond–Roy classification, and presence of reinforcement were significantly associated with recanalization but not associated with fetal-type Pcom. Aneurysm size was larger in patients with a fetal-type Pcom than in those with a normal Pcom. Pcom size was not related to recanalization rate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, endovascular treatment (EVT) has emerged as a feasible and acceptable option for the treatment of aneurysms [11, 12]; however, despite coil embolization proving effective, it has not shown promising recanalization rates. Posterior communicating artery aneurysms (PcomAs) are particularly susceptible to recanalization after coil embolization, with a 30% higher rate than aneurysms located elsewhere (P = 0.03) [1, 3]. Additionally, the posterior communicating artery (Pcom) has unique features in terms of coil embolization. PcomAs may be classified as either bifurcation or sidewall variants, depending on the caliber ratio of the Pcom and the P1 segment; therefore, treatment options vary accordingly [2].
This study sought to identify the risk factors and rates of recanalization that develop after coil embolization of PcomAs, with a special focus on the anatomic features of the Pcom and PcomA.
Methods
Patient selection
The need for institutional review board approval and to obtain informed consent from patients was waived due to the retrospective nature of this study. The electronic medical records of patients with PcomAs treated with EVT at two neurovascular centers were reviewed. A total of 3986 consecutive patients with ruptured and unruptured intracranial aneurysms were treated with microsurgical clipping or EVT at two tertiary hospitals between January 2013 and December 2019. Of these patients, 516 (13.0%) had a PcomA. PcomAs were diagnosed using computed tomography angiography, magnetic resonance angiography (MRA), and digital subtraction angiography (DSA). Of these cases, we included only those where the Pcom was located at the internal carotid artery (ICA)—Pcom junction. Patients who underwent microsurgical clipping were excluded, resulting in a total of 235 patients with PcomAs. The following patients were excluded for the following reasons: 41 due to lack of postoperative follow-up imaging, 16 who underwent repeated treatment after initial treatment that occurred before January 2013, 5 who underwent flow-diverter treatment, 1 who had a pseudoaneurysm, and 1 because of the presence of a dissecting aneurysm.
Data extraction
Demographic and clinical data were obtained from patient medical records; procedural details were collected from the procedural notes.
All patients had undergone prior cerebrovascular imaging using DSA. 3D DSA angiograms were reviewed to identify the following PcomA characteristics: aneurysm size (neck, depth, length, and width), maximum diameter, size ratio, relationship with the ICA, type of Pcom, and the occurrence of occlusion. Angiographic outcomes were classified using the Raymond–Roy classification system [10].
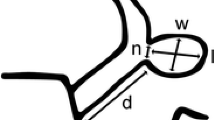
All aneurysms were evaluated and classified as either fetal, hypoplastic, or normal Pcom. A Pcom was defined as fetal-type when it had a size larger than or equal to that of the ipsilateral P1 segment of the posterior cerebral artery (PCA) and when it supplied the majority of blood flow to the PCA territory [3]. A Pcom was defined as hypoplastic-type when no vessel was found between the ophthalmic artery and the anterior choroidal artery. In these cases, the anterior choroidal artery was identified from the MRA source image. A Pcom was defined as normal-type when in had a size smaller than the ipsilateral P1 segment of the PCA (Fig. 1).

source image. B A fetal-type Pcom was defined as a Pcom larger than or equal in size to the ipsilateral P1 segment of the PCA. C A normal-type Pcom was defined as a Pcom smaller than the ipsilateral P1 segment of the PCA. (D) The white arrows indicate the anterior choroidal artery. Pcom, posterior communicating artery; MRA, magnetic resonance angiography; PCA, posterior cerebral artery
Posterior communicating artery type (hypoplastic/normal/fetal). We classified posterior communicating artery aneurysms according to Pcom size. A hypoplastic Pcom (A) was defined as that without a vessel between the ophthalmic artery and the anterior choroidal artery. In these cases, the anterior choroidal artery (arrow) was identified from the MRA
Recanalization was defined as new or increased contrast filling of an aneurysm with or without aneurysm growth. Major recanalization was defined as contrast filling of the aneurysm dome, significant coil compaction, or aneurysmal regrowth [14]. Retreatment was recommended for all patients with major recanalization.
All patients underwent neurological examination and were assigned to one of two groups based on the Hunt-Hess scale before treatment: good grade (I–III) or poor grade (IV–V). The modified Rankin Scale (mRS) was used to assess functional outcome measure after treatment; patients were divided into two groups: good prognosis (0–2) and poor prognosis (3–6).
The direction of the angle between the projection line of the aneurysmal dome and the communicating segment of the ICA was measured using the lateral and anteroposterior view. A superior direction was defined as an angle larger than 90 degrees and an inferior direction as an angle smaller than 90 degrees.
Reinforcement included stent and/or balloon-assisted coiling.
Procedure
All patients with unruptured aneurysms received periprocedural antiplatelet therapy and platelet function testing per the protocol of the relevant institution. Intraprocedural heparin was administered to achieve an activated clotting time of 2 to 3 times the baseline. Patients were treated by five interventionists with 5 to 20 years of experience. In all patients, aneurysm coiling was performed under general anesthesia using a biplane angiography unit.
After initial coiling, routine imaging follow-up was conducted using 3.0 T MRA at 6, 18, 30, and 60 months. Follow-up MRA was reviewed for recanalization of the PcomA; if recanalization was suspected, DSA was used for surveillance by the treating physician.
Retreatment was considered if the aneurysm volume of the recurred sac was ≥ 20.0 mm3 on 3D angiogram. The modality of retreatment was decided based on consensus by a multidisciplinary neuro-interventional team. The final decision was made by the neurosurgeon in charge.
Statistical analyses
Univariate analysis was performed to determine the association of recanalization with factors and characteristics associated with rupture status. The chi-square test was used for categorical variables and the logistic regression test for continuous numerical variables. Multivariate logistic regression analysis was performed for variables with an unadjusted effect, and a P < 0.20 in univariate analysis was used to determine independent associations between the recurrence and characteristics of rupture. The results of binary logistic regression were reported as odd ratios (ORs) with P < 0.05 for a 95% confidence interval (CI) considered statistically significant. We compared characteristics related to the Pcom size using chi-square tests and analyses of variance with post hoc analysis to determine associations between aneurysm characteristics. The program used for statistical analysis was SPSS (SPSS v19; IBM Corp, Armonk, NY, USA).
Results
A total of 171 patients (female: 151, 88.3%; mean age: 61.9 years) with a PcomA underwent an EVT procedure. Among these patients, 85 (49.4%) had hypertension and 54 (31.4%) presented with a ruptured aneurysm. Regarding aneurysmal factors, the mean maximum diameter was 6.12 mm (range 1.5–24.8), mean aspect ratio was 1.56 (range 0.7–5.6), and mean size ratio was 1.07 (range 0.4–2.0). Of the total patients, 17 (9.9%) showed superior dome projection, 75 (43.6%) showed a fetal-type Pcom, and 37 (21.5%) showed a hypoplastic Pcom. A normal Pcom was observed in 59 patients (34.3%). Of 171 patients, 117 patients had unruptured aneurysms, all with a Hunt-Hess scale 0 and mRS 0. Among the 54 patients with a ruptured aneurysm, 48 patients were good grade and 6 patients poor grade. In total, 47 had a good prognosis and 7 patients a poor prognosis. One patient with poor progression was that with vasospasm. Nine patients died within 6 months due to subarachnoid hemorrhage (7 patients) or other medical complications (2 patients, pneumonia and renal failure). These nine patients were excluded from our data analysis due to an insufficient follow-up imaging duration of at least 6 months.
Nine among 171 patients exhibited procedure-related thromboembolism (6 SAH patients/3 unruptured patients, 5.2%). Intra-arterial tirofiban was injected using a microcatheter. The major thrombus burden resolved away after injection of tirofiban in all 9 patients, and thus mechanical thrombectomy was not required. However, three patients (1.7%) showed diffusion restriction in the ipsilateral territory with neurological deterioration.
There were no arterial ruptures during the procedure or delayed hemorrhagic complications.
All aneurysms were successfully treated without (n = 102, 59.6%) or with (n = 69, 40.4%) a balloon and/or stent, and immediate post-procedural angiogram revealed 100 (58.1%) Raymond–Roy class I, 64 (37.4%) class II, and 7 (4.0%) class III aneurysms.
Retreatment was performed in 7 (4.0%) patients; 1 patient was treated with microsurgical clipping, while the remaining six patients underwent EVT. There were no recanalizations after retreatment at an average follow-up duration of 15.5 months (3.6–44). There were also no re-ruptures nor procedure-related complications.
Recanalization
Recanalization was noted after initial EVT in 53 patients (30.9%). Follow-up MRA was performed in all patients (mean: 27.7 months; range 3.5–78.6). In univariate analysis, significant risk factors for recurrence comprised ruptured status (P = 0.04), maximum diameter (P = 0.03), a Raymond–Roy classification of grade II or III (P = 0.02), and the presence of reinforcement in the form of a balloon or/and stent (P = 0.05). In multivariate logistic regression analysis, statistically significant factors included maximum diameter (OR 1.23, 95% CI 1.07–1.44, P = 0.01), a Raymond–Roy classification of grade II or III (OR 2.26, 95% CI 1.08–4.82, P = 0.03), and reinforcement in the form of a balloon or/and stent (OR 0.44, 95% CI 0.20–0.91, P = 0.03). These results are presented in Table 1.
In subgroup analysis based on Pcom size, maximum diameter differed significantly between those with fetal-type Pcoms and normal Pcoms (6.8 ± 3.5 vs 5.6 ± 1.9 mm; P = 0.03); however, no significant difference was found based on rupture status or the occurrence of recanalization (Table 2, Fig. 2).
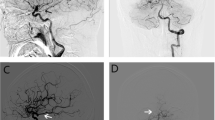
Initial coil embolization and recanalization of fetal-type (a and b), hypoplastic-type (c and d), and normal-type (e and f) posterior communicating arteries. Characteristics of the Pcom were not associated with recanalization. Raymond–Roy occlusion classification of these three patients was grade II. Recanalization occurred after 1 year (b), 9 months (d), and 29 months (f), respectively. The white arrow indicates the anterior choroidal artery and the black arrow indicates the Pcom. Pcom, posterior communicating artery
Kaplan–Meier analysis shows cumulative survival without recanalization by related risk factors. Log rank tests also showed that a maximal diameter over 7 mm (p = 0.037), lack of reinforcement (p = 0.016), and Raymond–Roy classification II or III (p = 0.048) were statistically significant risk factors for recanalization (Fig. 3).
Discussion
This study identified the incidence of and the risk factors for the recanalization of PcomAs after EVT. The only independent factors predisposing a PcomA to recanalization were the maximum diameter, a Raymond–Roy classification of grade II or III, and the presence of reinforcement. Furthermore, the anatomic characteristics of the Pcom were not associated with rupture status or recanalization after EVT.
A recent meta-analysis found a recanalization and retreatment rate of 20.8% and 10.3% after EVT of cerebral aneurysms, respectively [4]. The International Subarachnoid Aneurysm Trial found that 17.4% of ruptured aneurysms recurred after EVT. In particular, PcomAs are more susceptible to retreatment than aneurysms located at other sites (P = 0.03) [1]. The current study found that recanalization of PcomA occurs after EVT in 33.1% of cases [3]. Considering that PcomAs are subject to recurrence, we studied the recanalization of PcomAs after EVT.
The Pcom is a unique artery that connects the anterior and posterior cerebral circulation. If the embryonic Pcom fails to regress, the dominant blood supply to the occipital lobes originates from the ICA via a fetal-type Pcom instead of from the vertebrobasilar system [13]. Thiarawat et al. found that fetal-type Pcoms may be implicated in the formation of an aneurysm, as they are associated with a higher risk of PcomAs (p < 0.001) [15].
In recent studies, the association between PcomA rupture and fetal-type Pcom has remained controversial. Circle of Willis anomalies are more commonly found in ruptured than in unruptured cerebral aneurysms [5, 7, 16]. Thiarawat et al. found that Pcom variant was not associated with rupture status [15]. In our study, rupture status was also not related to Pcom type. Choi et al. demonstrated that Pcom type had no impact on the occurrence of recanalization [3]. In this study, aneurysmal features were classified into three groups depending on Pcom type: fetal, normal, or hypoplastic. Aneurysm size was significantly larger in fetal-type than normal-type Pcoms. Moreover, fetal-type Pcoms experienced the largest number of PcomAs and ruptures, although this was not a statistically significant finding. Fetal-type Pcom was thus associated with aneurysm size, but not with the risk of rupture or recanalization. Therefore, in terms of recanalization, fetal-type Pcoms with PcomAs can be treated using EVT.
The direction of the aneurysm is determined by the direction of the parent artery. On hemodynamic study, the wall shear stress in the treated neck of a totally embolized aneurysm susceptible to future recanalization was markedly higher than that in the original aneurysm neck prior to embolization [8, 9]. Therefore, we studied the relationship between the direction of the Pcom and the occurrence of recanalization. The direction of the Pcom had no correlation with the occurrence of recanalization.
Many studies have investigated the risk factors involved in the recanalization of PcomAs. Jeon et al. found that the deployment of a stent could prevent recanalization because of the reconstruction of the parent artery [6], and Son et al. demonstrated that the recanalization rate was higher in larger aneurysms (> 15 mm) [14]. Similar to previous studies, this study found that maximum diameter (7.3 ± 3.7 mm, P = 0.006) and the use of reinforcement such as a balloon and/or stent (P = 0.03) were significantly associated with the recanalization of PcomAs. Furthermore, initial coil embolization was significantly correlated with the occurrence of recanalization. Therefore, patients with a Raymond–Roy classification of grade II or III, those who undergo coil embolization without the use of a stent and/or balloon, and those who aneurysms larger than 5.2 ± 2.2 mm should be carefully monitored for recanalization.
Based on the results of the our study, it is important to increase packing density using a balloon or stent. In this study, the average size of recurrent aneurysm was 7 mm. In large aneurysms like this, it is worth considering treatment options other than coil embolization, like clipping, or other endovascular treatment. Pcom type does not have a significant effect on recurrence, so it is not a factor that needs to be considered when selecting treatment modality.
Limitations
Limitations of this study include its retrospective nature. Additionally, this study includes specifically selected patients from two tertiary hospitals. A multicenter prospective study is needed to increase the generalizability of the study results. In this study, the mean follow-up period was 27.7 months; therefore, the rate of recanalization could have been underestimated, as could recanalization after retreatment. Long-term follow-up is necessary. Furthermore, the complications of coil embolization, recanalization, and retreatment were not analyzed in detail.
Conclusion
The recanalization rate of PcomAs after EVT was 30.9%. Maximum diameter, Raymond–Roy classification, and the presence of reinforcement were significantly associated with the occurrence of recanalization; no association was found between Pcom type and recanalization, including fetal-type Pcoms. Therefore, careful monitoring of PcomAs after EVT is needed in in patient groups with the aforementioned risk factors.
Abbreviations
- EVT:
-
Endovascular treatment
- PcomA:
-
Posterior communicating artery aneurysm
- Pcom:
-
Posterior communicating artery
- MRA:
-
Magnetic resonance angiography
- DSA:
-
Digital subtraction angiography
- ICA:
-
Internal carotid artery
- PCA:
-
Posterior cerebral artery
References
Campi A, Ramzi N, Molyneux AJ, Summers PE, Kerr RS, Sneade M, Yarnold JA, Rischmiller J, Byrne JV (2007) Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the International Subarachnoid Aneurysm Trial (ISAT). Stroke 38:1538–1544
Cho YD, Lee WJ, Kim KM, Kang HS, Kim JE, Han MH (2013) Stent-assisted coil embolization of posterior communicating artery aneurysms. AJNR Am J Neuroradiol 34:2171–2176
Choi HH, Cho YD, Yoo DH, Lee HS, Kim SH, Jang D, Lee SH, Cho WS, Kang HS, Kim JE (2020) Impact of fetal-type posterior cerebral artery on recanalization of posterior communicating artery aneurysms after coil embolization: matched-pair case-control study. J Neurointerv Surg 12:783–787
Ferns SP, Sprengers ME, van Rooij WJ, Rinkel GJ, van Rijn JC, Bipat S, Sluzewski M, Majoie CB (2009) Coiling of intracranial aneurysms: a systematic review on initial occlusion and reopening and retreatment rates. Stroke 40:e523-529
Huhtakangas J, Lehecka M, Lehto H, Jahromi BR, Niemelä M, Kivisaari R (2017) CTA analysis and assessment of morphological factors related to rupture in 413 posterior communicating artery aneurysms. Acta Neurochir (Wien) 159:1643–1652
Jeon JP, Cho YD, Rhim JK, Yoo DH, Cho WS, Kang HS, Kim JE, Han MH (2016) Fate of coiled aneurysms with minor recanalization at 6 months: rate of progression to further recanalization and related risk factors. AJNR Am J Neuroradiol 37:1490–1495
Lazzaro MA, Ouyang B, Chen M (2012) The role of circle of Willis anomalies in cerebral aneurysm rupture. J Neurointerv Surg 4:22–26
Li C, Wang S, Chen J, Yu H, Zhang Y, Jiang F, Mu S, Li H, Yang X (2012) Influence of hemodynamics on recanalization of totally occluded intracranial aneurysms: a patient-specific computational fluid dynamic simulation study. J Neurosurg 117:276–283
Liu J, Jing L, Wang C, Zhang Y, Yang X (2016) Recanalization, regrowth, and delayed rupture of a previously coiled unruptured anterior communicating artery aneurysm: a longitudinal hemodynamic analysis. World Neurosurg 89:726.e725-726.e710
Mascitelli JR, Moyle H, Oermann EK, Polykarpou MF, Patel AA, Doshi AH, Gologorsky Y, Bederson JB, Patel AB (2015) An update to the Raymond-Roy occlusion classification of intracranial aneurysms treated with coil embolization. J Neurointerv Surg 7:496–502
McDougall CG, Spetzler RF, Zabramski JM, Partovi S, Hills NK, Nakaji P, Albuquerque FC (2012) The barrow ruptured aneurysm trial. J Neurosurg 116:135–144
Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, Sandercock P (2005) International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 366:809–817
Rhoton AL Jr (2002) Aneurysms Neurosurgery 51:S121-158
Son YJ, Kwon OK, Hwang G, Park NM, Oh CW, Bang JS (2016) Major recanalization occurs more often in young patients after unruptured aneurysm coil embolization. Acta Neurochir (Wien) 158:551–556
Thiarawat P, Jahromi BR, Kozyrev DA, Intarakhao P, Teo MK, Choque-Velasquez J, Niemelä M, Hernesniemi J (2019) Are fetal-type posterior cerebral arteries associated with an increased risk of posterior communicating artery aneurysms? Neurosurgery 84:1306–1312
Xu Z, Kim BS, Lee KS, Choi JH, Shin YS (2019) Morphological and clinical risk factors for the rupture of posterior communicating artery aneurysms: significance of fetal-type posterior cerebral artery. Neurol Sci 40:2377–2382
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (name of institute/committee) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Vascular Neurosurgery - Aneurysm
Rights and permissions
About this article
Cite this article
Kim, M.J., Chung, J., Park, K.Y. et al. Recurrence and risk factors of posterior communicating artery aneurysms after endovascular treatment. Acta Neurochir 163, 2319–2326 (2021). https://doi.org/10.1007/s00701-021-04881-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-021-04881-5